Leptin Regulates Appetite and Body Weight: Leptin is a hormone released by white adipose tissue (fat cells) located in the belly. Upon consuming food, leptin secreted from belly fat travels to the brain and activates the POMC-α-MSH-MC4R pathway in the arcuate nucleus of the hypothalamus to signal fullness. The function of leptin is extremely important to maintaining energy balance and preventing overeating.
Leptin Resistance Contributes to Obesity: Leptin signaling becomes disrupted during obesity and aging, and the loss of leptin signaling can make maintaining your body weight and preventing excess belly fat accumulation more challenging.
Disrupted Leptin Signaling in the Brain following Diet-Induced Obesity: Diet-induced obesity in mice is associated with increased activation of the mammalian target of rapamycin (mTOR) pathway, which directly disrupts the ability of leptin to activate the POMC-α-MSH-MC4R pathway during obesity. Chronic leptin resistance due to mTOR activation further increases food consumption, body weight, and belly fat accumulation.
Rapamycin Restores Leptin Signaling in Mice with Obesity: Rapamycin may function as a therapeutic strategy by inhibiting mTOR and restoring leptin’s ability to activate the arcuate nucleus’s POMC-α-MSH-MC4R pathway in the hypothalamus region of the brain. Rapamycin restoration of leptin sensitivity among mice with diet-induced obesity lowers their appetite, contributing to an overall reduction in body weight and fat mass. Importantly, rapamycin may help partially preserve lean muscle mass throughout the weight loss process.
Rapamycin Utilization: Rapamycin supplementation is effective even in the absence of dietary changes. However, weight and fat mass lost by mice during rapamycin treatment was quickly regained when treatment stopped, suggesting that continued rapamycin use may be required for sustained results. Likewise, rapamycin treatment was greatest among mice switching from a high-fat, obesity inducing diet to a standard chow diet, indicating rapamycin is most effective along with dietary changes.
Consequences of Rapamycin Use: Rapamycin may function as a prophylactic approach to maintain leptin signaling, prevent over consumption of calories, and prevent the development of obesity before pathology occurs. However, rapamycin has been shown to decrease lean mass negatively influence glucose metabolism by increasing insulin resistance. Rapamycin may be best utilized in combination with dietary changes, increased endurance and resistance exercise, and additional pharmacological approaches to overcome these potential drawbacks.
Translation to Humans with Obesity: The delivery of leptin-like molecules has been unsuccessful in humans with obesity, and no studies have examined rapamycin use to restore leptin resistance in humans.
Overview
The body functions as a highly choreographed set of systems, each interacting with multiple others to keep the body running smoothly. An important, yet underappreciated, example involves a molecular message that is released from fat cells in response to food intake and quickly travels to the brain, informing us that we are full. This one-way signaling highway is facilitated by the hormone leptin and is vital to regulating appetite and body weight.
If our bodily functions, appetite included, are so tightly regulated, why is it that we still gain weight with age? Likewise, why is it so challenging to lose weight when we restrict our calories with various diets?
For many individuals, the answer lies in leptin resistance—a breakdown in communication where the brain no longer responds to the fullness signal. Even as leptin levels rise, the signal fails to register. This failure quietly fuels overeating and makes sustained fat loss increasingly difficult. Leptin resistance is especially common in people with obesity, but it's not exclusive to them. In fact, aging itself is associated with changes in fat distribution—particularly the buildup of abdominal fat—that can impair leptin sensitivity even in those with stable body weight.
In other words, leptin resistance is not just a hallmark of obesity, but also a complication of aging.
Encouragingly, new research in mice offers a potential strategy for restoring leptin sensitivity and rebalancing appetite control. At the center of this research is rapamycin, a drug best known for its ability to slow aging and extend lifespan in animal models. In this review, we explore how rapamycin may help reawaken the brain’s ability to respond to leptin—leading to reduced food intake, lower fat mass, and new therapeutic possibilities for addressing obesity and age-related metabolic dysfunction.
Rapamycin: An Anti-Aging Drug with Anti-Obesity Potential
Rapamycin became a popular anti-aging treatment option after a 2009 landmark study in the journal Nature demonstrated that it increased the total lifespan of older mice [1]. In this study, mice began rapamycin supplementation at 600 days old (equivalent to ~40–60 years in humans) and lived up to 52% longer (from day 600 until death) compared to mice not receiving rapamycin (5–16% increase in total lifespan) [1].
In the 15 years since, an explosion of research has demonstrated improvements in immune, cardiovascular, neurocognitive, and skin health in humans [2]. Although the impact of rapamycin on other systems has not been thoroughly examined in humans, in mice, its potential benefits extend to numerous organ systems and have even shown promise in the treatment of several types of cancer [3].
What sets rapamycin apart from many other interventions is its ability to influence multiple hallmarks of aging at once. It improves mitochondrial efficiency, supports stem cell maintenance, slows cellular senescence, and reduces the pro-inflammatory secretions that accumulate in aged tissues [3, 4, 5] These wide-ranging effects have made rapamycin one of the most closely studied pharmacological tools in the field of longevity science.
Rapamycin is now understood to extend lifespan by modulating multiple hallmarks of aging. These include improvements in mitochondrial function, support for stem cell maintenance, attenuation of cellular senescence, and suppression of pro-inflammatory secretions associated with senescent cells [3, 4, 5]. The convergence of these effects has positioned rapamycin as one of the most extensively studied pharmacologic agents in the field of aging biology.
However, new evidence published by Dr. Jeffrey M. Friedman in Cell Metabolism identifies a new mechanism—leptin resistance—by which rapamycin improves the body’s ability to sense nutrient availability and enhance health and longevity [6].
Leptin is a hormone released by the stomach and white adipose tissue (fat cells) located in the abdomen. Upon consuming food, leptin secreted from both sites travels to the brain to signal fullness [7, 8]. Leptin function is critical for maintaining energy balance and preventing overeating. But in obesity—and, increasingly, with age—leptin’s signal becomes distorted. Despite high circulating levels of the hormone, the brain stops responding appropriately. [7]. This loss of leptin sensitivity can make maintaining a healthy body weight and preventing excess belly fat accumulation more challenging.
In this article, we review Dr. Friedman’s research from Princeton University that shows how rapamycin reduces food intake and fat mass in mice fed a high-fat diet who develop obesity [6]. In addition, we examine the mechanisms within the brain responsible for rapamycin’s restoration of leptin sensitivity and reversal of obesity.
What is Leptin, Leptin Resistance, and the Consequences for Obesity
As previously discussed, leptin is a hormone released by the stomach and abdominal fat cells in response to food intake. It functions as part of a negative feedback loop that helps regulate appetite and prevent overeating [7, 8]. Leptin secretion from the stomach appears to help regulate short-term appetite (immediately after a meal). In contrast, leptin secreted by abdominal fat cells is stimulated in part by rising insulin levels following a meal, contributing to longer-term appetite regulation (over several hours and general hunger levels) [8].
Once it crosses the blood-brain barrier, leptin binds to receptors in a specific region of the hypothalamus known as the arcuate nucleus (ARC). There, it activates a group of neurons that produce a protein called proopiomelanocortin (POMC). This precursor is processed into smaller signaling molecules, including alpha-melanocyte stimulating hormone (α-MSH), which then binds to MC4R receptors to suppress appetite and stabilize body weight [9, 10].
Simply stated, leptin enters the brain and triggers a chain reaction that suppresses appetite and helps us better recognize fullness during a meal.
In healthy individuals, this signaling chain works seamlessly. Leptin enters the brain, activates the POMC-α-MSH-MC4R pathway, and helps shut down hunger—both in the short term and over the course of several hours. It’s part of a tightly coordinated feedback loop that keeps food intake aligned with energy needs.
But when this system breaks down, the consequences are profound. In leptin resistance—a condition common in obesity and aging—the brain stops responding to the fullness signal. The POMC pathway becomes increasingly sluggish, and the brain behaves as though leptin were absent, even when it’s present in abundance. The result is persistent hunger, greater food intake, and gradual accumulation of fat—especially in the abdomen.
Over time, this dysfunction reinforces itself: more fat leads to more leptin secretion, but the brain becomes even less responsive. Although leptin resistance is difficult to measure directly in humans, one consistent marker is that blood leptin levels tend to rise in proportion to body fat, without corresponding decreases in appetite [11].
For years, researchers struggled to pinpoint the molecular source of this resistance. However, research by Tan and colleagues has provided a cellular and molecular basis for leptin resistance. Their research shows that mTOR, a nutrient-sensing protein complex, becomes overactive in the hypothalamus during obesity—and interferes with leptin’s ability to activate the POMC signaling pathway (Figure 1) [6]
Herein lies a potential solution to a major problem associated with both obesity and aging: rapamycin restores leptin sensitivity and improves the brain’s ability to recognize the “fullness” signal in mice. Although it is worth noting that additional research is required to corroborate these findings in humans, improvements in leptin sensitivity from rapamycin treatment reduces total food consumption, body weight, and fat mass in the process.
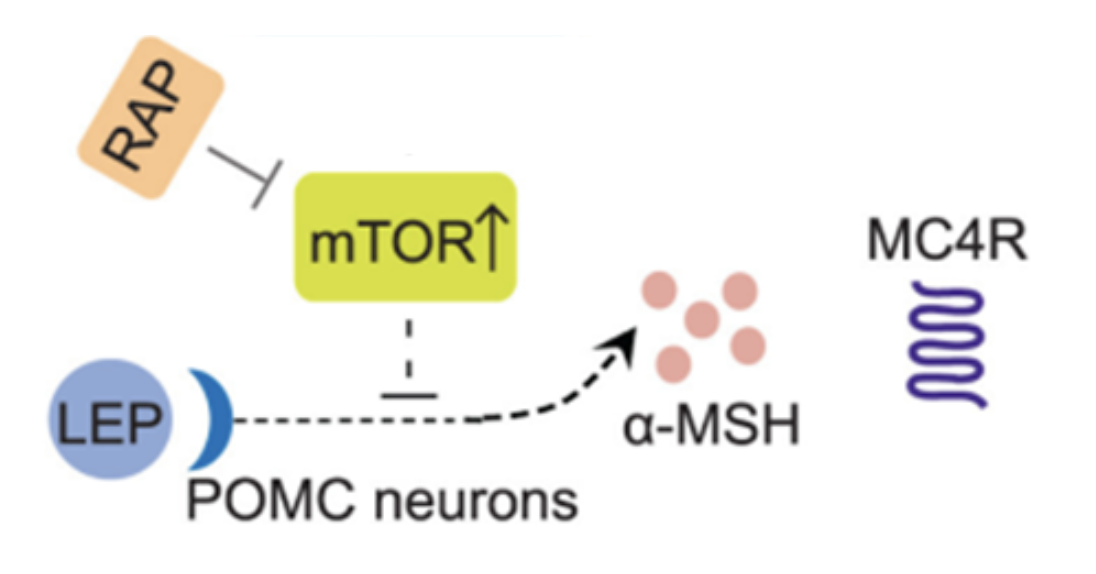
Figure 1. Leptin binds to its leptin receptor in the arcuate nucleus in the brain’s hypothalamus. Upon binding to its receptor, leptin activates proopiomelanocortin (POMC), which is processed into the alpha-melanocyte stimulating hormone (α-MSH) and binds to its MC4R receptor to reduce food intake and regulate body weight. During obesity, increased expression of the mammalian target of rapamycin (mTOR) inhibits the ability of leptin to regulate appetite and body weight. However, Tan and colleagues demonstrate that rapamycin (RAP) injection (2 mg/kg) inhibits mTOR and restores leptin’s ability to improve appetite regulation and reduction in body weight [6].
The mTOR Pathway May Induce Leptin Resistance Following High-Fat Diet
To investigate how leptin resistance develops—and how it might be reversed—Tan and colleagues designed a series of experiments comparing two foundational mouse models [6]. The first was a group of wild-type (WT) mice with normal physiology and intact leptin signaling. The second was a group of ob/ob mice, genetically modified to lack the Ob gene. These animals do not produce leptin and are predisposed to obesity, but they still have functioning leptin receptors and can respond to externally administered leptin.
Each mouse model was fed one of two diets. The first was a standard “chow diet” representing a well-balanced meal where rodents tend to remain weight stable. The second was a “high fat diet” (HFD) consisting of 60% fat, representing a Western diet known to give mice “diet-induced obesity” (DIO).
In summary, these four separate groups were evaluated in the study.
- Wild-type-chow (WT-C): A control population with normal leptin signaling. These rodents remain weight-stable and responsive to various experimental procedures.
- Wild-type-high fat diet (WT-HFD): Mice exposed to a Western diet. These mice develop obesity and elevated leptin levels but also become resistant to leptin’s appetite-suppressing effects.
- Ob/ob-chow (OB-C): Genetically leptin-deficient mice on a standard diet. These animals are prone to obesity due to constant hunger but remain sensitive to leptin if it’s administered therapeutically.
- Ob/ob fed a high-fat diet (OB-HFD): Leptin-deficient mice fed a Western diet. These mice gain even more weight than OB-C mice, though they still respond to leptin injections due to functional leptin receptors.
After 18 weeks, the researchers examined how these different groups responded to leptin. Mice on the high-fat diet—regardless of genotype—consumed more calories and gained more weight than their chow-fed counterparts. But the sequence of weight gain offered valuable insight: weight increased progressively across WT-C, OB-C, WT-HFD, and OB-HFD groups, suggesting that both genetic and dietary factors compound the problem.
Next, all mice were given daily injections of leptin for one week. The results were telling. As expected, leptin reduced food intake and body weight in WT-C mice, as well as in both OB-C and OB-HFD mice—confirming that these groups remained sensitive to leptin signaling. But in the WT-HFD group, leptin had no effect. Despite elevated leptin levels, the brain wasn’t registering the fullness signal. This was a clear demonstration that diet-induced obesity alone is sufficient to trigger leptin resistance.
To dig deeper, the researchers analyzed blood samples from the mice. Two amino acids stood out: methionine and leucine, both of which are known to activate the mTOR pathway. These metabolites were significantly elevated in the WT-HFD mice—the same group that showed resistance to leptin. Across all groups, higher levels of these mTOR-activating amino acids correlated with lower leptin sensitivity.
Thus, the authors hypothesized that mTOR activation may directly contribute to leptin resistance observed in the WT-HFD group of mice.
Rapamycin Helps Re-Sensitize Obese Mice to Leptin and Facilitate Weight Loss
In the next set of experiments, Tan and colleagues focused on wild-type mice that were fed a high-fat-diet (WT-HFD) for 18 weeks and developed both obesity and leptin resistance [6]. Researchers performed four experiments to understand the role of rapamycin, a molecule that inhibits mTOR activation, in restoring leptin sensitivity.
In the first experiment, mice fed a high-fat diet for 18 weeks were then injected with 2mg/kg rapamycin daily for 10 weeks. As a result, rapamycin decreased daily and total food intake, as well as reduced total body weight and fat mass compared to mice provided a placebo injection.
Researchers next addressed whether rapamycin increases leptin sensitivity. Leptin resistant mice fed a high-fat diet for 18 weeks were pretreated with rapamycin for 3 weeks, then provided two daily injections with leptin for 3 days. Leptin injection decreased high fat food intake and reduced total body weight and fat mass in mice pretreated with rapamycin, whereas mice lacking rapamycin pretreatment (placebo injection) remained unresponsive to leptin. These results confirmed that rapamycin likely has a role in restoring leptin sensitivity.
In the third experiment, researchers aimed to control for food intake as a potential variable. Thus, following 18 weeks of consuming a high-fat diet, mice pretreated with rapamycin had their food limited for 5 weeks. During this time, total food consumed was equal in mice with and without (placebo) rapamycin treatment. After 5 weeks, all mice were then injected with leptin twice per day for 3 days.
Despite similar amounts of food consumed, only mice treated with rapamycin and leptin exhibited reductions in food intake and body weight. Interestingly, total energy expenditure and fat metabolism were increased, and plasma leptin concentrations were reduced in mice receiving rapamycin and leptin.
Lastly, researchers switched the food consumed by the WT-HFD after 18 weeks from high fat to the standard, well-balanced chow for 4 weeks. As expected, body weight and fat mass significantly decreased in mice whose diets were switched compared to those whose diet remained unchanged. However, mice receiving rapamycin elicited a much larger reduction in daily and total caloric intake as well as larger reductions in total weight and fat mass compared to mice receiving the placebo injection.
Findings from this collection of experiments confirm that a high-fat diet that induces obesity also results in leptin resistance. Rapamycin appears to restore leptin sensitivity and decrease the amount of leptin secreted by fat cells required to regulate food consumption and facilitate weight loss in mice. Importantly, the weight lost in response to rapamycin came mostly from decreases in fat mass as opposed to lean mass, helping to preserve muscle mass throughout the intervention. Additionally, these responses, along with increased increases to overall energy expenditure, suggest that rapamycin may meet the criteria as an effective treatment strategy for sustained, long-lasting weight loss [12].
It is important to note that the dosing used in these experiments—2 mg/kg of rapamycin daily—is significantly higher and more frequent than doses used in human longevity protocols. In clinical practice, rapamycin is typically administered at a weekly dose of 0.075 to 0.1 mg/kg, which is approximately 10 to 20 times lower on a per-kilogram basis. The high daily dosing used in this animal study reflects a seemingly therapeutic approach specific to reversing obesity and leptin resistance, not a preventative or healthspan-oriented strategy.
Rapamycin Restores Leptin Sensitivity at the Molecular Level
In a deeper investigation of how rapamycin improves leptin sensitivity, Tan and colleagues examined a key region of the brain called the arcuate nucleus (ARC) of the hypothalamus [6]. This area acts as a control center for appetite, where hormonal signals like leptin are processed and converted into neural commands that influence food intake.
Using brain tissue from mice that had developed leptin resistance after 18 weeks on a high-fat diet (WT-HFD), the researchers found elevated levels of phosphoS6 (pS6), a molecular marker of mTOR activity [6]. This provided further evidence that hyperactive mTOR signaling was interfering with leptin’s ability to suppress appetite in the brain.
To determine whether rapamycin could reverse this disruption, the researchers administered the drug for just three days—long enough to influence molecular pathways but short enough to avoid changes in food intake or body weight [6]. They then performed RNA sequencing on the ARC to assess changes in gene expression. The results were revealing: rapamycin significantly altered the activity of POMC-expressing neurons that contain leptin receptors.
Specifically, rapamycin increased the expression of Pomc and Pcsk2, genes responsible for producing and processing the key peptides that mediate appetite suppression [6]. It also suppressed Gabrg3, a gene that inhibits POMC neuron activity, effectively removing a brake on the system. In parallel, rapamycin lowered the expression of Ptprm and Ptprt, two genes that deactivate STAT3, a central molecule in the leptin signaling pathway [6].
Together, these molecular changes suggest that rapamycin restores the brain’s ability to process leptin by both boosting the production of appetite-suppressing signals and preserving the integrity of downstream signaling.
To test whether these molecular changes translated into functional recovery, the researchers next measured POMC neuron activity using electrophysiology. In leptin-resistant mice on a high-fat diet, POMC neurons fired at sluggish rates—about 0.92 Hz—compared to 2.0 Hz in chow-fed controls (WT-C) [6]. But after three days of rapamycin treatment, firing rates in the high-fat diet group rebounded to 2.75 Hz, despite no changes in diet or weight.
The effect became even more pronounced when rapamycin was followed by leptin injections. In that case, neuron firing rates increased further, ranging from 3.58 to 4.81 Hz—levels well above baseline for either group [6]. These findings confirm that rapamycin doesn’t merely compensate for leptin resistance; it appears to repair the underlying signaling machinery in the hypothalamus, enabling the brain to respond to leptin accurately once again.
Rapamycin’s Effects on Leptin Depend on an Intact POMC–MC4R Pathway
To determine whether rapamycin’s appetite-suppressing effects depend on the brain’s leptin-responsive circuitry, researchers turned their attention to a key pathway: the POMC-α-MSH-MC4R axis. Prior work has shown that eliminating POMC neurons is sufficient to induce obesity in mice, underscoring their importance in energy balance [13]. These neurons produce alpha-melanocyte stimulating hormone (α-MSH), a peptide that binds to MC4R receptors to suppress appetite and reduce body weight [9, 10].
Because rapamycin appeared to act through POMC neurons, Tan and colleagues asked a simple but revealing question: What happens if you remove the receptor that α-MSH signals through?
To find out, they used a group of mice genetically engineered to lack the mc4r gene, which encodes the MC4R receptor. These mice ate similar amounts of food as their wild-type counterparts and gained weight at the same rate when placed on a high-fat diet. As expected, they also developed leptin resistance [6].
But what stood out was their response—or lack of one—to rapamycin. When treated with rapamycin for 14 days, these mc4r-deficient mice showed no reduction in food intake, body weight, or fat mass. In contrast, the wild-type high-fat diet mice treated with rapamycin experienced the expected weight loss and metabolic improvements [6]. This striking divergence suggested that rapamycin’s benefits depend on an intact POMC–MC4R pathway. Without the receptor, the signaling cascade halts—rendering rapamycin ineffective.
To further confirm this link, the researchers stopped rapamycin treatment in both groups and monitored the mice for an additional two weeks. The wild-type mice—those that had previously lost weight with rapamycin—quickly regained both body weight and fat mass, along with an increase in food intake. Meanwhile, the mc4r-knockout mice, who had shown no benefit from rapamycin in the first place, remained metabolically unchanged [6].
Taken together, these results underscore a critical point: rapamycin's effects on leptin sensitivity and weight regulation hinge on a fully functioning POMC–α-MSH–MC4R pathway within the hypothalamus. And while rapamycin can help restore this signaling in the face of leptin resistance, sustained treatment may be necessary to maintain those benefits—especially in the absence of dietary changes.
Rapamycin Restores Leptin Sensitivity by Inhibiting mTOR in POMC Neurons
The final set of experiments conducted by Tan and colleagues sought to answer a foundational question: Is mTOR overactivation in specific neurons responsible for leptin resistance? To test this, the researchers zeroed in on POMC-expressing neurons, the same population previously shown to be essential for appetite suppression and leptin responsiveness.
In their first experiment, they genetically modified wild-type mice to selectively delete the tsc1 gene in POMC neurons. TSC1 normally acts as a brake on mTOR activity, so removing it leads to unchecked mTOR activation—effectively creating a hyperactive mTOR state within those neurons. Notably, these mice were kept on a standard chow diet, which typically does not induce obesity or leptin resistance on its own.
Despite the healthy diet, the consequences of this genetic modification were dramatic. Mice lacking tsc1 in their POMC neurons consumed more food, gained more weight, and accumulated significantly more fat than their wild-type counterparts. When given leptin injections, these mice failed to respond: there was no reduction in appetite, body weight, or fat mass. In contrast, wild-type mice with intact tsc1 responded to leptin as expected.
To tease apart whether leptin resistance was driven by mTOR activation itself or simply a byproduct of weight gain, the researchers repeated the experiment but controlled for caloric intake. The tsc1-deficient mice were pair-fed to match the food consumption of control mice, eliminating differences in diet as a confounding factor. Even with identical food intake, the mice with mTOR overactivation remained leptin-resistant—providing strong evidence that hyperactive mTOR signaling in POMC neurons is sufficient to block leptin’s effects, independent of weight or diet.
This finding is crucial: it suggests that obesity-related leptin resistance may stem not only from fat accumulation but also from disrupted intracellular signaling within key neurons. Given the parallels in obesity mechanisms between mice and humans, this offers a compelling, targetable pathway for future interventions [6].
In a second, complementary experiment, the researchers turned to ob/ob mice—animals that do not produce leptin but have functional leptin receptors. These mice are extremely sensitive to externally administered leptin and serve as a useful model for testing central leptin signaling pathways. The team again deleted tsc1 in POMC neurons, this time in the ob/ob mice, and assessed how mTOR overactivation would affect leptin responsiveness.
After four weeks of leptin injections, both groups of ob/ob mice lost weight and fat mass, confirming that leptin was still effective overall. However, the ob/ob mice lacking tsc1—and thus exhibiting elevated mTOR activity—experienced a significantly weaker response. This suggested that excess mTOR activity blunted leptin’s ability to regulate energy balance, even in a context of extreme leptin sensitivity.
To test whether this effect could be reversed, researchers administered rapamycin alongside leptin. The results were striking: in both models, the combination restored full leptin responsiveness. Mice receiving both treatments showed reductions in food intake and significant weight and fat loss, despite the genetic manipulation [6].
Taken together, these experiments provide strong mechanistic evidence that rapamycin restores leptin signaling by inhibiting mTOR activity within POMC neurons. Importantly, they also confirm that mTOR hyperactivation alone is enough to induce leptin resistance—reinforcing the therapeutic potential of rapamycin in targeting this specific signaling bottleneck.
Restoring Leptin Sensitivity in Humans with Obesity – Is Rapamycin a Solution?
Traditional attempts to treat obesity with leptin-like therapies have shown limited success—except in a small subset of individuals with rare genetic mutations. These individuals, whose condition resembles the ob/ob mouse model, lack sufficient leptin production altogether. For them, providing leptin externally can lead to substantial weight loss [11].
But for the vast majority of people with obesity, the challenge isn’t a lack of leptin—it’s that the brain no longer listens to it. In these cases, fat mass accumulates slowly over time due to chronic energy imbalance, while leptin resistance develops in parallel. As a result, simply injecting more leptin has failed to produce consistent or meaningful outcomes in clinical settings.
To overcome this problem, researchers have explored ways to restore leptin sensitivity rather than increase leptin levels. These efforts include enhancing leptin’s ability to cross the blood-brain barrier, boosting downstream signaling molecules like pSTAT3, and reducing circulating leptin to increase the potency of what little signal gets through. Although many of these methods have shown promise in rodent models, their efficacy in humans remains uncertain.
This is where rapamycin offers a potentially novel solution. Unlike previous strategies, rapamycin doesn’t aim to boost leptin levels or transport—it works by quieting the molecular interference that disrupts leptin signaling at its destination. By inhibiting mTOR activity in the arcuate nucleus of the hypothalamus, rapamycin appears to reopen the POMC–α-MSH–MC4R signaling cascade that is otherwise blocked during obesity.
In animal models, this restored signaling allows leptin to once again suppress appetite and reduce fat mass—even in the face of ongoing high-fat feeding. If a similar mechanism holds true in humans, rapamycin could represent a targeted intervention for reversing one of obesity’s core physiological drivers: central leptin resistance.
That said, it’s critical to distinguish between the doses used in these animal experiments and those typically used in human longevity protocols. The mice in these studies received 2 mg/kg of rapamycin daily, a dose designed to produce therapeutic effects in the context of established obesity. In contrast, most human protocols for healthspan optimization use a much lower dose—typically 0.075 to 0.1 mg/kg administered weekly. These regimens are designed to modulate aging-related pathways over time, not to treat active metabolic dysfunction.
Whether such low, intermittent doses can restore leptin sensitivity in humans—or whether higher, therapeutic-level dosing is needed—remains an open question. The exciting results seen in mice offer a clear direction for future investigation, but more research is urgently needed to determine the safety, efficacy, and ideal dosing strategy in humans.
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009 Jul 16;460(7253):392-5.
- Lee DJW, Hodzic Kuerec A, Maier AB. Targeting ageing with rapamycin and its derivatives in humans: a systematic review. Lancet Healthy Longev. 2024 Feb;5(2):e152-e162.
- Selvarani R, Mohammed S, Richardson A. Effect of rapamycin on aging and age-related diseases-past and future. Geroscience. 2021 Jun;43(3):1135-1158.
- Weichhart T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology. 2018;64(2):127-134.
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023 Jan 19;186(2):243-278.
- Tan B, Hedbacker K, Kelly L, Zhang Z, Moura-Assis A, Luo JD, Rabinowitz JD, Friedman JM. A cellular and molecular basis of leptin resistance. Cell Metab. 2025 Mar 4;37(3):723-741.e6.
- Friedman JM. Leptin and the endocrine control of energy balance. Nat Metab. 2019 Aug;1(8):754-764.
- Picó C, Palou M, Pomar CA, Rodríguez AM, Palou A. Leptin as a key regulator of the adipose organ. Rev Endocr Metab Disord. 2022 Feb;23(1):13-30.
- D'Agostino G, Diano S. Alpha-melanocyte stimulating hormone: production and degradation. J Mol Med (Berl). 2010 Dec;88(12):1195-201.
- Chen KY, Muniyappa R, Abel BS, Mullins KP, Staker P, Brychta RJ, Zhao X, Ring M, Psota TL, Cone RD, Panaro BL, Gottesdiener KM, Van der Ploeg LH, Reitman ML, Skarulis MC. RM-493, a melanocortin-4 receptor (MC4R) agonist, increases resting energy expenditure in obese individuals. J Clin Endocrinol Metab. 2015 Apr;100(4):1639-45.
- Obradovic M, Sudar-Milovanovic E, Soskic S, Essack M, Arya S, Stewart AJ, Gojobori T, Isenovic ER. Leptin and Obesity: Role and Clinical Implication. Front Endocrinol (Lausanne). 2021 May 18;12:585887.
- Christoffersen BØ, Sanchez-Delgado G, John LM, Ryan DH, Raun K, Ravussin E. Beyond appetite regulation: Targeting energy expenditure, fat oxidation, and lean mass preservation for sustainable weight loss. Obesity (Silver Spring). 2022 Apr;30(4):841-857.
- Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci. 2013 Feb 20;33(8):3624-32.