Understanding HRV as a Real-Time Marker of Neural Responsiveness. How Does Oxytocin Change the Signal?
Autonomic Balance and HRV. Heart rate variability reflects the continuous interplay between the parasympathetic and sympathetic branches of the autonomic nervous system. The parasympathetic system—mediated largely by the vagus nerve—promotes relaxation and recovery, while the sympathetic system drives stress responses and mobilization. Greater variability between heartbeats typically indicates stronger vagal influence and greater autonomic flexibility. As a real-time physiological signal, HRV provides insight into how well the body can shift between states of rest and arousal—revealing not just momentary calm, but the capacity to adapt to internal and external demands.
Vagal Signaling and Cardiac Variability. HRV captures the shifting influence of autonomic inputs on the heart, particularly the fast-acting effects of parasympathetic activity and the slower, more sustained influence of sympathetic activation. Acetylcholine released by the vagus nerve rapidly slows heart rate and increases beat-to-beat variability, making high HRV a hallmark of parasympathetic (vagal) tone. In contrast, sympathetic stimulation via epinephrine increases heart rate and contractile force over several minutes, typically resulting in more uniform heart rhythms and reduced variability. While HRV is most reflective of vagal input, its changes offer a real-time, non-invasive window into the broader state of autonomic regulation.
HRV and Recovery Timing. Heart rate variability offers a real-time glimpse into autonomic recovery following physical exertion, reflecting the balance between sympathetic stress and parasympathetic rebound. Metrics like RMSSD often recover more quickly than muscular strength, inflammation, or subjective fatigue, making HRV a sensitive—but not comprehensive—recovery marker. In athletic contexts, persistently low HRV has been linked to overtraining and poor adaptation, with reductions of 20% or more serving as a potential threshold for excessive physiological load. By tracking HRV trends over time—rather than reacting to single-day dips—individuals can use HRV to autoregulate training, identify early signs of overreaching, and guide smarter recovery strategies.
HRV and Autonomic Dysregulation. Beyond its role in predicting cardiovascular events, HRV serves as a real-time signal of autonomic dysfunction—particularly when the sympathetic nervous system fails to mount appropriate responses to physical or psychological stress. From orthostatic intolerance to paradoxical stress reactions, blunted or exaggerated HRV responses can reveal impaired autonomic flexibility across conditions like POTS, Parkinson’s disease, fibromyalgia, and chronic pain. Significant interindividual variability exists in baseline autonomic tone, and HRV captures this resilience—or lack thereof—with high sensitivity. As such, HRV is a valuable non-invasive tool for identifying atypical stress responses in both clinical populations and high-performance settings. HRV, Inflammaging, and Biological Aging. Emerging research links reduced heart rate variability not only to cardiovascular risk but also to systemic inflammation and aging. As a downstream signal of autonomic imbalance, low HRV reflects increased sympathetic tone and diminished vagal modulation—factors that can drive or exacerbate chronic low-grade inflammation, also known as “inflammaging.” Studies suggest that HRV may serve as a non-invasive biomarker for biological aging, with persistent reductions in RMSSD correlating with higher inflammatory load, frailty, and mortality risk. While more research is needed to confirm causal pathways, HRV offers a promising lens through which to track aging-related physiological decline and the impact of healthspan-promoting interventions.
Improving HRV to Support Healthspan. Heart rate variability reflects autonomic flexibility and the body’s ability to recover from physiological and psychological stress. Lower HRV is strongly associated with cardiometabolic risk and mortality, but encouragingly, it can be improved through modifiable behaviors. Regular aerobic and resistance training enhance vagal tone and reduce sympathetic load, while adequate sleep and stress-reduction practices like mindfulness, therapy, and social connection further support autonomic recovery. Together, these interventions promote a higher, more stable HRV—signaling improved resilience, reduced inflammation, and greater capacity for long-term health maintenance. HRV and Oxytocin: Interpreting Adaptive Engagement. While HRV is often viewed as a marker of parasympathetic recovery, its meaning shifts in the context of neuromodulators like oxytocin. Rather than simply promoting calm, oxytocin enhances adaptive engagement—modulating autonomic tone to prepare the body for social, emotional, or environmental responsiveness. This can lead to transient reductions in HRV, not as a sign of dysfunction, but as evidence of motivational arousal and neural readiness. Recognizing this bidirectional effect is essential for interpreting HRV as a functional, rather than purely calming, biomarker—especially in therapeutic contexts.
HRV Suppression as a Marker of Engagement. In response to oxytocin, studies consistently show a short-term decrease in HRV—particularly in RMSSD and HF power—accompanied by increases in blood pressure, cardiac output, and salience network activity. Rather than signaling distress, this autonomic shift reflects a mobilized and engaged nervous system, similar to what occurs during focused attention or social approach. In individuals experiencing apathy, fatigue, or social withdrawal, this pattern may represent a therapeutic reactivation of engagement circuits, challenging the assumption that lower HRV always indicates dysfunction.
Interpreting HRV as a Longitudinal Response Signal. Oxytocin’s effects on the autonomic nervous system unfold over time, making HRV most useful as a trend-based biomarker rather than a single-point measurement. An initial drop in RMSSD or HF power post-dose may indicate central engagement, not dysfunction—especially in individuals with low motivation or burnout. A subsequent rebound during sleep or over weeks of consistent use reflects restored vagal tone, increased adaptability, and improved autonomic resilience. Rather than chasing daily highs, clinicians and patients should look for responsive patterns—transient dips followed by recovery—as evidence of oxytocin’s therapeutic effect on physiological flexibility.
Introduction
Heart rate variability (HRV) has quietly become one of the most widely tracked, yet poorly understood, physiological metrics in modern wellness. From elite athletes to sleep-optimization enthusiasts, HRV is now a fixture on health dashboards—a number meant to reflect how well we handle stress and recover from it. But what does it really measure? And how should we interpret it when experimenting with interventions that promise to restore balance, energy, or calm?
At its core, HRV offers a window into the autonomic nervous system, specifically, the push and pull between sympathetic activation (fight or flight) and parasympathetic recovery (rest and digest). More variability between heartbeats usually signals a flexible, adaptive system, while lower variability is often taken as a sign of stress or dysfunction. Yet this framework is more nuanced than it seems, especially when we bring in therapeutic agents like oxytocin, which can shift autonomic tone in unexpected ways.
Often described as the “bonding hormone,” oxytocin is increasingly being explored for its potential to enhance emotional engagement, motivation, and stress resilience. But its effects on HRV, and by extension, on the body’s physiological stress signature, don’t always fit cleanly into the “higher is better” narrative. In some cases, a drop in HRV might actually reflect the system waking up, not shutting down.
This article takes a closer look at HRV: how it’s generated, what it reflects about your nervous system, and how to interpret changes over time. Along the way, we’ll explore how HRV can serve as a dynamic biomarker, not just of stress, but of progress. Whether you’re using oxytocin, lifestyle interventions, or both, understanding the rhythms of your autonomic response may offer powerful insight into what’s working, when, and why.
By: Anthony G. Pinzone, Ph.D., CSCS*D
Anthony Pinzone is an exercise scientist and university lecturer specializing in sports performance analytics, cardiovascular physiology, and physiological signal processing. He is currently a full-time lecturer in the Department of Kinesiology at California State University San Marcos and holds a Ph.D. in Exercise Physiology from Kent State University. His research focuses on modeling the relationship between rest, recovery, and performance in athletes, with a broader interest in autonomic function. Dr. Pinzone’s work integrates advanced statistical modeling with practical applications, to translate physiological data into insights to enhance performance, recovery, and long-term well-being.
Background
Heart rate variability (HRV) has steadily gained popularity since the 1960s, when it was first identified as a potential predictor of adverse cardiovascular health outcomes [1, 2]. In the decades following, HRV was used primarily in clinical settings to identify individuals at risk for cardiovascular events and to monitor cardiovascular health in individuals with cardiovascular abnormalities [3]. Since then, HRV has moved beyond the clinic and into the mainstream and is widely employed in wellness, fitness, and performance circles.
Today, it’s everywhere. Chest straps, smartwatches, and rings all promise insights into your nervous system and recovery status by tracking HRV [4, 5]. But often, the numbers feel like a black box, wrapped in proprietary algorithms and vague metrics. This article aims to change that. By the end, you'll understand how HRV is measured, what your wearable is likely doing under the hood, and how to interpret HRV responsibly in the context of your health, stress, and recovery.
All visuals in this article come from unpublished pilot data collected using a 3-lead ECG in an exercise physiology lab at Kent State University. The data were processed using an open-source Python algorithm created by Dr. Pinzone [6].
More Variability = More Relaxation
To understand HRV, a brief physiological overview is in order.
You’ve probably heard of the autonomic nervous system (ANS), or at the very least, phrases such as “fight or flight” or “rest and digest”. The ANS is entirely responsible for regulating everything that we do involuntarily: breathing, thermoregulation, regulating heart rate, digestion control, and more. Our ANS is split into two primary branches: the parasympathetic nervous system (PNS) and the sympathetic nervous system (SNS) [7, 8].
The PNS is colloquially known as the “rest and digest” system because it is responsible for sending signals that relax us or calm us down (e.g. reducing our heart rate, dilating blood vessels, up-regulating digestion). The main conduit of PNS activity is the vagus nerve, the tenth cranial nerve, which extends from the brainstem to multiple organs, including the heart, lungs, and digestive tract [8, 9]. When people talk about “vagal tone” or “vagal activation,” they’re referring to how strongly the PNS is influencing bodily functions through this nerve.
Conversely, our SNS is predominantly active in kickstarting our stress response by releasing epinephrine/adrenaline to support our internal organs and skeletal muscle. SNS activity increases heart rate, constricts blood vessels to keep blood around our primary organs, and down-regulates digestion, along with many other responses that assist in ramping our system up to respond to threats or stress [8].
Both systems work in concert with each other to keep our body functioning optimally at rest and in response to stress.
How Each System Affects the Heart and HRV
Each branch of the ANS has specific effects on the heart, allowing for HRV to be a powerful window into ANS activity. The PNS controls heart rhythm using a chemical messenger called acetylcholine (Ach), which acts on receptors in the heart and lungs to slow heart rate and respiration rate [9]. This response occurs rapidly, eliciting subtle moment to moment alterations in heart rate and respiration rate within 10-15 seconds of Ach binding [9]. This variability is exactly what is captured by HRV. As such, greater variability in R-R interval distances (more HRV) is representative of more PNS (or vagal) activity, and, in turn, a more relaxed physiological state [3].
On the other end of the spectrum, SNS activity stimulates the release of epinephrine (adrenaline), which elicits a much more complex physiological response [8]. Epinephrine binds, most notably, to receptors on the heart that regulate both the force of contraction and heart rhythm, speeding up heart rate and increasing contractile force [10]. Moreover, epinephrine constricts our blood vessels, dilates our lungs, and speeds up our respiration rate to prepare our body for mobilization in response to stress [8, 11]. Unlike PNS activity, this process is slower, taking at least three to five minutes to fully take hold due to a cascade of hormonal signals and resultant enzymatic activity [10, 11, 12, 13]. However, physiological effects from sympathetic outflow are more sustained and uniform. As a result, less HRV is often a marker of SNS dominance, though this is more challenging to isolate directly [3].
These physiological underpinnings are the reason that HRV is often considered a window into vagal activity and recovery from stress. However, these metrics do not tell the whole story regarding ANS activity but do provide a remarkably accessible snapshot.
Can HRV Predict Exercise Recovery?
HRV provides a momentary snapshot of autonomic balance and is particularly useful for monitoring cardiac-autonomic recovery following intense training. By capturing shifts in autonomic nervous system activity—particularly the balance between sympathetic arousal and parasympathetic recovery—HRV offers a window into how the body responds to, and recovers from, physical exertion. For example, a study involving 10 rugby athletes examined recovery after a high-volume resistance training session to failure. Researchers tracked multiple recovery markers: Root Mean Square of Successive Differences (RMSSD)—a time-domain measure of HRV closely tied to vagal activity—alongside muscular power and subjective ratings of soreness and recovery over the next 48 hours [14].
From pre- to immediately post-training, RMSSD (a marker of greater PNS activity and reduced autonomic stress) significantly declined, along with muscular power, perceived soreness, and recovery. Interestingly, RMSSD returned to resting values by 24 hours, while muscular power did not recover until 48 hours and perceived soreness and recovery remained suppressed below baseline levels 48 hours later. These results underscore a key insight: HRV recovers along a different timeline than other physiological and perceptual recovery markers [14, 15, 16].
This phenomenon reflects the layered complexity of post-exercise recovery. While HRV offers a relatively rapid and sensitive indicator of autonomic rebound, it may not track perfectly with muscle repair, inflammation resolution, or psychological fatigue. Each of these operates on partially distinct biological clocks, influenced by factors such as immune signaling, neuromuscular repair, and even mood state.
More sustained shifts in HRV are often observed under chronic training stress, such as during pre-season periods of high volume or post-season cumulative fatigue. Athletes across multiple sports have displayed suppressed resting HRV during pre-season (when they incur their highest training volume) [17, 18] and during the post-season (after accumulating stress from the entire season [17], and even during the season [19] compared to off-season levels.
A compelling body of evidence has supported the use of HRV in identifying overtraining. Overtraining occurs when cumulative stress (extreme training volumes over weeks or months, sleep disruption, poor nutrition, psychological stress) outpaces recovery, leading to declines in performance, perceived recovery, and overall health [20, 21, 22] and poor recovery in terms of sleep, nutrition, and stress management. Multiple studies have demonstrated significant decreases in HRV at rest in individuals that are overtrained [20, 23, 24, 25], indicating that extended periods of high volume are when we can see cardiac-autonomic recovery be suppressed along with most other typical recovery metrics (soreness, perceived recovery, inflammation).
In athletic populations, overtraining has been associated with reductions in RMSSD of up to 50% [25, 26] or an average decrease of approximately 16 ms [25]. While there is substantial interindividual variability in HRV metrics, a drop of more than 20% in resting HRV can serve as a useful threshold for identifying potential periods of overexertion.
To make the most of HRV data, it’s crucial to use a reliable device, establish a personal baseline, and monitor trends over time rather than relying on single-day readings. HRV is a useful tool for autoregulating your training, but it should never be the only factor guiding decision-making. If your HRV is low and you also notice poor sleep, lingering soreness, recent training overload, or high perceived fatigue, it may be wise to scale back. Still, HRV can fluctuate due to various non-training factors (hydration, caffeine, stress, measurement timing), so interpret it in context. Think of HRV as one valuable piece of your recovery toolkit, not the whole picture.
Consequences of Autonomic Dysregulation
Low HRV was historically used as an identifier of risk for an acute cardiovascular event (e.g. heart attack or stroke) [3]. Today, we recognize that HRV can also reflect more subtle forms of autonomic dysfunction that affect daily life. These include poor regulation of the ANS in response to physical or psychological stress, something HRV can often help detect both at rest and during recovery from stress exposure.
Autonomic dysregulation can be complex and often misunderstood, in part because not all stress is harmful. In fact, short-term sympathetic nervous system (SNS) activation is essential for homeostasis and survival [8]. The key distinction lies in the appropriateness of the response: Is the SNS activating at the right time, with the right magnitude, and for the right reason?
Fainting during long periods of standing, such as at weddings or ceremonies, is a classic example of autonomic failure under orthostatic stress—the physiological challenge of maintaining blood pressure when standing for long periods. Here, gravity causes blood to pool in the legs, reducing venous return to the heart [27]. To compensate, the SNS increases heart rate and blood pressure to maintain adequate perfusion [8, 25]. If this reflex fails, the brain is briefly deprived of oxygen, triggering syncope (fainting)—a last-resort strategy to return the body to a horizontal position and restore circulation [28]. This scenario is a real-time test of SNS adequacy.
In clinical settings, this is mimicked using a tilt table test, where an individual is strapped to a near-vertical table to assess their ability to tolerate orthostatic stress [27]. Individuals with autonomic dysfunction may faint quickly, reflecting impaired SNS responsiveness. Exercise is another case where functional SNS activation is necessary. We rely on increases in heart rate and blood pressure to deliver oxygen to working muscles. Our only mechanism for achieving these responses is to increase SNS activity. Conversely, a non-functional stress response occurs when sympathetic activation (e.g., sweating, chest tightness) is triggered by purely psychological stimuli, or any type of stressful situation, without any real physiological demand.
A functional sympathetic response is essential during exercise: it ensures blood pressure rises, blood flow is redistributed, and oxygen delivery meets metabolic demand. But when SNS responses are blunted, inappropriately triggered, or excessively prolonged—as seen in various autonomic disorders—the result is systemic inefficiency. A variety of atypical responses are possible including: blunted or paradoxical HRV changes in response to stress when vagal withdrawal is needed (e.g., no change or even an increase in HRV when a drop is expected), poor tolerance to heat, cold, or exercise, or frequent fainting or dizziness upon standing. These symptoms are common in clinical conditions such as postural orthostatic tachycardia syndrome (POTS) [29], spinal cord injuries [30], Parkinson’s disease [31], chronic neuropathic pain or fibromyalgia [32]. In these populations, HRV is often suppressed at rest and responds atypically to stress, making HRV a valuable tool for tracking autonomic function both in clinical practice and personal wellness [33].
Everyone has a different level of baseline ANS function, and there is an immense amount of individual variability in the ability to mount a functional stress response. In a seminal paper by Fu et al. [34], researchers subjected healthy young adults to a tilt table test combined with hand immersion in ice water, both strong SNS stressors. While most showed the expected rise in heart rate and blood pressure, a subset paradoxically showed declines, suggesting fundamental differences in ANS responsiveness. We replicated this pattern with heart rate and blood pressure in my doctoral work at Kent State. Here we assessed HRV, blood pressure, and heart rate surrounding high-volume resistance exercise and also demonstrated that some individuals failed to display a typical autonomic response, despite exposure to robust physiological stress [35].
Because HRV reflects cardiac-autonomic function, it’s highly sensitive to individual differences in stress resilience. This is the same reason HRV is useful for detecting overtraining in athletes and why it holds promise as a non-invasive marker of chronic autonomic dysregulation in a wide range of settings.
HRV as a Marker of Age and Inflammation
The relationship between reduced HRV and adverse cardiovascular outcomes is well established [3] [36]. Yet this association is often interpreted too narrowly—confined to the heart and vasculature—when in fact the ANS plays a regulatory role across virtually every physiological domain. One area where its influence is particularly consequential, though less widely recognized, is inflammation.
Inflammation is the body’s primary response to injury, infection, or cellular stress. While essential for healing and host defense, inflammation must be tightly regulated—too much, too little, or mistimed activation can result in tissue damage, immune exhaustion, or chronic disease. The ANS, especially the SNS, acts as a central modulator of this inflammatory cascade.
For instance, the initial mobilization of immune cells in response to infection or injury is triggered by the release of epinephrine, the principal effector hormone of the SNS [37]. Similarly, as stress becomes prolonged, another axis comes into play: the hypothalamic–pituitary–adrenal (HPA) axis. Its chief output, the glucocorticoid cortisol, helps resolve inflammation by inhibiting pro-inflammatory cytokine production and orchestrating the withdrawal of immune cells from peripheral tissues [38]. Together, these hormonal signals ensure that the inflammatory response is both mobilized and terminated at the right time.
Given these relationships, researchers have increasingly explored whether HRV can serve as a surrogate marker of chronic inflammation, especially in the context of aging and overall health. Olivieri et al. [33] proposed an integrative model that positions HRV as a central indicator of inflammaging, where low HRV reflects reduced autonomic balance, elevated systemic inflammation, and an increased risk of age-related disease. Their review suggests that autonomic dysfunction may not simply be a byproduct of aging, but a contributing factor to its biological progression. As people age, they commonly exhibit elevated resting inflammatory markers [39] along with an increased shift in ANS balance that favors SNS activity [40].
Importantly, these patterns vary considerably among individuals and are not always linear [41]. However, individuals with greater magnitudes of ANS dysfunction with age do display a higher risk of mortality. Lower HRV (e.g. consistent resting RMSSD values less than 27 ms) has also been associated with decreased physical fitness, higher levels of inflammation, frailty, and impaired autonomic function in middle-aged and older adults [40, 42, 43].
Although this conceptual framework is promising, further longitudinal and mechanistic research is needed to determine whether low HRV directly contributes to the development of inflammaging or merely serves as a downstream indicator. Clarifying this relationship will be essential for establishing HRV as a clinically useful and predictive biomarker in the fields of aging and preventative medicine.
How is HRV Derived?
To truly understand HRV, it’s integral to first understand where it comes from, specifically, the electrocardiogram (ECG). An ECG is a simple, non-invasive technique where electrodes are placed on the chest to capture the electrical signals associated with heart function. These recordings are typically collected when an individual is at rest or exercising.
In a typical ECG graph, time is shown on the x-axis and the electrical activity of the heart in millivolts (mV) is presented on the y-axis. Each heartbeat is characterized by a wave of electrical activity, and different parts of the ECG wave reflect different phases of the cardiac cycle. For HRV analysis, the most critical portion is the R-wave, a sharp, prominent peak that marks the moment the heart’s ventricles contract to pump blood.
When your heart contracts, nerves supplying our heart muscle (myocardium), fire electrical signals that stimulate muscle contraction. The R-wave represents the electrical stimulation of the myocardium in the left and right ventricles of the heart, resulting in a strong contraction that sends blood to the lungs (from the right ventricle) and out to the rest of the body (from the left ventricle). In the ECG snippet below, we can clearly see eight R-waves captured over ~7.5 seconds.
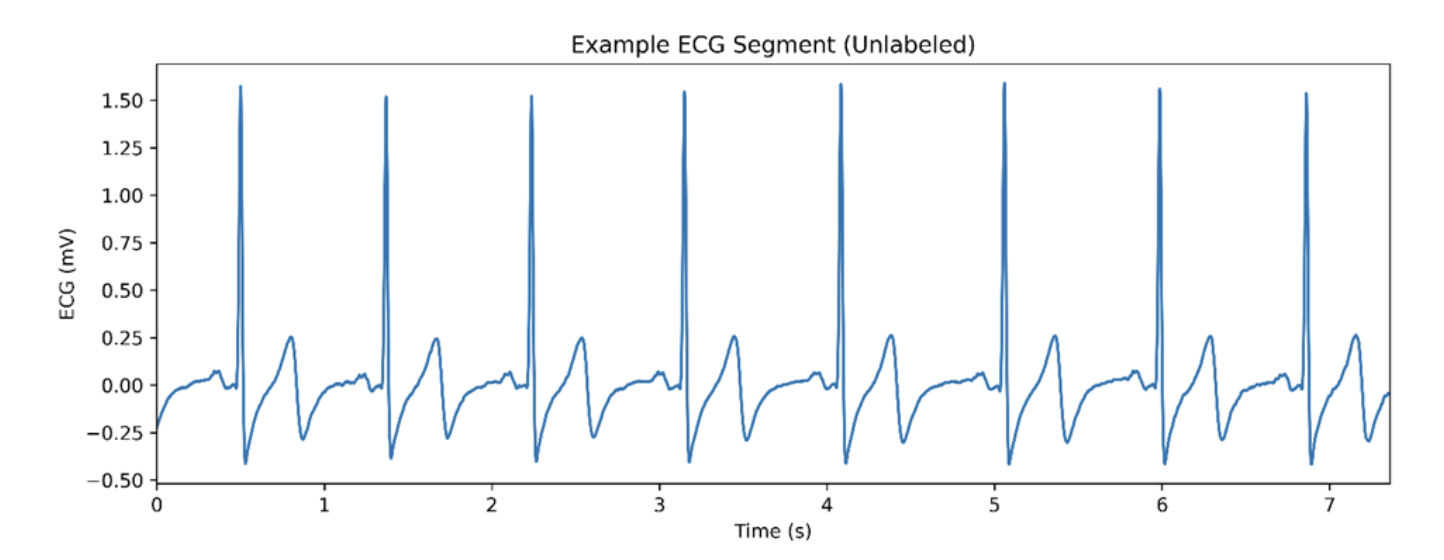
Figure 1. A raw ECG signal with recording time on the x-axis and ECG amplitude plotted on the y-axis.
At its core, every measure of HRV starts with one simple thing: the time between heart beats. To be specific, HRV calculations hinge on the time between consecutive R-waves on an ECG, known as R-R intervals [3]. Below, we can observe the same ECG snippet presented before with each R-wave annotated with an X and the time in ms denoting the distance in time comprising R-R intervals.
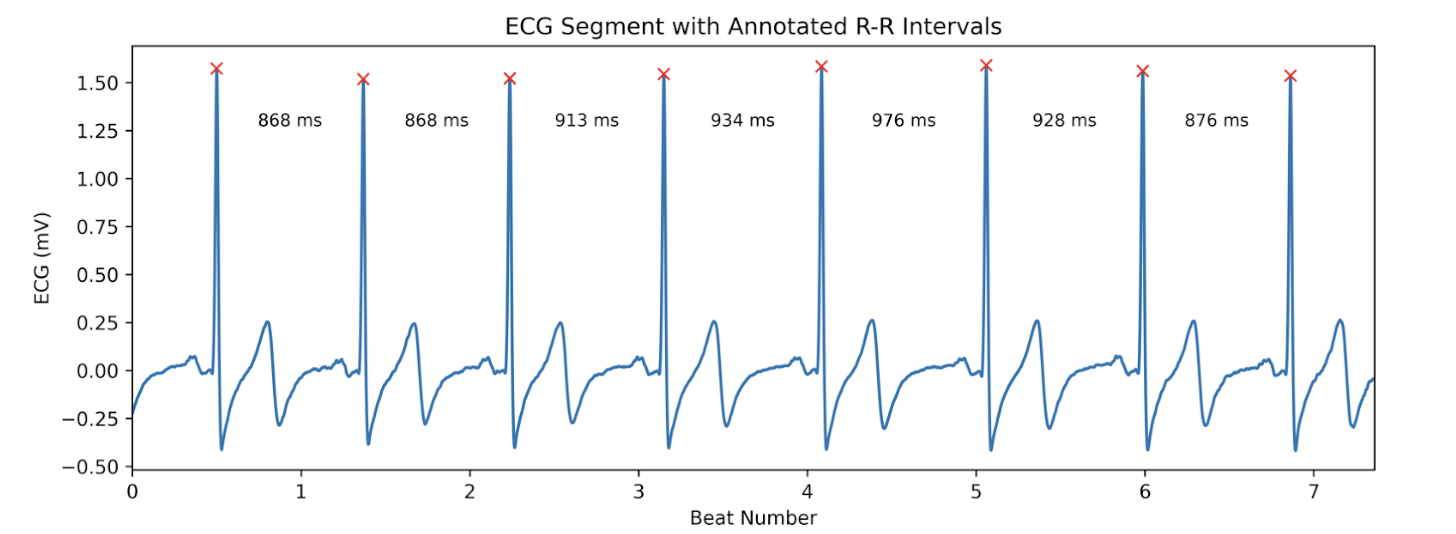
Figure 2: An annotated ECG recording with R-R intervals identified.
Whether a lab-grade ECG or wearable device is outputting HRV, all analysis boils down to identifying or estimating these R-R intervals. Devices that record ECG data can detect these peaks directly, while others, such as fitness trackers or smartwatches, can infer them from heart rate measurements [4, 44].
After capturing R-R intervals, we created a tachogram (visual representation) of how these intervals vary from beat to beat. In the example below, each beat is plotted with its corresponding time distance to the next beat. For our truncated sample, our R-R interval list looks like this: [868, 868, 913, 934, 976, 928, 876] ms
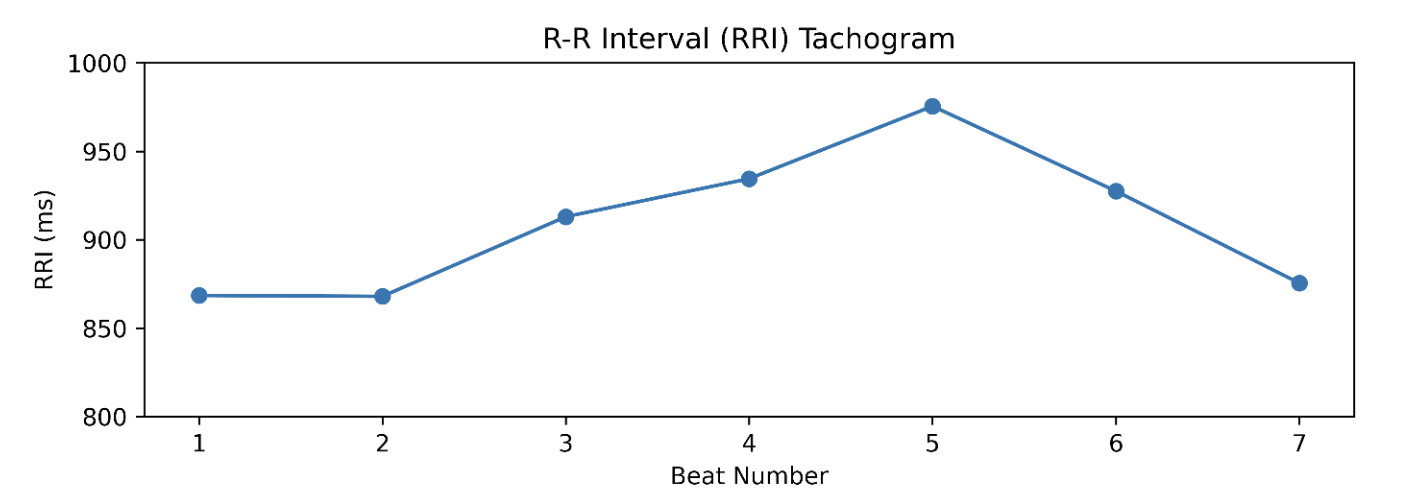
Figure 3. A visualization of R-R interval distances (tachogram) from the small ECG snippet mentioned above.
All HRV metrics, whether time domain, frequency domain, or geometric, are just different ways of quantifying the amount of variability that exists in a time-series of R-R intervals.
What HRV Metrics Do We Have?
Now that we have visualized a simple ECG segment and unpacked the physiology underpinning HRV, let’s explore what this looks like over a longer recording window. In clinical or research settings, a minimum of two minutes is typically required to accurately measure time-domain HRV metrics, while five minutes or more is preferred for capturing both time and frequency domain data [3, 45].
Below, you’ll see a tachogram from a five-minute ECG recording collected during quiet, seated rest. Each point on the graph represents the time in ms between consecutive R-waves, also known as R-R intervals. A total of 310 heartbeats are depicted here. This type of visualization offers a broader view of beat-to-beat variation and is the foundation for almost all modern HRV analysis.
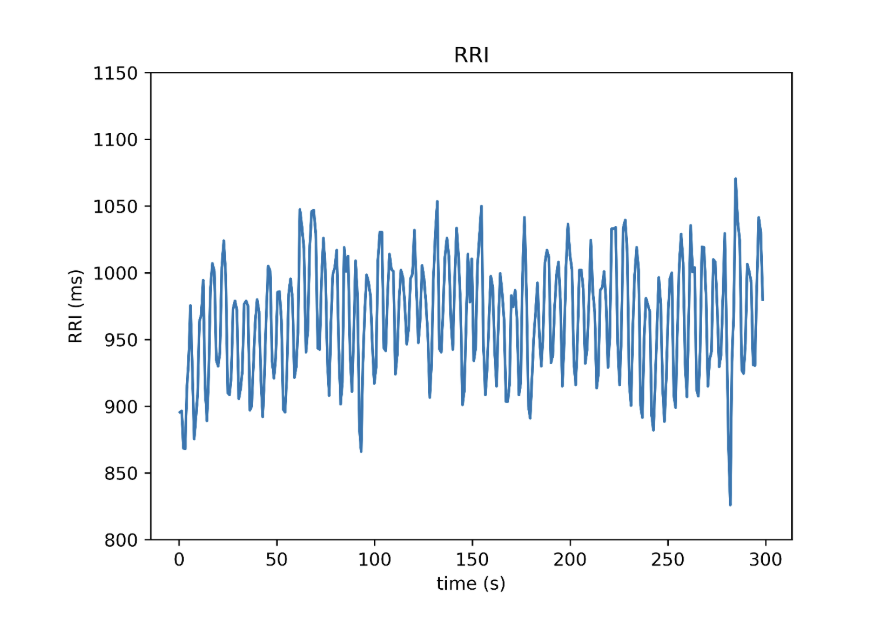
Figure 4. RRI Tachogram constructed from a 5-minute recording period, reflecting real-world HRV data. Once R-R interval data are gathered, the next step is to quantify the amount of variability present in the data; this is where HRV metrics come in. The most commonly employed method for calculating HRV is time-domain metrics [3], which applies simple calculations to measure fluctuations in R-R intervals. One of the most trusted and widely used metrics here is:
RMSSD (Root Mean Square of Successive Differences in R-R Intervals)
This may sound technical but is a very straightforward calculation. Let’s break it down:
- Start with your R-R intervals, for example: [868, 913, 934, 976, 928] ms
- Find the difference between each pair of adjacent intervals: [45, 21, 42, -48]
- Square the differences to remove negatives: [2025, 441, 1764, 2304]
- Average the squared values: (2025 + 441 + 1764 + 2304)/4 = 1633.5
- Take the square root of the result: 1633.5=40.4 ms
This final number, 40.4ms in our case, is RMSSD.
What it means: Higher RMSSD is representative of more PNS/vagal activity. In simple terms, it’s a sign that our body is in a more relaxed, recovered state. Most wearables use RMSSD under the hood as their primary HRV or “Stress” score. Typically, resting RMSSD ranges from 27-72ms. Other Key Time Domain Metrics
- The proportion of consecutive R-R intervals that differ by more than 50 ms (pNN50) is another time domain metric that is not used as frequently as RMSSD. Values greater than 3% for pNN50 indicate normal vagal activity at rest.
- The standard deviation of normal-to-normal R-R intervals (SDNN), or a number representing, on average, how much each R-R interval distance differs from the average in the whole sample, represents how much total ANS activity is occurring. Values ranging from 50-100ms are regarded as typical for SDSD.
Frequency-Domain Metrics
The last piece of the HRV puzzle is frequency-domain analysis, which, instead of assessing how your heart rate changes in time from beat to beat (like RMSSD, pNN50, or SDSD), looks at how these R-R intervals vary in terms of rhythms, like waves or oscillations [3]. The idea is simple: with more PNS activity, heart rate tends to fluctuate more in rhythmic, wave-like patterns. These fluctuations create distinct frequency “bands” that we can measure. In general, more PNS activity is indicative of more variability, which manifests as greater power in high-frequency waves within your heart rhythm.
The most often employed frequency domain HRV metrics are high-frequency power (HF) and low-frequency frequency power (LF) [3].
- High frequency power is closely tied to PNS or vagal activity, especially as they relate to breathing patterns and relaxation.
- LF power reflects a mix of PNS and SNS activity, though is understood to be more influenced by the SNS [3]. Together, these values sum to make up total power (TP).
To understand how these two systems work together, researchers often calculate the LF/HF ratio. This can sometimes be interpreted as an index of autonomic balance, or a way to estimate if your ANS is leaning more toward stress and activation (higher ratio) or recovery and relaxation (lower ratio). Typically, LF and HF values are reported in ms², which represents the power or amplitude of heart rate oscillations in each frequency band. However, they can also be converted into normalized units (n.u.), which reflect the relative proportion of each component [3]. This can make it easier to track shifts in autonomic balance over time, though it may oversimplify the underlying physiology.
Importantly, there are no universal reference ranges for these frequency domain metrics, as both LF and HF can vary widely between individuals [3]. That said, in a resting state, HF typically far exceeds LF, reflecting dominance of PNS activity. During stress or sympathetic activation, HF tends to drop, while LF may remain steady or rise modestly, shifting the balance toward lower HRV and increased physiological arousal.
Below is a table displaying the most common HRV metrics, calculated from the five-minute tachogram shown above.
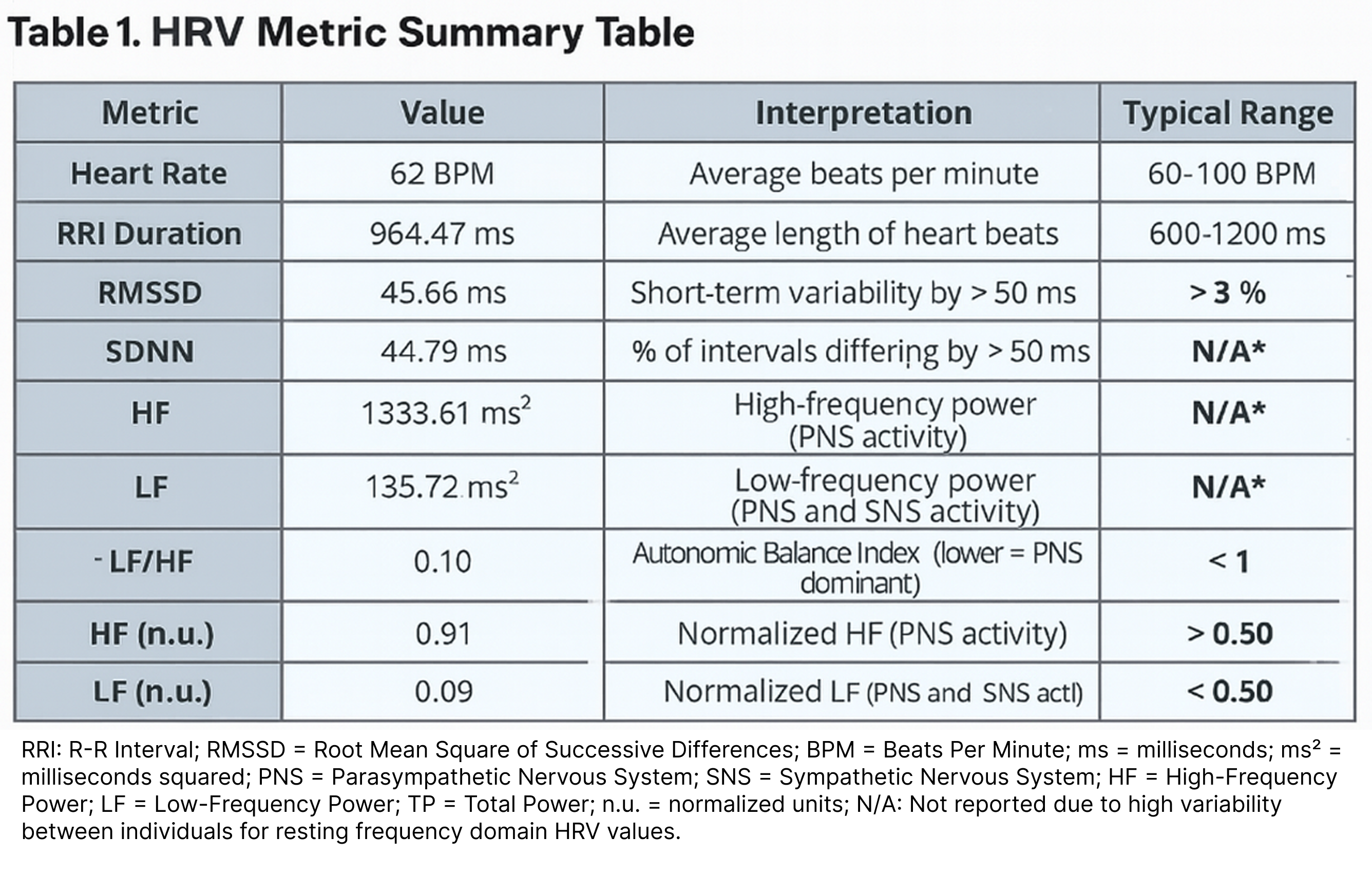
What Does This Mean? This individual is clearly in a PNS-dominant state, which is expected for a recording taken during seated rest. Let’s break it down and compare these values to reference ranges for HRV metrics:
- RMSSD falls well within the normal resting range (27-72ms) at 43.66 ms
- pNN50 far exceeds the normal minimum of 3% at 27.01%, indicating strong vagal activity
- SDNN is 44.79ms, a bit below the normal resting range of 50-100ms, a good teachable moment to highlight the inconsistency that can be observed, even in lab-grade HRV metrics
- Normalized HF of 0.91 and LF/HF ratio of 0.09 indicating that ~91% or more of the frequency-domain signal of HRV is dominated by PNS activity (made up of HF).
Together, these values indicate that the individual’s autonomic system is not under stress and is in a state of good recovery and physiological balance.
What is your Wearable Doing?
In research and clinical settings, HRV is typically derived from highly controlled ECG recordings. Participants are fitted with precisely placed electrodes, asked to breathe at a consistent rate (~12 breaths per minute), and instructed to avoid exercise, caffeine, alcohol, and stress prior to measurement to minimize physiological noise. We know that this is not remotely practical in the real world and diverges heavily from the goal of wearables. Wearables aren’t designed to provide clinical-grade diagnostics. Instead, they offer a practical way to monitor stress and recovery trends over time. Despite their limitations, reliable wearables can be valuable tools for tracking recovery and day-to-day stress.
Types of Wearables and How They Obtain HRV
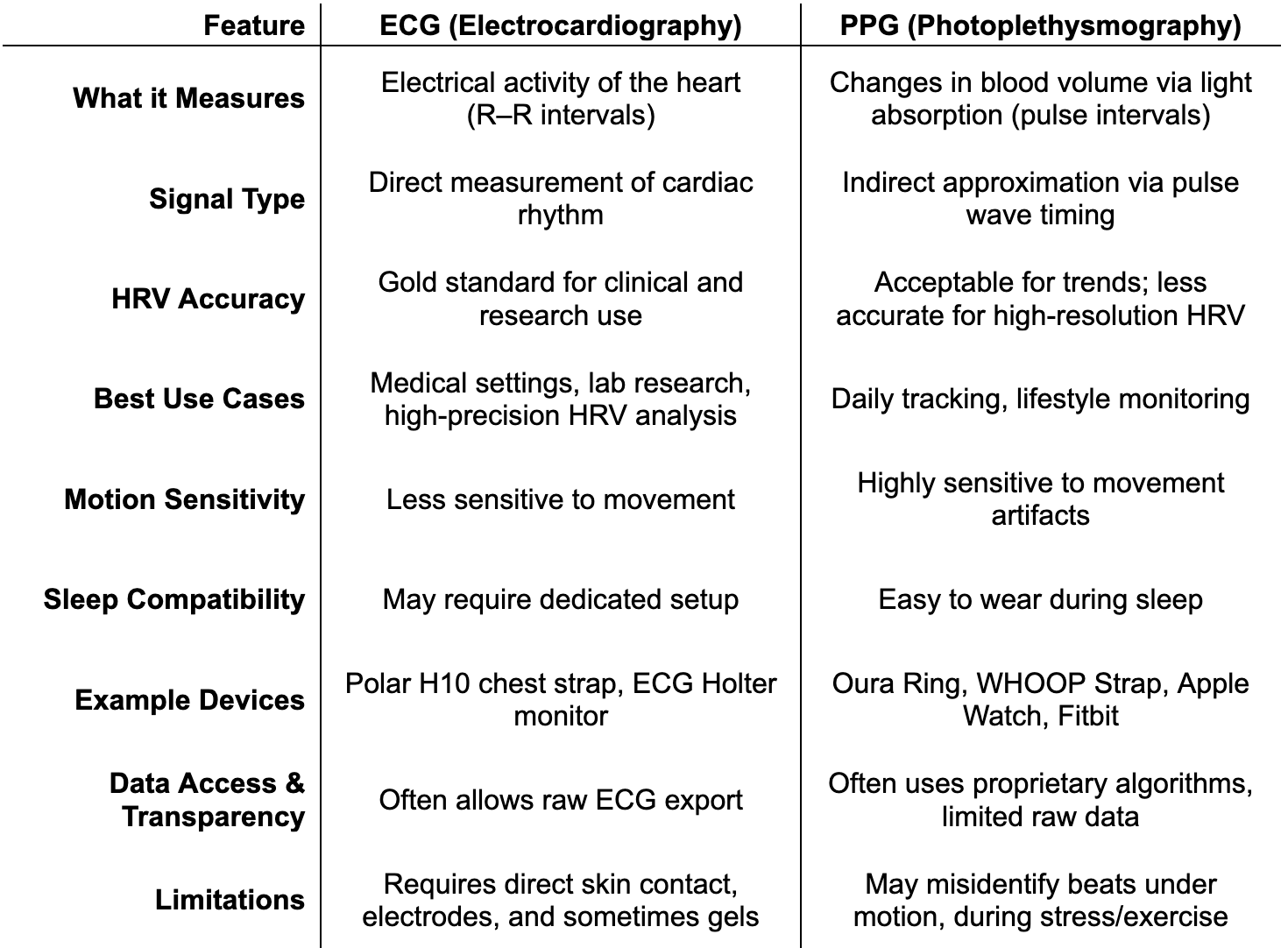
HRV can be measured through a variety of wearable devices, but not all methods are created equal. The key distinction lies in the type of sensor used to detect the heartbeat. The two approaches employed by these devices are ECG-based devices and photoplethysmography (PPG)-based devices.
ECG-based devices, like the Polar H10 chest strap, detect the heart’s electrical signal and precisely identify R peaks. This makes them the gold standard for HRV assessment, especially in research and clinical settings.
PPG-based devices such as the Oura Ring, Whoop Strap, Apple Watch, and many Garmin smartwatches use optical sensors to detect cyclical changes in peripheral blood volume [44, 46, 47]. These devices estimate the pulse interval, which serves as an indirect approximation of the R–R interval. While PPG enables convenient, passive tracking of HRV throughout the day and night, it is inherently more prone to timing inaccuracies, particularly during movement or in the presence of irregular heart rhythms. As a result, PPG-derived HRV can be heavily affected by motion artifacts, limiting its reliability in certain contexts, especially during exercise or periods of elevated SNS activity.
Wearable HRV monitors come in three main forms: chest straps like the Polar H10, which offer high accuracy during rest or exercise; wrist-worn devices (e.g., Apple Watch, Whoop, Garmin), which are comfortable for long-term use but more prone to signal artifacts; and rings like Oura, which balance comfort and accuracy, performing best during sleep or low-motion periods. Here’s how ECG and PPG technologies compare at a glance:
Limitations and Considerations:
Heart rate variability values from wearables can vary widely depending on sensor type, signal quality, and proprietary algorithms used to detect heartbeats. Wearables also tend to struggle during exercise, high movement, or irregular breathing, which can throw off beat detection [44]. It’s not uncommon to see implausibly high or low HRV readings due to motion artifacts, poor skin contact, or signal dropout. In practice, HRV is often most reliable when measured at rest or during sleep, times when artifacts are minimized and signal quality is strongest.
While wearable devices have made HRV monitoring far more accessible, reliability varies widely depending on the technology and the specific HRV domain (time vs. frequency). ECG-based chest straps (e.g., Polar H10) remain the most valid option for short-term HRV recordings, particularly in controlled settings or when assessing frequency-domain metrics like LF/HF ratio [4, 48]. Furthermore, PPG-based wearables—such as rings and smartwatches—can estimate time-domain measures like RMSSD with moderate to high accuracy [5, 49, 50], but struggle with frequency-domain reliability and are highly sensitive to motion artifacts, especially during wakefulness or activity [50]. However, both smartwatches and rings show improved agreement with ECG-derived HRV when used during sleep or prolonged periods of rest, making them most suitable for overnight monitoring or recovery tracking.
Recommendation: For casual daily HRV tracking, especially during sleep, wrist- or ring-based PPG devices are sufficient. However, for research, clinical decision-making, or high-resolution HRV metrics, an ECG-based chest strap is the most reliable and validated option.
Interpreting HRV Responsibly
There are important nuances of HRV interpretation, especially regarding the balance of PNS and SNS activity. One of the biggest misconceptions is that certain HRV metrics directly reflect SNS activity. In reality, metrics like RMSSD and HF are reliable indicators of PNS (vagal) activity, but we cannot say the same for LF [3].
LF contains both SNS and PNS input, and while it’s often hypothesized to reflect SNS activity predominantly, this is not definitive. Contrary to PNS activity, we currently lack an accessible and non-invasive way to monitor SNS activity in real time. Accurate lab-grade assessments, such as measuring blood or saliva for epinephrine levels or inserting electrodes into sympathetic nerves, are invasive and impractical for daily monitoring [3, 34]. These are clearly not feasible for day-to-day use or monitoring.
It is important to understand how to interpret HRV data. Lower HRV is probably indicative of more SNS activity (in most cases), but we can more confidently state that fluctuations in HRV are more representative of fluctuations in PNS activity (i.e., vagal withdrawal or vagal dominance. Because of this, most fluctuations in HRV, particularly short-term time domain metrics like RMSSD, are best understood as reflecting changes in PNS tone such as vagal withdrawal during stress or vagal dominance at rest. While lower HRV is often associated with higher SNS output, it’s more appropriate to view HRV as a proxy for PNS modulation, not a direct readout of total autonomic balance.
HRV Optimization for Healthspan Extension
HRV is a valuable marker of autonomic flexibility and general health, reflecting PNS activity and the body's capacity to recover from stress. Low resting HRV has been convincingly linked to increased risk for cardiometabolic diseases and all-cause mortality [36, 51]. Moreover, HRV is consistently impaired in individuals with both modifiable and non-modifiable cardiovascular risk factors including hypertension, smoking, obesity, diabetes, and high cholesterol [51].
Fortunately, while HRV is highly individually variable and influenced by fixed traits such as age and genetics, it is also modifiable through lifestyle behaviors that support cardiovascular and nervous system resilience across the lifespan. Among these, exercise is particularly well-supported.
A growing body of evidence shows that consistent physical training across a range of modalities can improve HRV. Resistance training has been shown to positively affect HRV [32], though aerobic or cardiovascular training tends to produce the most robust improvements [52].
This is consistent with known physiological adaptations to endurance training, including reduced resting heart rate, enhanced vagal tone, vascular remodeling, and increased mitochondrial density and efficiency [53], adaptations that collectively reduce sympathetic demand and increase PNS dominance at rest. Notably, individuals with cardiometabolic conditions also demonstrate HRV improvements following structured exercise interventions [54].
Sleep is another modifiable factor that significantly influences HRV. Adequate sleep not only supports autonomic recovery but appears to act synergistically with physical activity [55]. Individuals who regularly engage in physical activity and obtain sufficient sleep tend to exhibit the greatest HRV benefits. Moreover, one behavior may partially buffer the negative effects of the other—physically active individuals may attenuate the adverse HRV impact of sleep deprivation, and vice versa [55]. This underscores the importance of optimizing both sleep duration and quality in conjunction with physical activity for promoting autonomic health.
Psychological stress is another key contributor to autonomic imbalance. Chronic stress, whether from occupational demands, interpersonal conflict, or generalized anxiety, has been repeatedly associated with reduced HRV [56]. Furthermore, individuals diagnosed with depression or anxiety disorders often exhibit impaired autonomic regulation, as reflected by consistently lower HRV values [57, 58]. Fortunately, a variety of behavioral and psychosocial interventions, such as mindfulness [59], therapy [60], and time spent with loved ones or pets [61, 62], have shown promise in improving HRV by reducing sympathetic arousal and enhancing parasympathetic engagement.
HRV Optimization for Healthspan Extension
HRV is a valuable marker of autonomic flexibility and general health, reflecting PNS activity and the body's capacity to recover from stress. Low resting HRV has been convincingly linked to increased risk for cardiometabolic diseases and all-cause mortality [36, 51]. Moreover, HRV is consistently impaired in individuals with both modifiable and non-modifiable cardiovascular risk factors including hypertension, smoking, obesity, diabetes, and high cholesterol [51].
Fortunately, while HRV is highly individually variable and influenced by fixed traits such as age and genetics, it is also modifiable through lifestyle behaviors that support cardiovascular and nervous system resilience across the lifespan. Among these, exercise is particularly well-supported.
A growing body of evidence shows that consistent physical training across a range of modalities can improve HRV. Resistance training has been shown to positively affect HRV [32], though aerobic or cardiovascular training tends to produce the most robust improvements [52].
This is consistent with known physiological adaptations to endurance training, including reduced resting heart rate, enhanced vagal tone, vascular remodeling, and increased mitochondrial density and efficiency [53], adaptations that collectively reduce sympathetic demand and increase PNS dominance at rest. Notably, individuals with cardiometabolic conditions also demonstrate HRV improvements following structured exercise interventions [54].
Sleep is another modifiable factor that significantly influences HRV. Adequate sleep not only supports autonomic recovery but appears to act synergistically with physical activity [55]. Individuals who regularly engage in physical activity and obtain sufficient sleep tend to exhibit the greatest HRV benefits. Moreover, one behavior may partially buffer the negative effects of the other—physically active individuals may attenuate the adverse HRV impact of sleep deprivation, and vice versa [55]. This underscores the importance of optimizing both sleep duration and quality in conjunction with physical activity for promoting autonomic health.
Psychological stress is another key contributor to autonomic imbalance. Chronic stress, whether from occupational demands, interpersonal conflict, or generalized anxiety, has been repeatedly associated with reduced HRV [56]. Furthermore, individuals diagnosed with depression or anxiety disorders often exhibit impaired autonomic regulation, as reflected by consistently lower HRV values [57, 58]. Fortunately, a variety of behavioral and psychosocial interventions, such as mindfulness [59], therapy [60], and time spent with loved ones or pets [61, 62], have shown promise in improving HRV by reducing sympathetic arousal and enhancing parasympathetic engagement.
HRV as a Biomarker for Oxytocin Responsiveness
Heart rate variability (HRV) is typically interpreted as a marker of parasympathetic activity; the higher it is, the more “relaxed” and recovered your autonomic system appears to be. Metrics like RMSSD and high-frequency (HF) power are especially sensitive to vagal tone, which is why HRV has become a favored biomarker in stress science and recovery monitoring. But this interpretation, while useful in many contexts, becomes more complex and even misleading when applied to interventions like oxytocin.
Oxytocin is often described as calming, but this framing doesn’t fully capture its physiological role. As a neuromodulator, oxytocin doesn't just promote relaxation, it enhances adaptive engagement. Depending on the context, oxytocin can either increase parasympathetic activity or amplify sympathetic arousal, especially when social or emotionally relevant stimuli are involved. In other words, oxytocin isn't simply sedating. It prepares the nervous system to respond—to stimuli, to stress, to people, and to one’s environment. And that means HRV may go down, not up, when oxytocin is working well. [63, 64]
This flexibility is what makes oxytocin biologically powerful—but it also complicates its interpretation via HRV. Because HRV is typically seen as a unidirectional proxy for relaxation, a decrease in HRV during or after oxytocin administration might be erroneously viewed as a negative outcome. In reality, such a change may reflect appropriate autonomic engagement, particularly if oxytocin is facilitating approach-oriented behaviors or increasing attention to emotionally significant stimuli.
Put differently, a drop in HRV may be a sign that oxytocin is working as intended, not blunting arousal, but mobilizing the nervous system to respond. This has been observed in studies where oxytocin administration leads to decreased vagal activity in anticipation of social evaluation, or increases sympathetic tone during emotionally relevant tasks. These findings highlight the bidirectional nature of oxytocin's effects on autonomic output and caution against simplistic interpretations of HRV as a marker of therapeutic efficacy
What Happens to HRV When Oxytocin Is Active?
In several controlled studies, researchers have observed a temporary reduction in HRV, specifically in RMSSD and HF power, following oxytocin administration. This typically occurs within 15 to 45 minutes after dosing. At the same time, markers like systolic blood pressure and cardiac output increase, indicating not distress, but mobilization. This state of arousal aligns with increases in sympathetic tone and metabolic readiness, not unlike what occurs during focused attention or social approach behavior.
Interestingly, in these same studies, participants also show signs of heightened engagement: increased pupil diameter, elevated galvanic skin response (GSR), and fMRI activation in regions like the amygdala, anterior cingulate, and insula, all key hubs in the brain’s salience network. These regions are involved in scanning the environment for emotionally or socially relevant information. So while HRV may decrease, cognitive-affective engagement increases. This is particularly relevant for individuals struggling with apathy, burnout, depression, or social withdrawal, states in which vagal dominance may paradoxically reflect disengagement or blunted responsiveness [63, 64].
How to Use HRV as a Feedback Tool for Oxytocin Response
Because oxytocin produces both acute and long-term effects, HRV should be interpreted as part of a trajectory, not a moment-to-moment score. A brief suppression in HRV after dosing — for instance, a 10–20% drop in RMSSD within the first hour, is often a sign that the central nervous system is responding, shifting the body into a more alert, engaged state. This is not a stress response in the negative sense, but rather a motivational upshift, part of what makes oxytocin potentially valuable in longevity protocols, especially where energy, social motivation, or affective engagement are lagging.
Overnight HRV, measured during sleep, is a better gauge of restorative parasympathetic tone. If HRV rebounds after the initial dip, or starts to trend higher over the course of a few weeks, it likely reflects increased resilience and recovery capacity. Individuals may also experience improved sleep quality, faster subjective recovery from stress, or enhanced emotional clarity — all of which align with improved autonomic regulation, even if not immediately evident in daytime HRV snapshots.
Here's a simplified summary of this trajectory:
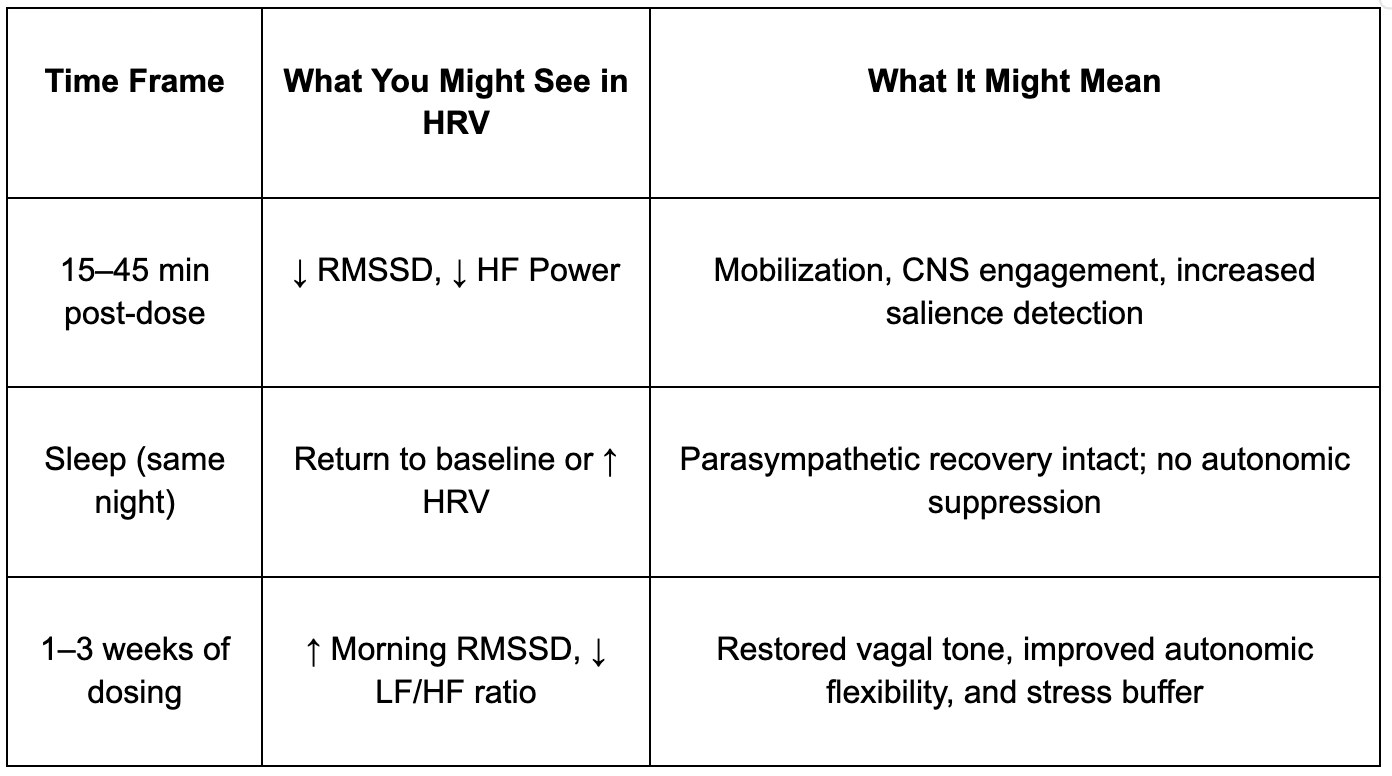
Oxytocin affects multiple brain and body systems that regulate autonomic output. Its receptors are expressed in key parasympathetic control centers, like the nucleus tractus solitarius (NTS) and vagal motor nuclei, but it also enhances activity in the locus coeruleus, the brain’s principal source of norepinephrine. This helps explain why oxytocin can enhance alertness while still improving mood or reducing anxiety in the long term. It’s not pushing the system in one direction, sympathetic or parasympathetic, but improving the range and adaptability of autonomic responses. [65, 66, 67]
In aging populations, or in individuals with burnout, fatigue, or inflammation-linked dysregulation, this adaptive flexibility is often compromised. Oxytocin’s ability to gently shift the system into a more engaged, motivated state, even if it reduces HRV in the short term, may be exactly what’s needed to restore physiological plasticity.
The best way to use HRV as a biomarker for oxytocin efficacy is to zoom out. Don’t fixate on daily highs or lows, instead, look for signs of responsiveness. A healthy pattern might include a small dip in HRV right after oxytocin is taken, followed by a rebound later in the day or overnight. Over time, morning HRV should trend higher or become more stable, a sign that autonomic balance is improving, and the body is becoming more adaptable.
Conclusion
Heart rate variability is a powerful lens into the dynamic balance of the autonomic nervous system, offering insight into how the body responds to stress, recovers, and adapts over time. While inter-individual variability in HRV is high, consistent tracking — especially through validated wearables during periods of rest or sleep — allows for meaningful trend analysis. A sustained drop in RMSSD (Root Mean Square of Successive Differences between heartbeats), a time-domain metric closely linked to parasympathetic or vagal activity, by 20 percent or more from baseline can serve as an early signal of autonomic strain. This may reflect overtraining, poor recovery, psychological stress, or underlying dysfunction.
Beyond athletics and clinical diagnostics, HRV is emerging as a promising marker of broader physiological resilience. It may reflect not only momentary stress but also chronic inflammation, maladaptive aging, and the body's capacity to rebound from internal and external challenges. When tracked consistently and interpreted in context, HRV can help guide more intelligent, individualized decisions about recovery, therapeutic protocols, and long-term health.
- Hon EH, Lee ST. Electronic evaluation of the fetal heart rate. VIII. Patterns preceding fetal death, further observations. Am J Obstet Gynecol. 1963;87:814–26.
- Sayers BM. Analysis of heart rate variability. Ergonomics. 1973;16(1):17–32.
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–65.
- Georgiou K, Larentzakis AV, Khamis NN, Alsuhaibani GI, Alaska YA, Giallafos EJ. Can wearable devices accurately measure heart rate variability? A systematic review. Folia Med (Plovdiv). 2018;60(1):7–20.
- Jerath R, Syam M, Ahmed S. The future of stress management: integration of smartwatches and HRV technology. Sensors (Basel). 2023;23(17).
- Pinzone AG. OS HRV 2025. Available from: https://github.com/apinzone/OS_HRV.
- Ernsberger U, Rohrer H. Sympathetic tales: subdivisions of the autonomic nervous system and the impact of developmental studies. Neural Dev. 2018;13(1):20.
- McCorry LK. Physiology of the autonomic nervous system. Am J Pharm Educ. 2007;71(4):78.
- Heilbronn E, Bartfai T. Muscarinic acetylcholine receptor. Prog Neurobiol. 1978;11(3–4):171–88.
- Mohyuddin R, Dietrichs ES, Sundaram P, et al. Cardiovascular effects of epinephrine during experimental hypothermia (32°C) with spontaneous circulation in an intact porcine model. Front Physiol. 2021;12.
- Sassone-Corsi P. The cyclic AMP pathway. Cold Spring Harb Perspect Biol. 2012;4(12).
- Morady F, Nelson SD, Kou WH, et al. Electrophysiologic effects of epinephrine in humans. J Am Coll Cardiol. 1988;11(6):1235–44.
- Stratton JR, Halter JB, Hallstrom AP, et al. Comparative plasma catecholamine and hemodynamic responses to handgrip, cold pressor and supine bicycle exercise testing in normal subjects. J Am Coll Cardiol. 1983;2(1):93–104.
- Flatt AA, Globensky L, Bass E, et al. Heart rate variability, neuromuscular and perceptual recovery following resistance training. Sports. 2019;7(10):225.
- Djaoui L, Haddad M, Chamari K, Dellal A. Monitoring training load and fatigue in soccer players with physiological markers. Physiol Behav. 2017;181:86–94.
- Mann TN, Lamberts RP, Nummela A, Lambert MI. Relationship between perceived exertion during exercise and subsequent recovery measurements. Biol Sport. 2017;34(1):3–9.
- Naranjo J, De la Cruz B, Sarabia E, et al. Heart rate variability: a follow-up in elite soccer players throughout the season. Int J Sports Med. 2015;94(11):881–6.
- Oliveira RS, Leicht AS, Bishop D, et al. Seasonal changes in physical performance and heart rate variability in high-level futsal players. Int J Sports Med. 2013;34(5):424–30.
- Flatt AA, Allen JR, Keith CM, et al. Season-long heart-rate variability tracking reveals autonomic imbalance in American college football players. Int J Sports Physiol Perform. 2021;16(12):1834–43.
- Purvis D, Gonsalves S, Deuster PA. Physiological and psychological fatigue in extreme conditions: overtraining and elite athletes. PM&R. 2010;2(5):442–50.
- Kellmann M. Preventing overtraining in athletes in high-intensity sports and stress/recovery monitoring. Scand J Med Sci Sports. 2010;20 Suppl 2:95–102.
- Kellmann M, Bertollo M, Bosquet L, et al. Recovery and performance in sport: consensus statement. Int J Sports Physiol Perform. 2018;13(2):240–5.
- Mourot L, Bouhaddi M, Perrey S, et al. Decrease in heart rate variability with overtraining: assessment by the Poincaré plot analysis. Clin Physiol Funct Imaging. 2004;24(1):10–8.
- Hynynen ESA, Uusitalo A, Konttinen N, Rusko H. Heart rate variability during night sleep and after awakening in overtrained athletes. Med Sci Sports Exerc. 2006;38(2).
- Baumert M, Brechtel L, Lock J, et al. HRV, blood pressure variability, and baroreflex sensitivity in overtrained athletes. Clin J Sport Med. 2006;16(5):412–7.
- Thiel C, Vogt L, Bürklein M, et al. Functional overreaching during preparation training of elite tennis professionals. J Hum Kinet. 2011;28:79–89.
- Wieling W, Kaufmann H, Claydon VE, et al. Diagnosis and treatment of orthostatic hypotension. Lancet Neurol. 2022;21(8):735–46.
- Mark AL. The Bezold-Jarisch reflex revisited: clinical implications of inhibitory reflexes originating in the heart. J Am Coll Cardiol. 1983;1(1):90–102.
- Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. 2019;285(4):352–66.
- Eldahan KC, Rabchevsky AG. Autonomic dysreflexia after spinal cord injury: systemic pathophysiology and methods of management. Auton Neurosci. 2018;209:59–70.
- Zesiewicz TA, Baker MJ, Wahba M, et al. Autonomic nervous system dysfunction in Parkinson’s disease. Curr Treat Options Neurol. 2003;5(2):149–60.
- Kingsley JD, Panton LB, McMillan V, Figueroa A. Cardiovascular autonomic modulation after acute resistance exercise in women with fibromyalgia. Arch Phys Med Rehabil. 2009;90(9):1628–34.
- Olivieri F, Biscetti L, Pimpini L, et al. HRV and autonomic nervous system imbalance: potential biomarkers and detectable hallmarks of aging and inflammaging. Ageing Res Rev. 2024;101:102521.
- Fu Q, Witkowski S, Levine BD. Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation. 2004;110(18):2931–7.
- Kingsley JD, Pinzone AG, Humm SM, Elsey GE. Hemodynamic and autonomic modulation in response to additive sympathetic stressors in young, healthy individuals. Int J Exerc Sci. 2025. In Review.
- Vinik AI, Maser RE, Ziegler D. Autonomic imbalance: prophet of doom or scope for hope? Diabet Med. 2011;28(6):643–51.
- Dimitrov S, Lange T, Born J. Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol. 2009;184(1):503–11.
- Geiger AM, Pitts KP, Feldkamp J, et al. Cortisol-dependent stress effects on cell distribution in healthy individuals and individuals with chronic adrenal insufficiency. Brain Behav Immun. 2015;50:241–8.
- Li X, Li C, Zhang W, et al. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct Target Ther. 2023;8(1):239.
- Kop WJ, Stein PK, Tracy RP, et al. Autonomic nervous system dysfunction and inflammation contribute to cardiovascular mortality risk associated with depression. Psychosom Med. 2010;72(7):626–35.
- Abboud FM. The Walter B. Cannon Memorial Award Lecture, 2009. Physiology in perspective: in search of autonomic balance. Am J Physiol Regul Integr Comp Physiol. 2010;298(6):R1449–67.
- Li L, Li H, He L, et al. Orthostatic hypotension and its relation to HRV, pulse wave velocity, and frailty in the elderly. Front Cardiovasc Med. 2020;7:603957.
- Alen NV, Parenteau AM, Sloan RP, Hostinar CE. HRV and circulating inflammatory markers in midlife. Brain Behav Immun Health. 2021;15:100273.
- Pinheiro N, Couceiro R, Henriques J, et al. Can PPG be used for HRV analysis? IEEE EMBC. 2016:2945–9.
- Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258.
- Hoog Antink C, Mai Y, Peltokangas M, et al. Accuracy of HRV estimated with wrist-PPG in elderly vascular patients. Sci Rep. 2021;11(1):8123.
- Esgalhado F, Batista A, Vassilenko V, et al. Peak detection and HRV feature evaluation on ECG and PPG signals. Symmetry. 2022;14(6):1139.
- Hinde K, White G, Armstrong N. Wearable devices suitable for monitoring 24-hour HRV in military populations. Sensors (Basel). 2021;21(4).
- Kinnunen H, Rantanen A, Kenttä T, Koskimäki H. Accuracy of nocturnal HR and HRV via ring PPG compared to ECG. Physiol Meas. 2020;41(4):04nt1.
- Cao R, Azimi I, Sarhaddi F, et al. Accuracy of Oura Ring HR and HRV compared with ECG. J Med Internet Res. 2022;24(1):e27487.
- Thayer JF, Yamamoto SS, Brosschot JF. Autonomic imbalance, HRV, and cardiovascular disease risk. Int J Cardiol. 2010;141(2):122–31.
- Yang F, Ma Y, Liang S, et al. Effect of exercise modality on HRV in adults. Rev Cardiovasc Med. 2024;25(1):9.
- Hellsten Y, Nyberg M. Cardiovascular adaptations to exercise training. Compr Physiol. 2015;6(1):1–32.
- Routledge FS, Campbell TS, McFetridge-Durdle JA, Bacon SL. HRV improvements with exercise therapy. Can J Cardiol. 2010;26(6):303–12.
- Onimaru LJ, Christofaro DGD, Valente HB, et al. Sleep quality and cardiac autonomic modulation by activity level. Sleep Breath. 2025;29(4):216.
- Kim HG, Cheon EJ, Bai DS, et al. Stress and HRV: a meta-analysis. Psychiatry Investig. 2018;15(3):235–45.
- Levine JC, Fleming R, Piedmont JI, et al. HRV and GAD during worry and aversive imagery. J Affect Disord. 2016;205:207–15.
- Brunoni AR, Kemp AH, Dantas EM, et al. HRV as a trait marker of major depression. Int J Neuropsychopharmacol. 2013;16(9):1937–49.
- Natarajan A. HRV during mindful breathing meditation. Front Physiol. 2023;13.
- Blanck P, Stoffel M, Bents H, et al. HRV in psychotherapy: associations with alliance and outcome. J Nerv Ment Dis. 2019;207(6):451–8.
- Pourmand V, Froidevaux NM, Williams DP, et al. Attachment, HRV, and social support in young adults. Front Psychol. 2023;14.
- Ortmeyer HK, Katzel LI. Effects of proximity between companion dogs and older adults on HRV. Int J Environ Res Public Health. 2020;17(8).
- Lawson EA. Understanding oxytocin in human physiology and pathophysiology. Compr Psychoneuroendocrinol. 2024;19:100242.
- Jankowski M, Broderick TL, Gutkowska J. The role of oxytocin in cardiovascular protection. Front Psychol. 2020;11:2139.
- Michelini LC, Marcelo MC, Amico J, Morris M. Oxytocinergic regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2003;284(6):H2269–76.
- Norman GJ, Cacioppo JT, Morris JS, et al. Oxytocin increases autonomic cardiac control: moderation by loneliness. Biol Psychol. 2011;86(3):174–80.
- Kemp AH, Quintana DS, Kuhnert RL, et al. Oxytocin increases HRV at rest. PLoS One. 2012;7(8):e44014.
- Cohen R, Bakhshi S. Oxytocin supplementation for longevity: benefits and mechanisms. Healthspan. 2024. Available from: https://gethealthspan.com/science/article/oxytocin-supplement-benefits