The Biology of Cold Exposure: A Hormetic Stressor That Activates Autophagy, Improves Metabolism, and Reduces Inflammation—But Only If You Shiver
Shivering as a Metabolic Signal. Shivering is more than an involuntary response to cold—it’s a potent metabolic trigger. When sustained, shivering initiates a cascade of physiological responses, including increased caloric expenditure, sympathetic nervous system activation, and brown fat thermogenesis. It also enhances glucose uptake in skeletal muscle, independent of insulin, mimicking key aspects of exercise. These effects are not observed with brief cold exposure that fails to elicit shivering, underscoring the importance of intensity and duration in achieving metabolic adaptation.
Cold-Induced Improvements in Glucose and Lipid Metabolism. Ten days of repeated cold exposure leading to sustained shivering improved glucose control and lipid metabolism in overweight adults. Participants experienced reduced fasting glucose, lower glucose AUC, and significant decreases in triglycerides—effects that paralleled reductions in circulating free fatty acids. These changes suggest that shivering supports insulin sensitivity and lipid clearance, potentially offering cardiometabolic benefits when practiced consistently.
Energy Expenditure and Cold Acclimation. Shivering initially produces a high thermogenic load, increasing energy expenditure by over 50% above resting levels. However, this effect diminishes with repeated exposure, reflecting metabolic acclimation. Over time, the body becomes more efficient at thermoregulation—likely through increased non-shivering thermogenesis and vascular adaptations—highlighting cold’s capacity to drive both acute and long-term physiological change.
Cold Stress and Muscle Remodeling. Skeletal muscle biopsies taken before and after cold acclimation reveal upregulation of key metabolic pathways, including GLUT4 expression and mitochondrial gene activation. These findings indicate that cold exposure reprograms skeletal muscle for improved substrate utilization and cellular energy efficiency—mechanisms that may complement or even substitute for traditional endurance exercise in certain contexts.
Autophagy, Apoptosis, and Hormetic Adaptation. Cold stress influences core longevity pathways by modulating autophagy and apoptosis. Initial exposures impair autophagic flux and increase apoptotic signaling, reflecting cellular stress. With repeated exposure, however, autophagy improves while apoptosis diminishes, indicating a hormetic adaptation. The cold, when sustained and repeated, appears to recalibrate cellular cleanup systems to favor repair over cell death.
Immune Resilience and Inflammatory Control. Immune cells exposed to ex vivo cold stress mirrored skeletal muscle in their adaptation: early signs of dysfunction gave way to increased stress tolerance after repeated cold immersion. Cold acclimation also suppressed inflammatory cytokines like TNF-α and IL-6, suggesting that cold exposure exerts systemic anti-inflammatory effects—effects that may be mediated through heat shock proteins and improved autophagic function.
Heat Shock Proteins and Cellular Defense. Heat shock proteins HSP70 and HSP90 spiked early in response to cold but did not continue to increase with repeated exposure. This suggests that cold rapidly activates these molecular chaperones, which help protect and refold damaged proteins. Their transient activation implies that even early cold exposures may confer cellular benefits—though full adaptation requires ongoing stress.
Shivering Is Necessary, Not Optional. Across metabolic, muscular, and immune systems, the physiological benefits of cold exposure appear tightly linked to the presence of shivering. Brief, non-shivering dips may produce a subjective “high” via neurotransmitters, but they fall short of activating the deeper repair pathways shown to influence the hallmarks of aging. For cold to act as a true hormetic stressor, it must be long and cold enough to challenge the system.
Cold Exposure and the Hallmarks of Aging. Through its effects on autophagy, inflammation, mitochondrial function, and metabolic signaling, shivering-induced cold exposure intersects with multiple hallmarks of aging. These findings support the idea that deliberate thermal stress, when appropriately applied, may extend beyond biohacking trends to serve as a meaningful longevity intervention.
Cold Exposure: Beyond the Plunge
Cold plunges have become a fixture of the modern wellness movement. Scroll through any social media feed and you’ll likely encounter influencers submerging themselves into sleek tubs of near-freezing water, often touting benefits ranging from mental clarity to metabolic health. These quick dips are invigorating, triggering a surge of neurochemicals—including adrenaline, cortisol, and dopamine—that leave people feeling energized and alert.
But beneath the surface lies a more complex physiology than most cold-plunge reels reveal.
Short bouts of cold exposure primarily stimulate the sympathetic nervous system, producing an acute stress response. This activates the “fight or flight” cascade—raising heart rate, boosting focus, and releasing feel-good neurotransmitters. While these effects are not without merit, they represent just the tip of the iceberg when it comes to the potential benefits of cold exposure.
Emerging research suggests that longer and more sustained cold exposure—particularly when it leads to shivering—may provide deeper adaptations that go far beyond a transient mood boost. These adaptations involve fundamental shifts in how our bodies regulate temperature, expend energy, and metabolize fat. The key distinction? Duration and intensity matter. Achieving the metabolic and cellular benefits of cold requires moving beyond the brief plunge and into a more sustained thermal challenge.
In this review, we’ll explore two recent studies that probe the molecular and physiological effects of cold exposure. Specifically, we’ll examine how sustained cold stress reshapes muscle metabolism, enhances cellular cleanup mechanisms, and alters glucose and fat handling—and what it means for people looking to optimize metabolism and longevity through environmental stressors like cold.
The Physiology of Shivering: Cold as a Metabolic Stimulus
While the sharp jolt of a cold plunge may grab attention, it’s the sustained exposure to cold—and the involuntary act of shivering—that activates some of the body’s most profound metabolic adaptations.
Why is shivering so important?
Shivering is more than just a discomfort reflex. It is a highly coordinated thermogenic process, triggered when the hypothalamus detects a drop in core temperature. In response, the body initiates rapid, low-amplitude muscle contractions to generate heat. These contractions are energy-intensive, significantly increasing caloric expenditure—much like moderate physical activity.
Beyond simply burning calories, shivering also appears to act as a metabolic signal. It stimulates the sympathetic nervous system and triggers the release of catecholamines like norepinephrine, which in turn activate brown adipose tissue (BAT)—a specialized form of fat packed with mitochondria. BAT doesn’t just store energy; it burns it, converting chemical energy into heat through a process known as non-shivering thermogenesis. This dual mode of heat production—muscle-driven and fat-driven—may amplify cold’s effects on energy balance and fat metabolism.
Intriguingly, shivering also enhances glucose uptake in skeletal muscle, independent of insulin. Much like exercise, cold-induced muscle activity mobilizes glucose transporters (GLUT4) to the cell surface, allowing muscles to absorb circulating glucose and lower blood sugar levels.
In short: cold exposure that’s intense or prolonged enough to induce shivering activates a multi-system metabolic response—burning more energy, mobilizing fat, and improving glucose regulation. These effects are unlikely to be fully realized through brief, non-shivering cold dips alone.
Two new studies provide additional insights into how these shivering-induced adaptations may extend even further, beyond metabolism to cellular stress resilience and mitochondrial remodeling. But to be clear, as we’ll find out, you have to spend a lot of time in the cold to get these benefits.
Shivering for Metabolic Health
In a recent proof-of-concept study, researchers explored whether structured cold exposure—specifically one that would induce shivering—could produce measurable improvements in metabolic health. The results suggest that the answer is yes, with potential implications for glucose regulation, lipid metabolism, and even cardiovascular function.
The study enrolled 15 adults, all with body mass indexes (BMIs) in the overweight to mildly obese range (27 to 35). Over the course of 10 consecutive days, participants were fitted with a full-body, water-perfused suit designed to steadily reduce skin temperature from a thermoneutral baseline (32°C) down to 10°C. Once skin temperature dropped enough to trigger sustained shivering, that state was maintained for an hour each day.
Importantly, the researchers personalized the cooling protocol for each participant to ensure consistent physiological responses. Using indirect calorimetry, surface electromyography (EMG), and visual observation, they verified that the participants entered and sustained a true shivering state. A key benchmark was a ≥50% increase in energy expenditure above resting levels, confirming that the cold stress was sufficiently intense to mobilize metabolic systems.
After 10 days of cold acclimation, the researchers observed a modest but statistically significant 6% decrease in glucose area under the curve (AUC) during an oral glucose tolerance test—a metric that reflects how efficiently the body handles a glucose load. Fasting glucose levels declined by 3%, and postprandial (2-hour) glucose dropped by 11%. Perhaps most strikingly, the number of participants classified as glucose intolerant dropped from nine at baseline to five following the intervention.
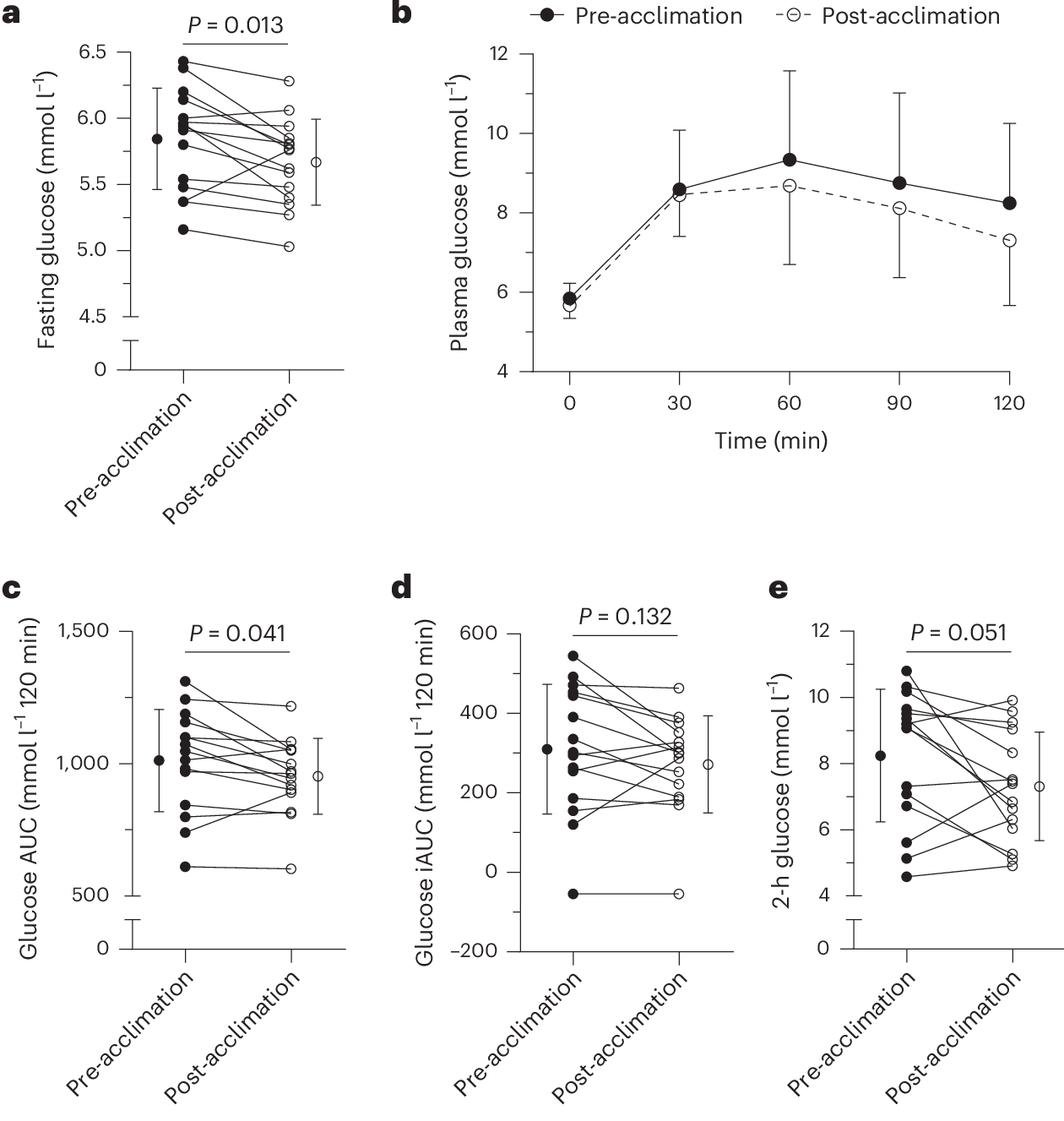
These improvements were accompanied by a 14% reduction in non-esterified fatty acids (NEFAs)—circulating free fatty acids derived from white adipose tissue. Chronically elevated NEFAs are known to impair insulin signaling and contribute to insulin resistance. Their suppression suggests that cold-induced shivering may somewhat help re-tune the body’s lipid-glucose axis toward a more insulin-sensitive state.
Lipid markers shifted even more dramatically. After just one cold session, fasting triglyceride levels dropped by 17%. After 10 sessions, they had plummeted by 32%. Given the strong association between elevated triglycerides and cardiovascular risk, this finding could have relevance for cardiometabolic health.
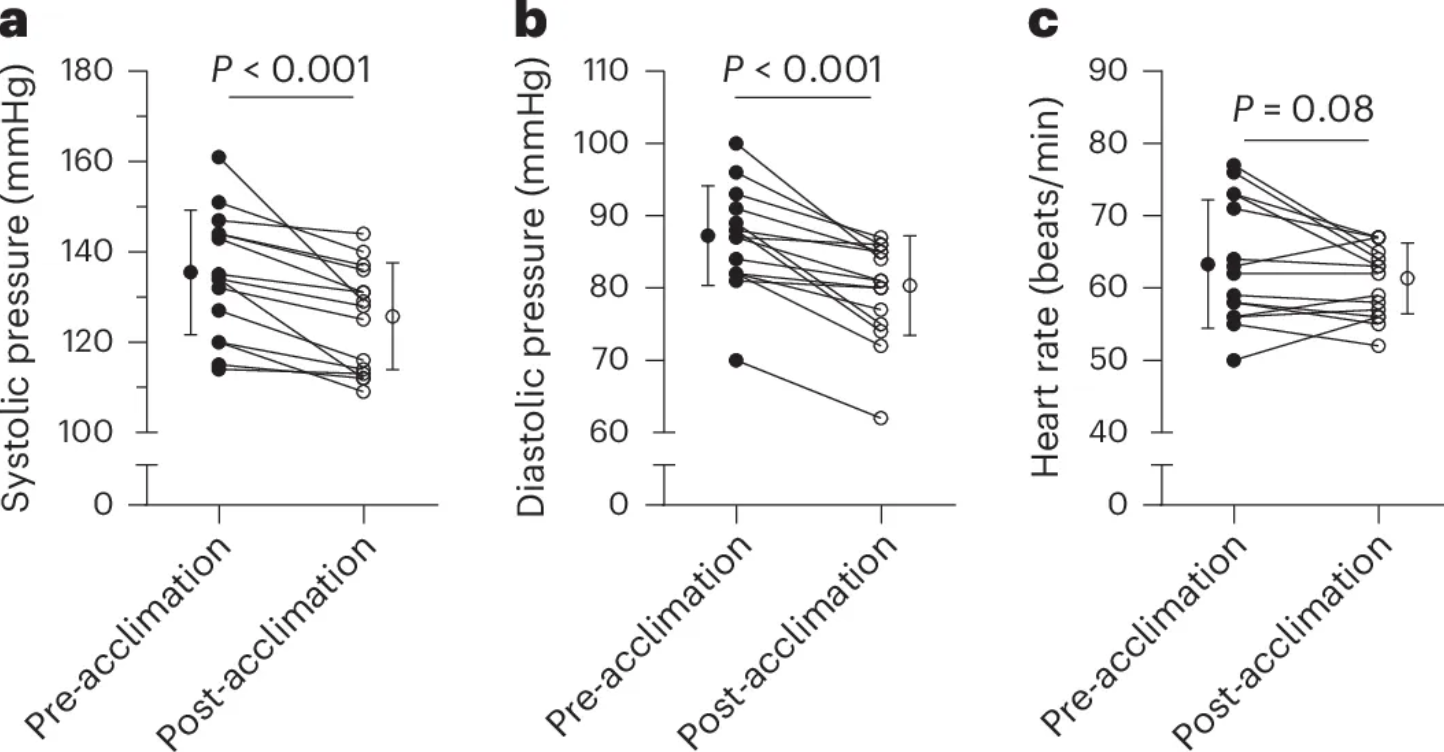
Finally, cold exposure also yielded blood pressure benefits. Following the 10-day acclimation period, participants experienced an average decrease of 10 mmHg in systolic and 7 mmHg in diastolic pressure—effects that rival those of many first-line antihypertensive medications. Remarkably, even a single session produced detectable reductions, hinting at an immediate vascular response to cold-induced shivering.
Despite the impressive metabolic shifts observed after repeated cold exposure, participants did not lose fat mass over the 10-day intervention. Instead, researchers noted a modest 0.5-kilogram reduction in fat-free mass—a somewhat unexpected outcome that raises intriguing questions about how cold stress influences body composition.
The decline in fat-free mass likely does not reflect true muscle loss, given the short duration of the study. One hypothesis is that the observed reduction may stem from transient changes in muscle glycogen stores and associated water content. Cold-induced shivering increases glucose uptake and accelerates substrate turnover, and muscle tissue may transiently lose intracellular water as glycogen is depleted, creating the appearance of lean mass reduction on standard body composition scans. Alternatively, repeated cold stress could induce subtle shifts in muscle protein metabolism, though this remains speculative and was not directly assessed in the study.
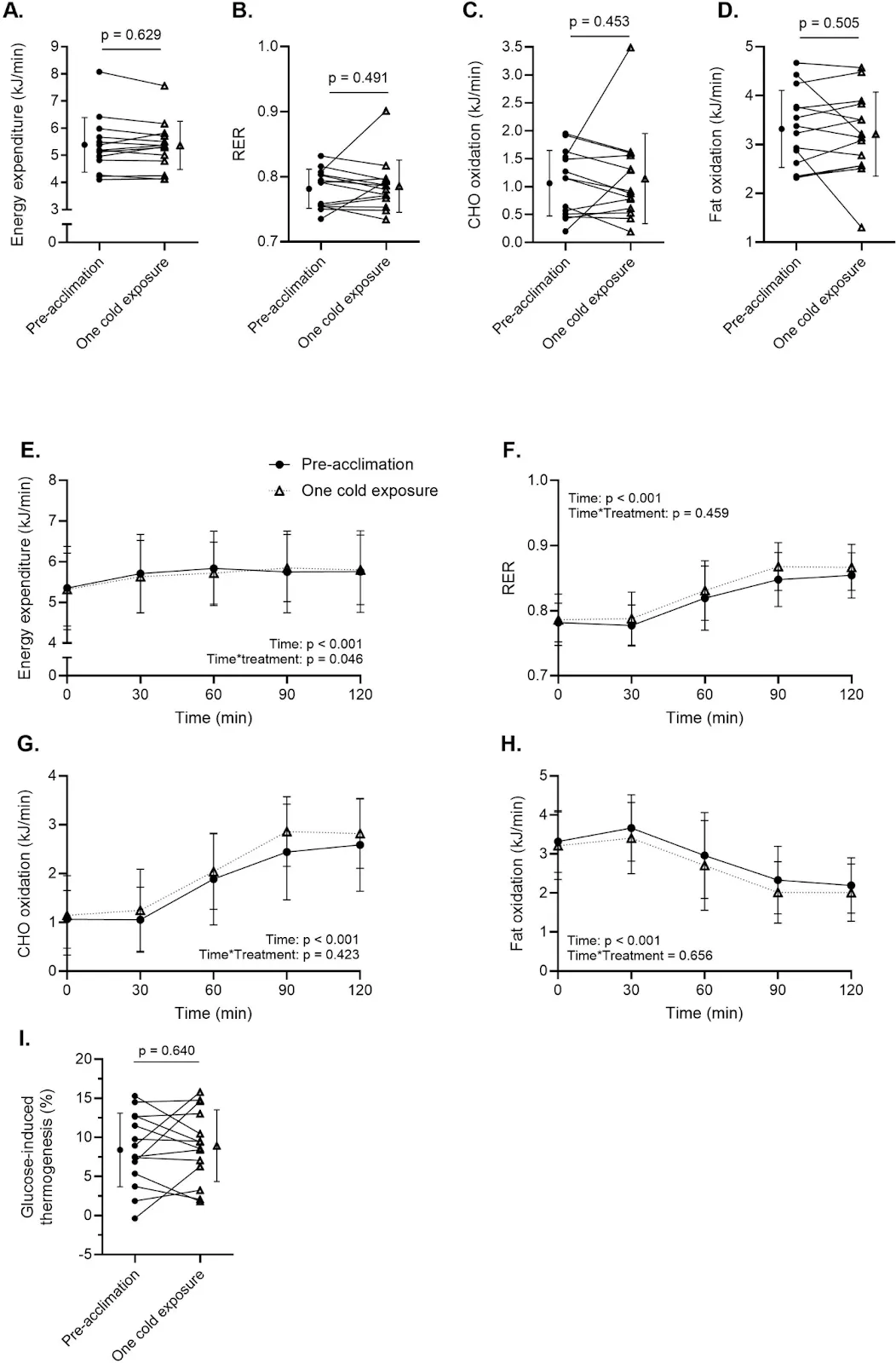
What is clear is that shivering sharply elevated energy expenditure. During the first cold exposure, participants expended an average of 823 kilojoules—approximately 200 kilocalories—equivalent to the energy burned during a brisk 60-minute walk. However, by the tenth session, energy expenditure had dropped to 659 kilojoules. This decline suggests that the body was adapting to repeated cold exposure, becoming more efficient at generating heat with fewer metabolic demands.
This form of metabolic acclimatization—sometimes referred to as “cold adaptation”—is a hallmark of human physiological plasticity. Over time, repeated cold stress appears to blunt the initial energy spike, likely through improved vasoconstriction, increased non-shivering thermogenesis via brown fat, and a dampened sympathetic nervous response. While this efficiency may reduce the calorie-burning appeal of cold exposure over time, it may also signal deeper adaptations that promote thermoregulatory and metabolic resilience.
In other words, the body's ability to adapt may be a feature—not a bug—of cold-based interventions.
Cold Exposure, Cellular Function, and Autophagy
The benefits of cold exposure may reach well beyond metabolism. In a second study, researchers investigated how repeated cold-water immersion influences two of the body’s most fundamental cellular maintenance systems: autophagy and apoptosis.
Autophagy, often referred to as the cell’s internal recycling program, plays a critical role in maintaining cellular health. At Healthspan, we often explore this process in the context of nutrient deprivation—when food is scarce, cells initiate autophagy to break down and repurpose damaged organelles and protein aggregates for energy and rebuilding. But autophagy is more than just an emergency backup system. It’s a continuous housekeeping mechanism that prevents the accumulation of cellular debris. When autophagy becomes impaired, dysfunctional components begin to pile up, accelerating the onset of many age-related diseases—from neurodegeneration to metabolic dysfunction. So, maintaining healthy autophagy is essential for prolonging a healthy lifespan.
Apoptosis, by contrast, is the mechanism of programmed cell death. It’s how the body safely eliminates cells that are too damaged to repair—cells that, if left unchecked, might become cancerous or fuel chronic inflammation. When a cell experiences irreparable stress or DNA damage, apoptosis ensures it is quietly and cleanly dismantled. Like autophagy, this process is essential for tissue health and cellular turnover.
When these two systems function in harmony, they maintain the integrity of our tissues by recycling what can be salvaged and removing what cannot. But when they fall out of balance—when autophagy stalls or apoptosis is triggered inappropriately—cells begin to accumulate damage, inflammation rises, and the risk of chronic disease increases. This imbalance is a hallmark of aging and is tightly linked to conditions like sarcopenia, insulin resistance, and even cognitive decline.
To test how cold influences this delicate balance, researchers enrolled 10 healthy, physically active men in a seven-day protocol. Each participant underwent daily cold-water immersion—one hour per day in water cooled to 14°C (57°F). The researchers then collected muscle tissue samples at key time points to track molecular signatures of autophagy and apoptosis over the course of the intervention.
The cellular response to cold, it turns out, is not immediate.
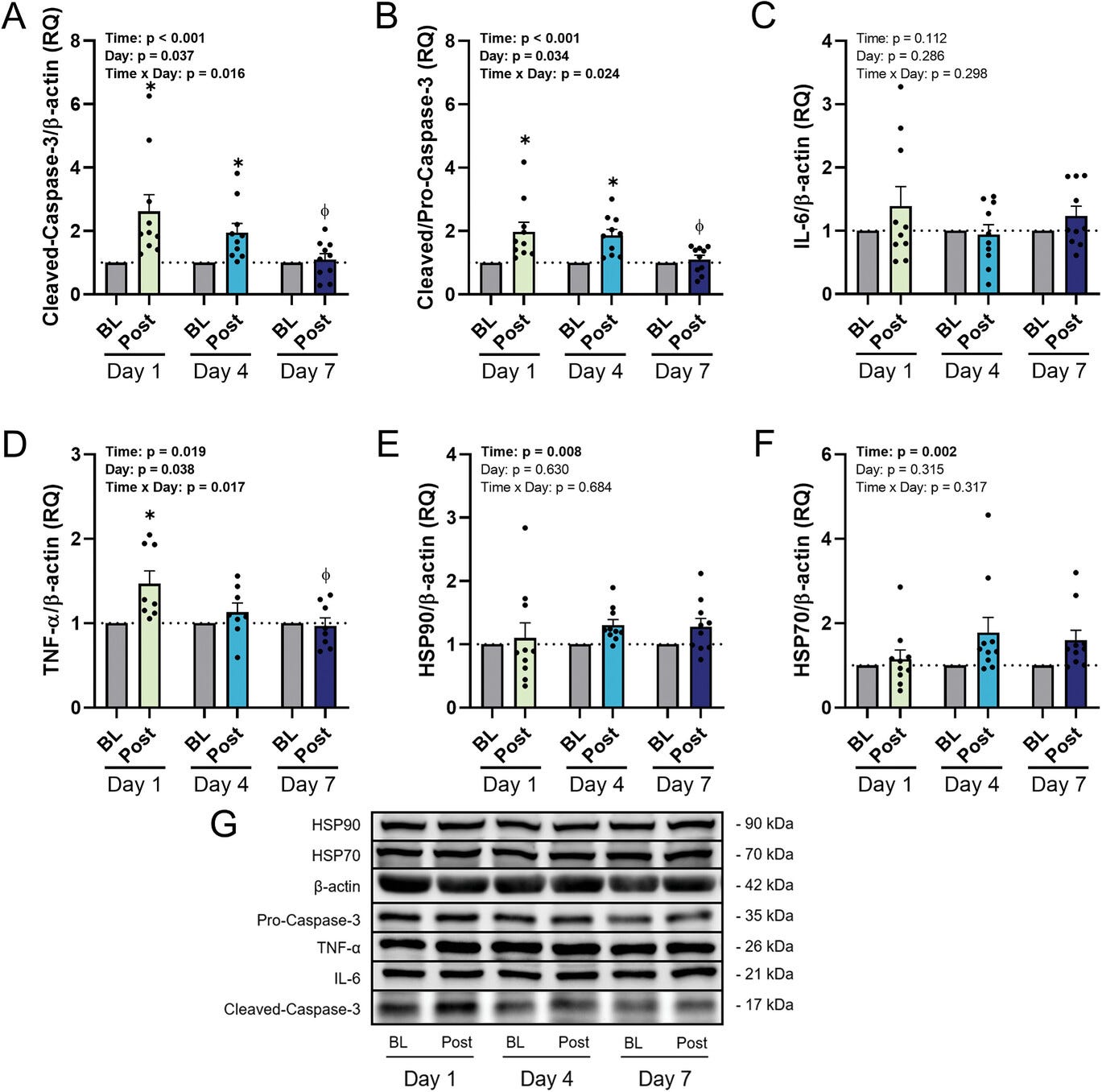
After just a single cold exposure, the data showed signs of autophagic dysfunction. A key protein called p62, which accumulates when damaged cellular components are not properly degraded, was significantly elevated. At the same time, markers of apoptosis—specifically cleaved caspase-3, an enzyme that triggers the self-destruction of cells—also rose sharply. These results suggest that the sudden stress of cold immersion overwhelmed the cells’ cleanup systems, prompting them to initiate damage control through cell death rather than repair.
But by the fourth day, a shift began to take place. Autophagy started to improve. p62 levels began to decline, and LC3-II, a protein that marks the formation of autophagosomes (the vesicles responsible for delivering cellular waste to the lysosome), increased. While apoptotic signaling remained elevated, it appeared to plateau. The cells were beginning to adapt—not fully recovered, but showing signs of resilience.
By the end of the week-long protocol, the transformation was more complete. LC3-II levels were significantly elevated, p62 levels had markedly decreased, and caspase-3 activity had dropped. These changes indicated that autophagy was now functioning more effectively, while the need for apoptosis had diminished. The cold stress that had initially overwhelmed the system had now trained it. In essence, the cells had been conditioned to withstand the cold and respond with enhanced cleanup rather than capitulation.
This adaptive trajectory mirrors a broader principle in biology known as hormesis—the idea that low doses of stress can provoke a beneficial response by challenging the system to grow stronger. In this case, repeated cold exposure appeared to recalibrate the muscle’s quality-control networks, nudging cells toward repair and renewal instead of damage and decline.
How Cold Acclimation Trains the Immune System
To explore whether the benefits of cold exposure extended beyond muscle tissue, the researchers conducted a series of ex vivo experiments—testing immune cell responses to cold stress in a controlled laboratory setting. Using blood samples taken before and after the seven-day immersion protocol, they isolated immune cells and subjected them to extreme cold (4°C) to observe how well the cells could withstand environmental stress outside the body.
Before cold acclimation, the cells faltered. They mirrored the same molecular distress signals seen in muscle tissue after the initial cold exposures: elevated levels of p62, indicating impaired autophagy, and heightened activity of cleaved caspase-3, a marker of apoptosis. This confirmed that the immune system, like muscle tissue, was initially unprepared for the cold and responded with dysfunction and cell death.
But after a week of repeated cold exposure, the picture changed. Immune cells taken from participants showed enhanced autophagic activity and reduced apoptotic signaling when re-exposed to cold in the lab. The improvements paralleled the adaptations seen in skeletal muscle, suggesting a systemic enhancement of cellular stress resilience. In essence, the cold-trained immune cells were better able to maintain their function and integrity in the face of environmental extremes.
Another key player in this adaptation process appeared to be the heat shock proteins—specifically HSP70 and HSP90. These molecular chaperones help stabilize proteins under stress and facilitate proper folding and repair. Both spiked acutely during the early phases of cold exposure, consistent with their role as first responders in cellular defense. However, their levels didn’t increase further over the course of the week, implying that their protective effects are rapidly activated by cold, but may not require progressive upregulation with ongoing exposure. This could help explain why many of cold’s benefits appear early—even before full acclimation sets in.
Finally, the study revealed a broader immunological shift: inflammatory markers, including tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), declined significantly by the end of the week. This suggests that regular cold exposure doesn’t merely provoke a stress response—it ultimately dampens chronic inflammation, a root contributor to many age-related diseases. What begins as a physiological challenge may, with repetition, evolve into an anti-inflammatory intervention.
Taken together, these findings point to a broader narrative: cold acclimation doesn’t just toughen the body—it trains the cells, improving their ability to manage stress, preserve function, and reduce damaging inflammation.
Why This Matters: Cold as a Catalyst for Important Cellular Adaptations
The implications of these studies are not revolutionary—but they are revealing. They demonstrate that repeated cold exposure, when it leads to sustained shivering, initiates a cascade of physiological and cellular adaptations that go far beyond mental toughness. Autophagy was enhanced, and markers of apoptosis and inflammation were regulated in ways that suggest improved cellular health and metabolic function. Even in young, physically active individuals, these changes were measurable.
This matters because many of the diseases we associate with aging—metabolic dysfunction, chronic inflammation, and cellular senescence—begin with a breakdown in these same pathways. While cold exposure is not a cure-all, it appears to nudge the body toward a more resilient state, priming muscles, immune cells, and mitochondria to better withstand stress and maintain function over time.
To be clear: this isn’t about quick fixes. A one-minute plunge into a cold bath may be exhilarating, but these studies suggest it is the repeated, sustained exposure that makes the difference—particularly when it elicits shivering. Shivering isn’t just a sign that you’re cold; it’s a physiological signal that your body is working to adapt. And it seems to be both necessary and sufficient to drive many of the metabolic and cellular effects observed.
For those interested in adding cold to their health regimen, it doesn’t require high-end plunge tubs or laboratory-grade cooling suits. Practical approaches abound. Walking outside in minimal clothing on a cold winter morning, submerging in a lake or unheated bath, or even spending extended periods in a chilly room—provided it’s cold enough to induce mild shivering—may offer similar benefits.
Cold exposure, like many lifestyle interventions, works not because of intensity but because of consistency. The magic lies not in the first plunge, but in the body’s repeated efforts to adapt. And as the research shows, those adaptations may extend to the molecular level—retraining our cells to clean, repair, and function more efficiently.
In a world where comfort is ubiquitous and temperature is tightly regulated, deliberately courting discomfort might seem counterintuitive. But as these studies remind us, stress—when applied wisely and repeatedly—can be a catalyst for resilience.
- Sellers, A.J., van Beek, S.M.M., Hashim, D. et al. Cold acclimation with shivering improves metabolic health in adults with overweight or obesity. Nat Metab (2024). https://doi.org/10.1038/s42255-024-01172-y
- King KE, McCormick JJ, Kenny GP. The Effect of 7-Day Cold Water Acclimation on Autophagic and Apoptotic Responses in Young Males. Adv Biol (Weinh). 2024 Nov 27:e2400111. doi: 10.1002/adbi.202400111. Epub ahead of print. PMID: 39601474.