Linking Aerobic Fitness to Increased Brain Myelination: A Strategy Against Neurodegeneration
Higher VO₂ Max Linked to Increased Myelination: The study found a significant positive association between aerobic fitness levels and brain myelin content across nearly all regions examined. Participants with higher maximal oxygen consumption (VO₂ max) exhibited increased myelination, indicated by higher myelin water fraction (MWF) values.
Pronounced Effect in Middle-Aged and Older Adults: This relationship was particularly pronounced among middle-aged and older adults, where higher fitness levels corresponded with markedly higher MWF values compared to less fit counterparts.
Protective Effects Against Age-Related Demyelination: These findings suggest that aerobic fitness may offer protective effects against age-related demyelination, potentially mitigating the typical decline in myelin integrity that accompanies aging. Overall, the data support the notion that physical fitness not only benefits cardiovascular and metabolic health but also plays a crucial role in maintaining neural structure, enhancing myelin integrity, and contributing to more efficient neural communication and better cognitive function in older adults.
Aerobic fitness benefits myelination across all ages, but the magnitude of its impact grows with increasing age, highlighting the importance of maintaining fitness throughout the lifespan for neural health. Participants aged 80–94 showed the most significant increase in myelination with higher fitness levels. Participants aged 60–79 also exhibited a notable increase in myelination with higher fitness levels. Younger participants displayed flatter increases.
In the low VO₂ max group (lowest fitness level), peak myelination occurred before age 22, and these individuals experienced a more rapid decline in myelin content thereafter. This early peak suggests that poor aerobic fitness may accelerate the maturation and subsequent aging of myelin, leading to an earlier onset of decline in neural efficiency and cognitive function.
For participants in the middle VO₂ max group (moderate fitness level), peak myelination occurred at age 28. This indicates a slight delay in the peak compared to the low fitness group, suggesting that moderate levels of fitness can modestly extend the period of myelin development.
Most notably, participants in the highest VO₂ max group (high fitness level) did not reach peak myelination until age 41—a full decade later than the average for the entire cohort and nearly two decades later than the low fitness group. This significant delay implies that higher levels of aerobic fitness may prolong the developmental phase of myelination, thereby extending the period during which neural communication is most efficient.
Just as regular physical exercise leads to increased muscle mass, delayed onset of muscle atrophy, and a slower decline in muscle strength with age, aerobic fitness appears to have a parallel effect on brain myelination. Muscle mass typically reaches its peak in early adulthood and gradually declines with age, but consistent physical activity can modify this trajectory by enhancing muscle strength and endurance. Similarly, aerobic fitness may modulate the trajectory of myelination, contributing to sustained cognitive function and neural health.
The potential mechanisms behind these observations could involve increased activity of oligodendrocytes—the cells responsible for myelin production—in response to aerobic exercise. Aerobic activity has been shown to elevate levels of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor 1 (IGF-1), which support neuron and oligodendrocyte survival and function. Additionally, improved cerebral blood flow resulting from aerobic exercise enhances oxygen and nutrient delivery to the brain, facilitating myelin synthesis and repair processes.
Background
Cognitive decline, including conditions like Alzheimer's disease and other forms of dementia, stands as one of the leading causes of death and disability worldwide. According to the World Health Organization, over 55 million people globally are living with dementia—a number projected to rise to 78 million by 2030 due to aging populations. This alarming increase underscores the critical need for effective strategies to combat cognitive deterioration.
Emerging research suggests that exercise may be one of the most impactful interventions for promoting brain health. Regular physical activity has been associated with enhancements in brain structure and function, such as increased neurogenesis, improved synaptic plasticity, and elevated levels of neurotrophic factors like brain-derived neurotrophic factor (BDNF). Epidemiological studies have shown that individuals who engage in consistent exercise exhibit better cognitive function, particularly in later stages of life. Acute bouts of physical activity, especially high-intensity exercise, have been demonstrated to boost cognitive performance in the short term by enhancing cerebral blood flow and neurotransmitter availability.
Despite these positive associations, the exact mechanisms by which exercise exerts its neuroprotective effects are still being explored. In previous reviews, we have highlighted several underlying mechanisms of exercise and other geroprotective interventions that promote brain health and functional integrity:
One prevailing hypothesis is that physical fitness mitigates structural brain changes that contribute to cognitive decline. Recent evidence points to a potential role involving myelin—the insulating sheath surrounding neurons that is crucial for efficient neural signaling and overall cognitive health. Myelination facilitates rapid signal transmission and supports neural network integrity.
The degeneration of myelin in the brain is increasingly recognized as a critical factor contributing to disruptions in neural communication, which may play a significant role in the cognitive decline observed in Alzheimer's disease and other neurodegenerative disorders. Emerging research suggests that myelin breakdown may even precede the formation of amyloid-beta plaques and neurofibrillary tangles—the hallmark pathological features of Alzheimer's disease. Advanced imaging studies have detected early myelin degeneration in individuals who later develop Alzheimer's, indicating that myelin damage could be an initial event in the disease's progression.
Age-related deterioration of myelin is closely associated with cognitive decline. Reduced white matter integrity—often resulting from myelin damage—is correlated with declines in memory, executive function, and processing speed in older adults. As myelin degradation leads to the slowing of cognitive processes and disrupts the synchronization of neural networks, preserving myelin integrity is essential for sustaining cognitive health across the lifespan.
Could exercise enhance brain myelination? That’s what a new study suggests [1]. Recent research indicates that physical fitness may promote myelin repair and increase myelination in the adult brain. Let’s review the study to understand its significance.
The Study Design
In the study, researchers drew participants from two well-established cohorts: the Baltimore Longitudinal Study of Aging (BLSA) and the Genetic and Epigenetic Signatures of Translational Aging Laboratory Testing (GESTALT). The sample comprised 125 cognitively healthy adults with an average age of 56 years, of whom 54% were men. The primary objective was to quantify myelin content across various brain regions to explore its relationship with aerobic fitness levels.
To achieve this, participants underwent advanced brain imaging techniques that measured whole-brain and regional myelin water fraction (MWF). MWF is a highly specific and direct proxy for myelin content in neural tissue. It quantifies the fraction of water molecules associated with the myelin sheath, providing a reliable indicator of myelination levels. While the technical intricacies of MWF imaging are complex and primarily of interest to neuroscientists, the essential takeaway is that higher MWF values reflect greater myelin integrity—a positive indicator for neural communication and cognitive function.
Aerobic fitness was assessed using a maximal graded treadmill exercise test to determine each participant's maximal oxygen consumption (VO₂ max). This method is considered the gold standard for evaluating cardiorespiratory fitness because it provides a direct and objective measurement of the body's maximum capacity to uptake and utilize oxygen during intense exercise. The use of VO₂ max testing enhances the study's validity by avoiding reliance on self-reported physical activity levels or indirect estimations of fitness.
Maximum oxygen consumption (VO2 max) is not only an indicator of endurance exercise performance but also a strong predictor of cardiovascular disease and mortality. In fact, cardiorespiratory fitness is the single greatest predictor of health and functional capacity.
Participants were stratified into three fitness categories based on their VO₂ max percentiles: low fitness (below the 30th percentile), moderate fitness (between the 30th and 70th percentiles), and high fitness (above the 70th percentile). This classification allowed the researchers to examine potential dose-response relationships between aerobic fitness and myelin content. Additionally, to investigate the effects of aging on myelination and fitness, the participants were divided into four age groups: young adults (22–29 years), middle-aged adults (40–59 years), older adults (60–79 years), and the oldest adults (80–94 years). [1]
By combining precise measurements of brain myelination with direct assessments of aerobic fitness across a diverse age range, the study aimed to uncover how physical fitness may influence myelin integrity throughout the lifespan. Understanding this relationship is crucial, as maintaining or enhancing myelin levels could be a key factor in preventing cognitive decline associated with aging. [1]
Now that we understand the experimental design, let’s review the results of the study.
The Results
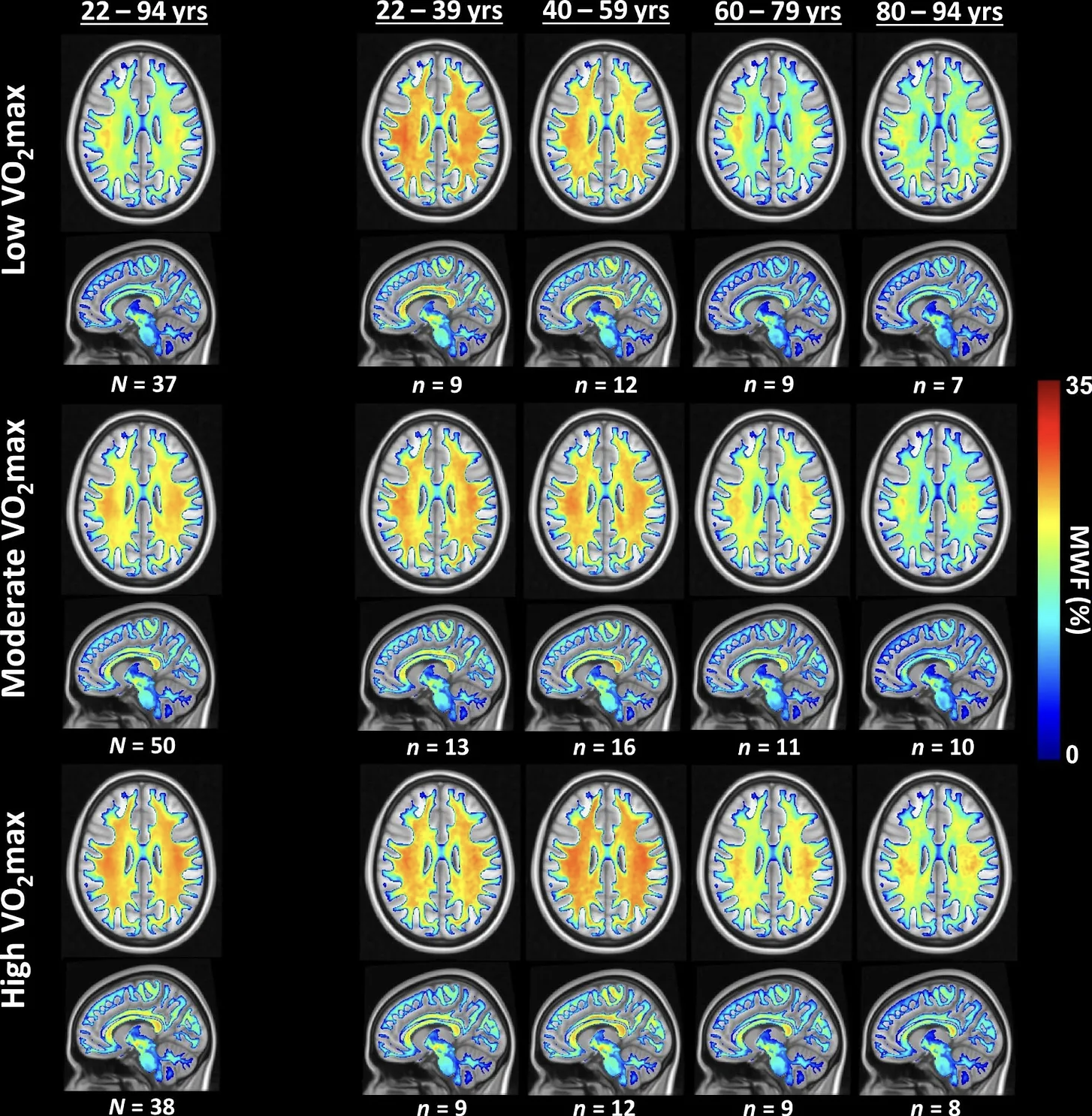
The study revealed a significant association between aerobic fitness levels and myelin content in the brain. Participants with lower maximal oxygen consumption (VO₂ max) exhibited reduced myelination, as indicated by lower myelin water fraction (MWF) values—reflected by more green and blue regions on their brain scans. Conversely, those with higher VO₂ max showed increased myelination, depicted by more red and orange areas on the scans. This gradient suggests that individuals with greater aerobic fitness have better myelin integrity throughout various brain regions.
This relationship was particularly pronounced among the middle-aged (40–59 years) and older adults (60–79 years and 80–94 years). In these age groups, higher aerobic fitness levels corresponded with markedly higher MWF values compared to their less fit counterparts. This finding implies that aerobic fitness may offer protective effects against age-related demyelination, potentially mitigating the decline in myelin integrity that typically accompanies aging.
The data support the notion that physical fitness not only benefits cardiovascular and metabolic health but also plays a crucial role in maintaining neural structure. By enhancing or preserving myelination, higher aerobic fitness could contribute to more efficient neural communication and better cognitive function in older adults.
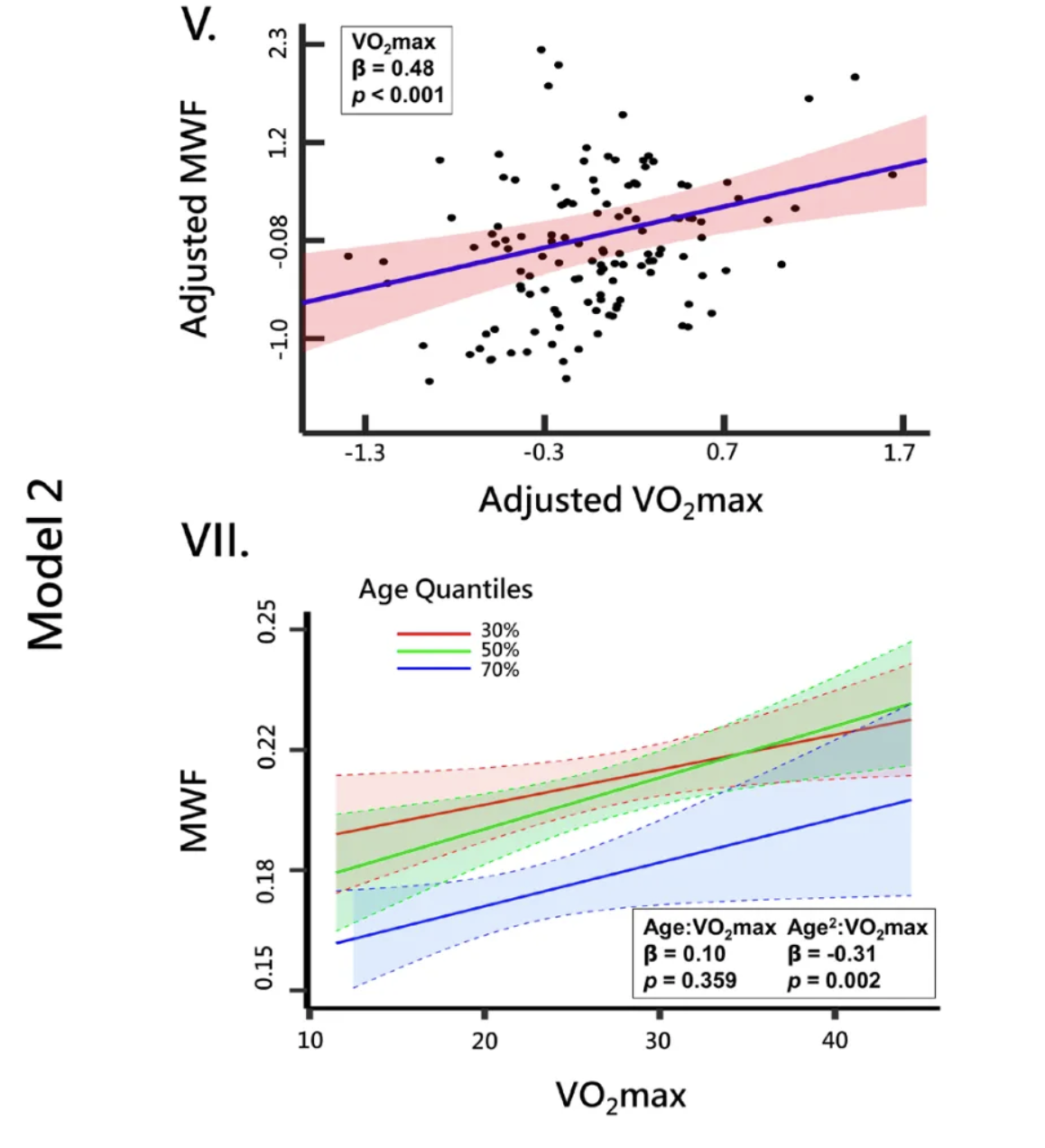
The study demonstrated a positive correlation between aerobic fitness and brain myelination across nearly all imaged brain regions. Specifically, as participants' maximal oxygen consumption (VO₂ max) increased, so did their levels of myelin content, as measured by the MWF. This indicates that individuals with higher aerobic fitness tend to have greater myelin integrity, reinforcing the idea that physical fitness is beneficial for brain health.
Importantly, the influence of aerobic fitness on myelination was more pronounced in older age groups. The oldest participants in the study (ages 80–94) exhibited the greatest increase in myelination with higher fitness levels. This is illustrated by the steep slope of the blue line (labeled VII) in the accompanying graph, which represents this age group. The middle-aged participants (ages 60–79), represented by the green line in the same plot, also showed a significant positive relationship between fitness and myelination, albeit to a lesser extent than the oldest group. The younger age groups displayed flatter slopes, suggesting that while aerobic fitness benefits myelination at any age, its impact becomes increasingly significant as individuals grow older.
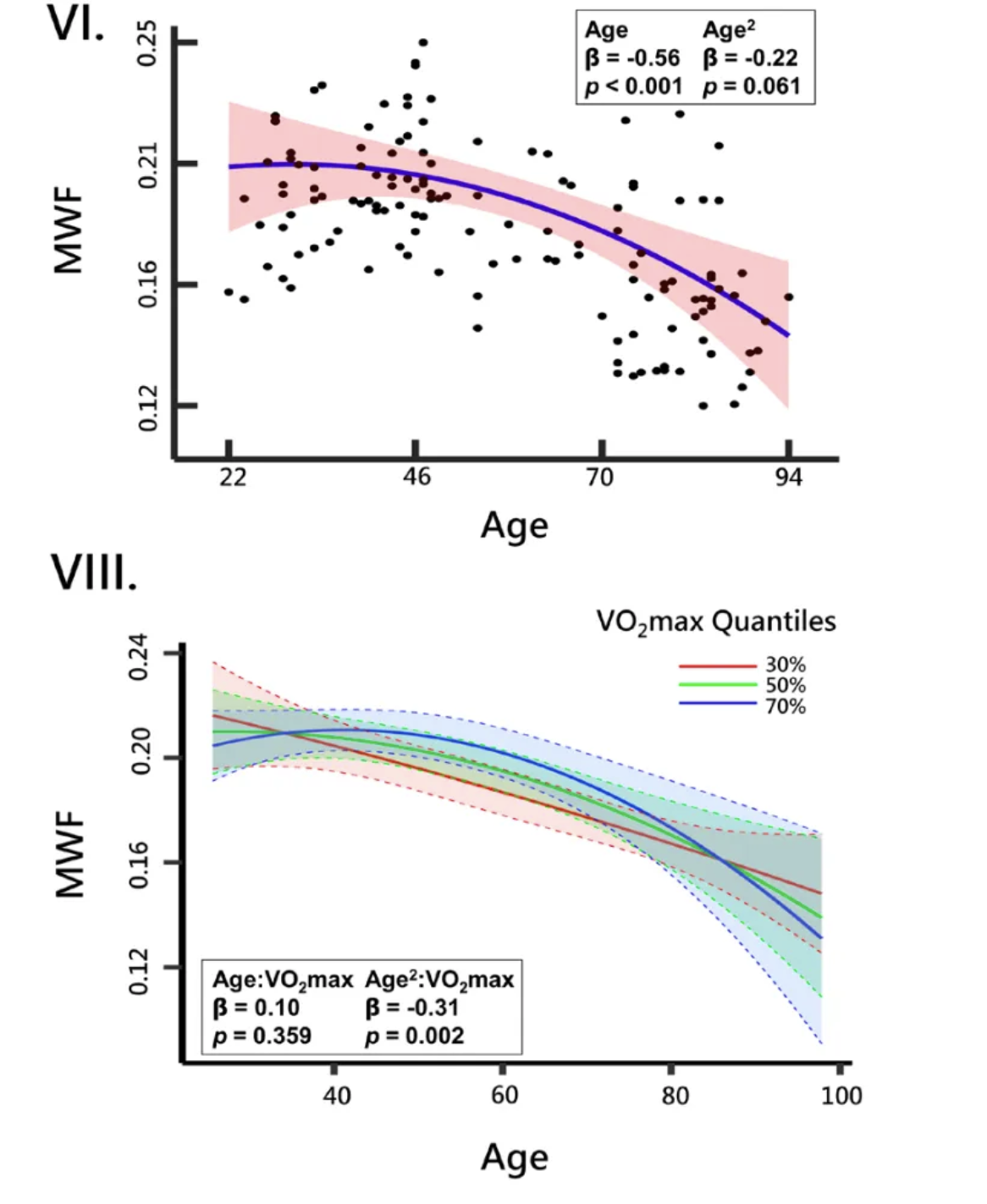
Moreover, higher aerobic fitness was associated with a slower decline in brain myelination over time and a delayed peak in myelin levels. This suggests that maintaining or improving fitness can not only enhance current myelin integrity but also mitigate the typical age-related decline in myelination. Again, these findings highlight the potential of aerobic exercise as a non-pharmacological strategy to preserve neural health and cognitive function in aging populations.
Further analysis of the data revealed that the age at which peak myelination occurs is significantly influenced by aerobic fitness levels. For the entire group of participants, peak myelination was observed around age 31. After this peak, myelin levels began a gradual decline, consistent with the typical aging process of the brain. However, when the participants were stratified based on their VO₂ max levels, distinct patterns emerged.
In the low VO₂ max group (lowest fitness level), peak myelination occurred before age 22, and these individuals experienced a more rapid decline in myelin content thereafter. This early peak suggests that poor aerobic fitness may accelerate the maturation and subsequent aging of myelin, leading to an earlier onset of decline in neural efficiency and cognitive function.
For participants in the middle VO₂ max group (moderate fitness level), peak myelination occurred at age 28. This indicates a slight delay in the peak compared to the low fitness group, suggesting that moderate levels of fitness can modestly extend the period of myelin development.
Most notably, participants in the highest VO₂ max group (high fitness level) did not reach peak myelination until age 41—a full decade later than the average for the entire cohort and nearly two decades later than the low fitness group. This significant delay implies that higher levels of aerobic fitness may prolong the developmental phase of myelination, thereby extending the period during which neural communication is most efficient.
The graphical representation of these findings illustrates the varying slopes of myelin decline across fitness levels. The steep slope of the line representing the low fitness group underscores the rapid decline in myelination post-peak, while the more gradual slopes of the moderate and high fitness groups indicate a slower rate of decline.
The findings from this study provide compelling mechanistic support for the hypothesis that aerobic fitness directly induces beneficial changes in the brain, promoting enhanced cognitive resilience with aging. Traditionally, numerous studies have established associations between physical activity, fitness levels, and improved cognitive function. However, this research allows us to delve deeper and speculate—with empirical evidence—on the underlying biological mechanisms that facilitate these cognitive benefits.
One of the key revelations is that higher aerobic fitness levels are associated with a later peak in brain myelination, a higher maximum level of myelin content, and a slower rate of myelin decline over time. Specifically, individuals with the highest VO₂ max did not reach peak myelination until around age 41, compared to those with lower fitness levels who peaked much earlier. This delay in peak myelination suggests that aerobic fitness may extend the period of myelin development, thereby prolonging optimal neural communication efficiency.
Just as regular physical exercise leads to increased muscle mass, delayed onset of muscle atrophy, and a slower decline in muscle strength with age, aerobic fitness appears to have a parallel effect on brain myelination. Muscle mass typically reaches its peak in early adulthood and gradually declines with age, but consistent physical activity can modify this trajectory by enhancing muscle strength and endurance. Similarly, aerobic fitness may modulate the trajectory of myelination, contributing to sustained cognitive function and neural health.
The potential mechanisms behind these observations could involve increased activity of oligodendrocytes—the cells responsible for myelin production—in response to aerobic exercise. Aerobic activity has been shown to elevate levels of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and insulin-like growth factor 1 (IGF-1), which support neuron and oligodendrocyte survival and function. Additionally, improved cerebral blood flow resulting from aerobic exercise enhances oxygen and nutrient delivery to the brain, facilitating myelin synthesis and repair processes.
Mechanisms: How Might Fitness Protect Myelin in the Brain?
Understanding the biological mechanisms by which aerobic fitness enhances myelin integrity is essential for developing interventions to preserve cognitive function during aging. Several pathways have been identified through which physical activity may exert protective effects on myelination:
- Stimulation of Oligodendrocyte Activity. Exercise has been shown to stimulate the production and activity of oligodendrocytes, the glial cells responsible for the formation and maintenance of myelin sheaths around neuronal axons. Physical activity promotes the proliferation of oligodendrocyte precursor cells (OPCs) and their differentiation into mature oligodendrocytes. This process enhances the ability of these cells to form new myelin sheaths, aiding in the repair of damaged myelin and supporting overall neural network efficiency [2].
- Elevation of Brain-Derived Neurotrophic Factor (BDNF). Aerobic exercise increases the levels of brain-derived neurotrophic factor (BDNF), a protein crucial for the survival, growth, and maintenance of neurons. BDNF plays a significant role in promoting myelination by supporting oligodendrocyte survival and stimulating myelin sheath formation [3]. Elevated BDNF levels enhance synaptic plasticity and neurogenesis, contributing to improved cognitive function and resilience against neurodegenerative processes.
- Reduction of Neuroinflammation Chronic inflammation in the brain is a known contributor to myelin degradation and neurodegenerative diseases. Exercise has anti-inflammatory effects, reducing levels of pro-inflammatory cytokines while increasing anti-inflammatory mediators. By mitigating neuroinflammation, physical activity helps protect oligodendrocytes and preserves myelin integrity, thereby maintaining efficient neural communication. [4]
- Enhancement of Metabolic Function Physical activity improves cerebral blood flow and enhances metabolic function within the brain. Increased blood flow delivers more oxygen and nutrients to neural tissue, supporting the high energy demands of myelination processes [5]. Exercise also enhances mitochondrial function and glucose metabolism in neurons and glial cells, providing the energy required for myelin synthesis and maintenance.
- Modulation of Insulin Signaling Pathways Emerging research suggests that metabolic dysfunction, including impaired insulin signaling, may contribute to neurodegenerative diseases like Alzheimer's disease. Some studies have referred to Alzheimer's as "type 3 diabetes" due to the brain's insulin resistance observed in the condition [6]. While this term is not universally accepted, it underscores the role of metabolic health in cognitive function. Exercise improves systemic insulin sensitivity and may enhance insulin signaling pathways in the brain, potentially protecting against metabolic factors that lead to myelin degradation and cognitive decline.
By influencing these mechanisms, aerobic fitness contributes to the preservation and enhancement of myelin in the brain. The cumulative effect of stimulating oligodendrocyte activity, elevating neurotrophic factors, reducing inflammation, improving metabolic function, and modulating insulin signaling creates an environment conducive to maintaining myelin integrity. This multiprong approach seems to support efficient neural communication and may delay or mitigate age-related cognitive impairments. This is really important for those of us interested in enhancing healthspan.
Limitations and Future Directions
While the findings of this study are compelling, several important questions and caveats warrant consideration. One pertinent question is whether there is a limit to the protective effects of aerobic fitness on brain myelination. Notably, the participants in this study were not elite athletes; their VO₂ max values ranged from approximately 10 mL·kg⁻¹·min⁻¹ on the lower end to about 50 mL·kg⁻¹·min⁻¹ on the higher end. These values represent a spectrum of fitness levels within a general population but do not encompass the extreme aerobic capacities seen in superior endurance athletes.
This raises the question: Are the brains of elite endurance athletes even more heavily myelinated than those of average individuals? Currently, there is insufficient data to answer this definitively. Investigating whether exceptionally high levels of aerobic fitness confer additional benefits to myelin integrity could provide valuable insights into the upper limits of exercise-induced neuroprotection.
Furthermore, we must exercise caution in interpreting the association between aerobic fitness and myelination. Causation cannot be implied from this cross-sectional study design. While a positive correlation exists, we do not know whether increasing fitness levels will lead to enhanced brain myelin content. It is also unclear whether the decline in myelination parallels the age-related decline in aerobic fitness. Longitudinal studies are necessary to determine whether interventions aimed at improving VO₂ max can causally affect myelination trajectories over time.
Implications for Brain Health
Despite these uncertainties, the study contributes to a growing body of literature suggesting that cardiorespiratory fitness benefits brain health. The association between higher aerobic fitness and increased myelin integrity adds neural resilience to the list of physiological systems positively influenced by physical activity.
From a practical standpoint, enhancing VO₂ max through regular aerobic exercise may serve as a non-pharmacological strategy to support brain health. Even for individuals not primarily motivated by longevity or athletic performance, improving aerobic fitness could be a prudent decision for cognitive well-being. By prioritizing activities that boost cardiorespiratory fitness, individuals may enhance neural communication efficiency and potentially mitigate age-related cognitive decline.
- Faulkner ME, Gong Z, Bilgel M, Laporte JP, Guo A, Bae J, Palchamy E, Kaileh M, Bergeron CM, Bergeron J, Church S, D'Agostino J, Ferrucci L, Bouhrara M. Evidence of association between higher cardiorespiratory fitness and higher cerebral myelination in aging. Proc Natl Acad Sci U S A. 2024 Aug 27;121(35):e2402813121. doi: 10.1073/pnas.2402813121. Epub 2024 Aug 19. PMID: 39159379.
- Fields, R. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat Rev Neurosci 16, 756–767 (2015). https://doi.org/10.1038/nrn4023
- Voss, M. W., Vivar, C., Kramer, A. F., & van Praag, H. (2013). Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Sciences, 17(10), 525-544.
- Gleeson, M., et al. (2011). The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nature Reviews Immunology, 11(9), 607-615.
- Maass, A., et al. (2015). Vascular hippocampal plasticity after aerobic exercise in older adults. Molecular Psychiatry, 20(5), 585-593
- de la Monte, S. M., & Wands, J. R. (2008). Alzheimer's disease is type 3 diabetes–evidence reviewed. Journal of Diabetes Science and Technology, 2(6), 1101-1113.