The Role of the Glymphatic System and Autophagy in Neurodegenerative Disease Prevention
The glymphatic system functions as the brain's waste management service: It clears metabolic waste and toxins by using cerebrospinal fluid (CSF) that circulates through peri-vascular spaces, similar to how a city's sewage system removes debris. This system is critical for maintaining brain health and preventing the buildup of harmful substances.
Astrocytes and AQP4 channels play a crucial role in facilitating fluid movement: Astrocytes, through their specialized extensions (end-feet) equipped with AQP4 channels, regulate the flow of CSF and interstitial fluid (ISF), ensuring efficient waste clearance. Disruption in this system, such as through blocked or misaligned AQP4 channels, can lead to the accumulation of toxic proteins, contributing to neurodegenerative diseases like Alzheimer's.
Aging leads to a decline in AQP4 channel function, reducing the brain's ability to clear waste: As we age, AQP4 channels, which regulate fluid flow in the brain, lose their alignment and become less effective, leading to a sluggish flow of cerebrospinal fluid (CSF). This reduces the brain's capacity to flush out metabolic waste and toxic proteins, increasing the risk of waste accumulation.
Astrocytes also degrade with age, further impairing glymphatic function: Astrocytes, which support fluid movement around blood vessels, undergo structural changes with aging, including reduced branching and polarization of their end-feet. This weakening limits their ability to maintain proper CSF flow, compounding the inefficiencies caused by declining AQP4 function and contributing to the buildup of harmful substances that increase the risk of neurodegenerative diseases. The decline of glymphatic function with age results in the accumulation of toxic proteins: As the glymphatic system becomes less efficient, it struggles to clear harmful proteins such as amyloid-beta, alpha-synuclein (α-syn), and tau—key contributors to neurodegenerative diseases like Alzheimer's and Parkinson's. The buildup of these proteins disrupts normal neuronal communication and accelerates the onset and progression of these diseases.
The glymphatic system plays a crucial role in waste clearance after brain injury: Impaired glymphatic function significantly hinders the brain’s ability to clear waste after traumatic brain injury (TBI), leading to the accumulation of toxic biomarkers in brain tissue and potentially worsening recovery outcomes. Targeting the glymphatic system could offer a promising therapeutic approach for improving recovery from both brain injuries and neurodegenerative diseases.
AQP4 channels, vascular pulsatility, and sleep are critical regulators of glymphatic function: AQP4 channels in astrocytes help control cerebrospinal fluid (CSF) flow, but their function declines with age and is disrupted by brain injuries. Vascular pulsatility, which helps propel CSF, also weakens with age, reducing waste clearance efficiency. Sleep, particularly slow-wave sleep, is essential for expanding interstitial spaces and enhancing glymphatic activity.
Maintaining vascular health and sleep quality is vital for preserving glymphatic function: Aging leads to reduced vascular flexibility and sleep disturbances, which impair glymphatic function. Regular exercise, a balanced diet, and maintaining good sleep quality are crucial for preserving the brain’s ability to clear waste and reduce the risk of neurodegenerative diseases.
Autophagy is crucial for maintaining cellular health, especially in neurons, by clearing damaged proteins and organelles: Autophagy acts as the cell’s internal cleanup system, removing malfunctioning components that, if left unchecked, can lead to cellular dysfunction or death. In neurons, which do not regenerate easily, autophagy prevents the accumulation of harmful proteins and damaged mitochondria, which is vital for maintaining brain health.
Inducing autophagy early in disease progression can help prevent neurodegeneration: Research using the 3×Tg-AD mouse model demonstrated that early treatment with rapamycin, an autophagy inducer, significantly reduced the formation of amyloid-beta plaques and tau tangles, preserving cognitive function. This highlights the importance of maintaining normal autophagy to prevent or mitigate the progression of neurodegenerative diseases like Alzheimer’s.
Autophagy and the glymphatic system work together to remove cellular and extracellular waste, protecting against neurodegenerative diseases: Autophagy clears intracellular waste by breaking down and recycling damaged components, while the glymphatic system removes extracellular waste, such as amyloid-beta and tau, from the brain. This coordinated defense is essential for maintaining a healthy brain environment and preventing the buildup of toxic proteins that contribute to neurodegenerative diseases like Alzheimer’s and Parkinson’s.
The decline of both autophagy and glymphatic function with age leads to waste accumulation, chronic inflammation, and accelerated brain degeneration: As both systems become less efficient with age, waste begins to accumulate inside and around neurons, creating a vicious cycle of damage. This waste buildup triggers an inflammatory response, further harming neurons and exacerbating the progression of neurodegenerative diseases.
Research on the glymphatic system faces challenges, particularly with postmortem studies and conflicting results regarding AQP4 channels: Many studies rely on postmortem data, which may not accurately reflect glymphatic function in living brains due to changes in cerebrospinal fluid (CSF) flow after death. Conflicting research on the role of AQP4 channels in CSF flow also complicates our understanding of glymphatic function, with some studies showing reduced CSF flow after AQP4 removal, while others do not.
Translating findings from animal studies to humans and understanding the interaction between autophagy and glymphatic function remain complex issues: Much of what is known about the glymphatic system comes from animal studies, but applying these findings to humans is difficult due to species differences in brain structure, blood flow, and metabolism. Additionally, more research is needed to explore how autophagy and glymphatic function interact, particularly in the context of neurodegenerative diseases.
Lifestyle interventions, particularly exercise, can enhance glymphatic function and reduce the risk of neurodegenerative diseases: Studies have shown that physical activity improves AQP4 function, increases waste clearance, and reduces amyloid-beta buildup in the brain. These benefits highlight the potential of exercise as a non-pharmacological intervention to protect against cognitive decline. Additional research is needed to determine the most effective exercise routines for different populations.
Combining autophagy-promoting strategies with glymphatic function optimization offers a dual approach to improving brain health: Interventions such as caloric restriction, intermittent fasting, and autophagy-inducing drugs, like rapamycin, can enhance cellular cleanup processes. This complements efforts to optimize glymphatic function, leading to more efficient waste removal from the brain. Sleep optimization, particularly during slow-wave sleep, also plays a critical role in enhancing glymphatic efficiency, making it an important factor in integrated brain health strategies.
Introduction
Most people are familiar with the lymphatic system, which is responsible for removing waste from the body and is often associated with lymph nodes. However, the brain lacks this traditional lymphatic system for clearing waste. Instead, it relies on a specialized mechanism known as the glymphatic system. This unique system is essential for brain health, serving as a clearance pathway to remove metabolic byproducts, soluble proteins, and other potentially toxic substances that accumulate during normal brain function.
The glymphatic system is most active during sleep, using cerebrospinal fluid (CSF) to flush out harmful waste, including amyloid-beta, tau proteins, and other compounds linked to neurodegenerative diseases. Recent research has identified impaired glymphatic clearance as a major contributor to cognitive decline and the progression of neurodegenerative diseases like Alzheimer's. These conditions are marked by the buildup of misfolded proteins, particularly amyloid-beta and tau, which form plaques and tangles in the brain. These toxic aggregates disrupt synaptic communication and damage neurons, ultimately leading to cell death. When the glymphatic system's function declines, the accumulation of these proteins accelerates, increasing the risk of neuronal damage and cognitive impairment. [1]
In addition to its role in waste removal, the glymphatic system is closely linked to another critical cellular process: autophagy. While the glymphatic system clears waste from the spaces surrounding brain cells, autophagy is responsible for breaking down and recycling damaged or unnecessary components within cells. Together, these systems form a dual defense against the buildup of toxic substances that contribute to neurodegeneration. When either system fails, waste accumulates, setting the stage for further neuronal damage and cognitive decline.
Because of the crucial role the glymphatic system plays in brain health, enhancing its function has become a promising therapeutic target for reducing cognitive decline and combating neurodegenerative diseases. Researchers are actively investigating interventions to improve glymphatic efficiency, with the goal of reducing the buildup of neurotoxic proteins and promoting overall brain health. If successful, these therapies could delay or even prevent the onset of disorders like Alzheimer's. [1]
In this review, Shreshtha Jolly from the Johns Hopkins University Department of Molecular Biology will explore the structure and function of the glymphatic system, its impact on aging and longevity, and its potential as a therapeutic target for improving brain health. The review will also discuss current challenges and controversies in glymphatic research and what these findings could mean for patients at risk of cognitive decline.
Glymphatic Clearance: Understanding Cerebrospinal Fluid Flow and Brain Waste Removal
The glymphatic system acts as the brain’s own waste management service, clearing harmful substances and maintaining brain health. Imagine the brain as a bustling city: just as a city depends on a sewage system to prevent blockages and maintain order, the brain relies on the glymphatic system to clear metabolic waste and toxins. At the core of this system are peri-vascular spaces (spaces surrounding the brain’s blood vessels), which act like the pipes of a city's sewer network. These spaces allow cerebrospinal fluid (CSF)—the brain’s cleaning fluid—to circulate, sweeping away debris in much the same way water flows through pipes to carry away waste. [1]
However, the brain’s "pipes" don’t work alone. Surrounding these blood vessels are astrocytes, the brain’s maintenance workers. These star-shaped glial cells support the flow of fluids, much like street cleaners ensuring the roads stay clear of debris. Astrocytes help regulate fluid dynamics around neurons, keeping the brain’s environment functioning smoothly by directing waste-clearing traffic. [1]
A particularly important feature of astrocytes is their "end-feet," specialized extensions that wrap around blood vessels like a network of small hoses. These end-feet contain aquaporin-4 (AQP4) channels—tiny valves that allow water and small molecules to pass through. Much like turning on a faucet enables water flow through a home’s plumbing, AQP4 channels control the flow of CSF into the peri-vascular spaces, enabling the brain’s waste removal system to function efficiently. Without these AQP4 "valves" working properly, waste would accumulate in the brain, much like a city’s plumbing system backing up and causing blockages. This disruption can lead to the buildup of toxic substances, including proteins associated with neurodegenerative diseases like Alzheimer's. [1]
The placement of AQP4 channels on the astrocytic end-feet is strategic, similar to how fire hydrants are placed at key locations throughout a city. Concentrated around blood vessels, these channels ensure that fluids can enter and exit the brain’s waste-clearance pathways effectively, keeping the system running smoothly. If these channels are blocked or misaligned, it’s like a city’s pipes becoming clogged—waste starts to back up, which can harm the overall system and contribute to the buildup of harmful proteins in the brain. [1]
The glymphatic system relies on two key fluids: cerebrospinal fluid (CSF), which flows around the brain, and interstitial fluid (ISF), found between brain cells. These fluids must mix together to efficiently flush out waste. CSF enters the spaces around blood vessels, mixes with ISF, and together they carry away toxic proteins, such as amyloid-beta, which can accumulate and lead to diseases like Alzheimer’s. [2]
Astrocytes, equipped with their AQP4 channels, play a central role in facilitating this mixing by allowing water to flow through and ensuring CSF mixes with ISF. This mixing is vital because it helps remove harmful waste, such as amyloid-beta, which, if left unchecked, can cause significant damage to brain cells. [2]
The flow of CSF through the glymphatic system follows a specific pathway. It begins by moving along the arteries, which bring oxygen-rich blood to the brain, collecting waste from brain cells. From there, the fluid moves into the veins, which carry blood back to the heart, and eventually reaches the lymphatic system. The lymphatic system acts as the body's filtration network, transporting fluids and filtering out harmful substances. As the waste-filled fluid passes through the lymph nodes—checkpoints that filter out toxins, including harmful proteins—it is cleansed. Finally, the filtered fluid is returned to the bloodstream, completing the waste-clearance process. [3, 4]
Using this analogy, the process of glymphatic clearance can be summarized in these key steps:
- CSF Entry: The Cleaning Crew Arrives. CSF enters the brain through spaces around arteries, much like a cleaning crew arriving at a building through designated pathways. This fluid acts as the brain’s cleaning solution, ready to sweep away accumulated waste.
- Mixing with ISF: Gathering the Trash. Once inside, the CSF mixes with ISF, the fluid that surrounds brain cells. As these fluids combine, the CSF collects waste products—metabolic byproducts, harmful proteins like amyloid-beta, and other debris—just like a cleaning crew gathering trash scattered throughout different rooms.
- Astrocyte Facilitation: Directing Traffic. Astrocytes, the brain’s traffic controllers, guide the flow of these fluids. Using AQP4 channels, they ensure that the "cleaning solution" reaches all necessary areas, directing it to where waste has accumulated so it can be efficiently cleared away.
- Waste Removal: Taking Out the Trash. After the fluids have collected waste, they are funneled out of the brain through spaces around veins, much like taking out the trash from different rooms and sending it down a chute for removal.
- Lymphatic System: The Recycling Center. The waste-filled fluid enters the body’s lymphatic system, which acts as a recycling center. Here, harmful substances are filtered out and processed, preventing them from causing further damage.
- Clean Fluid Return: Fresh Supplies. Once filtered, the now clean fluid is returned to the bloodstream—like a cleaning crew replenishing supplies for the next round—ensuring the brain remains clean, healthy, and ready for continued function.
Now that we’ve outlined the ideal functioning of the glymphatic system, let’s explore how this process becomes impaired as we age.
Glymphatic System and Aging
As we age, the glymphatic system’s efficiency naturally declines, significantly impacting the brain’s ability to clear waste. Research shows that aging leads to a reduction in both the expression and alignment (polarization) of AQP4 channels in astrocytes. AQP4 channels are like valves in a plumbing system, critical for regulating the flow of water and other fluids through the brain. They allow cerebrospinal fluid (CSF) to mix with interstitial fluid (ISF), flushing out metabolic waste and toxic proteins. In youth, these "valves" operate smoothly, ensuring that fluid flows freely, carrying away debris. However, as we age, these valves start to rust and lose their alignment—just like the aging AQP4 channels. As a result, the flow becomes sluggish, making it harder for the brain to clear out waste efficiently. [1, 2]
Beyond the decline in AQP4 function, aging also affects the structural integrity of astrocytes, which play a crucial role in maintaining CSF flow and supporting glymphatic clearance. Astrocytes act as scaffolding around blood vessels, helping to direct fluid movement. With age, these astrocytes undergo morphological changes, such as reduced branching and polarization of their end-feet—the extensions that normally wrap around blood vessels to facilitate fluid flow. Imagine these astrocytes as scaffolding that begins to weaken and collapse over time, reducing their ability to keep the "pipes" (blood vessels) clear and functional. When both the valves (AQP4 channels) and the scaffolding (astrocytes) degrade, the system struggles to flush out waste properly, leading to the accumulation of toxic materials.
The reduced branching and misalignment of astrocyte end-feet limit their interaction with blood vessels, further impairing the movement of CSF through the peri-vascular spaces. This structural decline exacerbates the inefficiencies already caused by reduced AQP4 function, making the brain less capable of removing waste. As a result, toxic proteins are more likely to build up, contributing to increased vulnerability to neurodegenerative diseases as we age. [1, 2]
Vascular health is a critical factor that compounds the age-related decline in glymphatic function. The movement of CSF through the glymphatic system is partially driven by the rhythmic pulsing of blood, known as vascular pulsatility, which is controlled by the heart's contractions. Think of this like a water pump. Healthy vascular pulsatility creates the pressure needed to push CSF through the perivascular spaces surrounding the brain’s blood vessels, where it mixes with ISF and carries away waste.
In younger, healthier individuals, blood vessels remain flexible, and the heart pumps with enough force to maintain strong vascular pulsatility. This consistent pressure drives CSF through the brain’s “cleaning system,” allowing it to effectively flush out waste. However, as we age, blood vessels naturally stiffen, and the heart's efficiency diminishes—much like a water pump losing power over time. This reduction in vascular pulsatility weakens the movement of CSF, causing less fluid to flow through the brain’s waste-clearing pathways.
As CSF flow weakens, the glymphatic system struggles to remove harmful substances from the brain, allowing toxic proteins to accumulate. These waste products, which include proteins linked to neurodegenerative diseases, gradually build up and contribute to age-related cognitive decline. Essentially, the deterioration of vascular health with age disrupts the brain’s "plumbing," reducing the force required to push waste out and maintain optimal brain function. [1, 6]
The consequences of these age-related changes are profound. As the glymphatic system’s efficiency declines, it becomes less effective at clearing toxic proteins such as amyloid-beta, alpha-synuclein (α-syn), and tau—proteins strongly linked to the development and progression of neurodegenerative diseases like Alzheimer's and Parkinson's. Amyloid-beta and tau, for instance, form plaques and tangles in the brain, which disrupt normal neuronal communication, while α-syn aggregates in Parkinson’s patients, contributing to the death of dopaminergic neurons. The aging glymphatic system’s diminished capacity to remove these proteins creates an environment that favors their buildup, accelerating the onset and progression of these diseases. [7]
Research has demonstrated that impaired glymphatic function accelerates the buildup of toxic proteins, such as amyloid-beta, alpha-synuclein (α-syn), and tau—proteins closely associated with neurodegenerative diseases (NDDs) like Alzheimer’s and Parkinson’s. One significant study by Kress et al. (2014) investigated how aging affects the glymphatic system’s ability to exchange CSF and ISF to remove waste, a process critical for clearing harmful substances like amyloid-beta from the brain. [7]
Using advanced imaging techniques, the researchers tracked how well the brain cleared amyloid-beta in mice of different ages: young (2-3 months), middle-aged (10-12 months), and old (18-20 months). They also analyzed the functionality of AQP4 channels in astrocytes, which, as discussed, are essential for transporting CSF through the brain’s perivascular spaces to facilitate waste clearance. [7]
The study revealed a marked decline in glymphatic efficiency as the mice aged. Specifically, older mice were 40% less effective at clearing amyloid-beta compared to younger mice. This reduced efficiency was linked to two major age-related changes: (1) a significant 27% decline in the pulsation of blood vessels, which is critical because the rhythmic pulsations help propel CSF through the glymphatic system, and (2) a deterioration in the functionality of many AQP4 channels in astrocytes. Without these channels functioning properly, the brain’s ability to flush out toxic proteins and metabolic waste was severely compromised. [7]
These findings highlight how the aging glymphatic system struggles to clear waste effectively, leading to the accumulation of harmful proteins. This impaired clearance may increase the risk of developing NDDs such as Alzheimer’s. [7]
In a similar study, Cui et al. (2021) explored the role of AQP4 in Parkinson's disease (PD) by developing a mouse model that lacked AQP4 channels. Given AQP4’s critical role in facilitating CSF flow, the absence of this channel mimics the dysfunctional glymphatic system observed in aging. The researchers injected the mice with α-synuclein, a protein that forms toxic clumps in the brain and is linked to PD. The absence of AQP4 led to an increased buildup of α-synuclein, a greater loss of dopamine-producing neurons (which are essential for motor function), and more severe PD symptoms, including movement impairments. Since dopamine-producing neurons are key to controlling motor activity, their death is a hallmark of PD. [8]
The study also highlighted a broader issue: as astrocytes age, they experience reduced branching and polarization, leading to fewer functional AQP4 channels. These changes mimic the natural decline of the glymphatic system seen in older individuals, where impaired AQP4 function increases vulnerability to toxic protein buildup. The research demonstrates how the absence of AQP4 exacerbates the accumulation of harmful proteins like α-syn, underscoring the glymphatic system’s vital role in clearing these proteins and slowing the progression of neurodegenerative diseases like Parkinson’s. [8]
Beyond traditional neurodegenerative diseases like Alzheimer's and Parkinson's, the glymphatic system has also been implicated in recovery from traumatic brain injury (TBI). TBI occurs when brain tissue is physically damaged by a sudden impact or rapid acceleration, such as from a car accident or sports injury. These injuries often lead to long-term disability, and understanding the role of the brain's waste clearance mechanisms in recovery is essential for improving outcomes. [9]
In a study by Plog et al. (2015), researchers investigated how disruptions to the glymphatic system affected recovery from TBI in mice. The study used four methods to impair glymphatic function:
- Removing AQP4 channels from astrocytes (mimicking what happens in aging or disease).
- Administering acetazolamide, a drug that reduces CSF production.
- Performing a cisternotomy which drains CSF and disrupts its flow.
- Depriving the mice of sleep reduced glymphatic activity because the glymphatic system is most active during sleep.
The researchers used a Controlled Cortical Impact (CCI) device to induce brain injury, which delivers a precisely measured force to the brain to simulate a TBI. The study aimed to determine how well the brain could clear TBI-related waste under conditions of glymphatic dysfunction. They used a special protein tracer to track how effectively waste was removed from the brain, particularly focusing on TBI biomarkers like S100b, GFAP, and NSE, which are proteins that indicate the severity of brain injury. [9]
The results showed that mice with impaired glymphatic systems—whether through AQP4 removal, CSF reduction, cisternotomy, or sleep deprivation—were significantly worse at clearing waste. In normal mice with intact glymphatic function, these TBI biomarkers were elevated in the blood following injury, indicating that the waste had been effectively cleared from the brain and transported into circulation.
However, in mice with impaired glymphatic function, these markers remained concentrated in brain tissue, indicating that they were not being cleared effectively. This suggests that when the glymphatic system is compromised, the brain is less capable of removing toxic substances after injury, which could lead to prolonged inflammation and worse recovery outcomes. These findings, therefore, emphasize the potential of targeting the glymphatic system for therapeutic interventions in both neurodegenerative conditions and brain injuries. [9]
Factors Influencing Glymphatic Function
Several factors, including AQP4 channels, vascular pulsatility, and sleep, play critical roles in regulating glymphatic function.
AQP4 channels in astrocytes are essential for controlling water movement in the brain, facilitating the flow of CSF through the glymphatic system to remove waste. These channels are typically polarized, meaning they are strategically positioned and aligned around blood vessels to optimize fluid flow. However, with age, this polarization is lost—AQP4 channels become disorganized and less concentrated in their usual locations. As a result, the channels are no longer as effective at directing fluid movement, which significantly reduces the glymphatic system’s ability to clear out toxic proteins and metabolic waste. Additionally, brain injuries such as concussions and strokes can disrupt AQP4 function, further impairing glymphatic efficiency and exacerbating waste accumulation in the brain. [10]
Vascular pulsatility, or the rhythmic pulsing of blood vessels, is another critical factor in glymphatic function. The pulsation of arteries helps propel CSF through the brain, promoting effective waste clearance. However, as we age, blood vessels tend to stiffen, reducing the pulsatility required for efficient CSF movement. While hypertension can paradoxically increase glymphatic flow by temporarily enhancing these pulsations, the long-term effects of high blood pressure—such as an elevated risk of neurodegenerative diseases and cognitive decline—far outweigh this short-term benefit. Therefore, maintaining vascular health through regular exercise and a balanced diet is crucial for preserving glymphatic function. [10]
Sleep, particularly slow-wave sleep, is one of the most important factors for glymphatic activity. During this deep sleep phase, the brain's interstitial spaces expand, allowing for increased CSF flow and more efficient clearance of waste products, including amyloid-beta and tau proteins, linked to Alzheimer's and other neurodegenerative diseases. However, aging often brings sleep disturbances such as insomnia or sleep apnea, which reduce the amount and quality of slow-wave sleep, diminishing glymphatic function and increasing the risk of waste accumulation in the brain.[10]
Finally, there is an important connection between autophagy—the process by which cells break down and recycle damaged components—and glymphatic function. A well-functioning autophagy system reduces cellular damage and inflammation, decreasing the burden of waste for the glymphatic system to handle. We’ll dig deeper into this in the next section. [5, 10]
Autophagy, The Glymphatic System, and Brain Health
Autophagy is the body’s internal cleanup crew, responsible for sweeping away and recycling damaged or unnecessary components within cells to ensure they remain healthy and function properly. Think of it as an internal janitorial team within a factory, constantly removing defective parts and malfunctioning machinery to keep operations running smoothly. In neurons, which are long-lived and do not regenerate easily, this cleanup process is especially critical. Autophagy helps prevent the buildup of damaged proteins and malfunctioning organelles, like worn-out mitochondria—much like clearing broken machines off the factory floor. If these faulty components aren’t removed, they can cause cellular disruptions that may lead to "shutdown" or even cell death.
Recent experiments using the 3×Tg-AD mouse model, which is genetically engineered to develop Alzheimer’s-like symptoms, have provided valuable insights into the role of autophagy in preventing neurodegeneration. These mice develop amyloid plaques, tau tangles, and cognitive deficits similar to those seen in human Alzheimer's disease (AD). In groundbreaking research, J20 AD mice treated with rapamycin, a well-known autophagy inducer, showed remarkable retention of cognitive abilities, in stark contrast to untreated mice, who exhibited cognitive decline as plaques and tangles accumulated in their brains. [18]
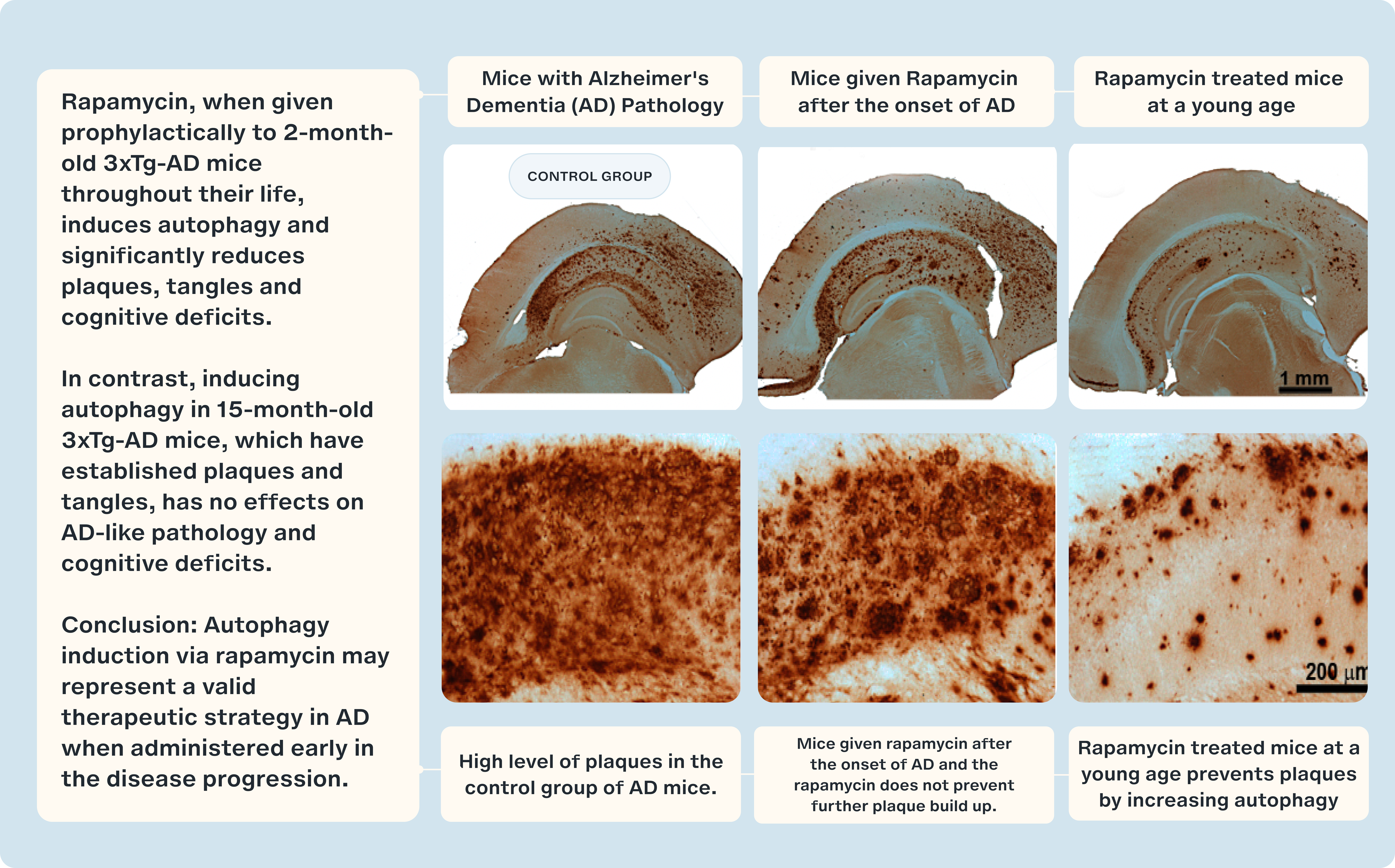
Notably, the most significant findings emerged when rapamycin was administered before the formation of plaques had begun. Early treatment led to a significant reduction in the formation of amyloid-beta plaques and tau tangles, along with the preservation of cognitive function. This underscores the critical importance of normal autophagy in maintaining neurological health. These experiments demonstrate that inducing autophagy early in disease progression can prevent, or at least mitigate, the damaging effects of protein buildup in the brain. [18]
However, autophagy alone cannot manage all the waste generated in and around brain cells. The glymphatic system complements autophagy by removing waste from the spaces surrounding brain cells. It relies on CSF to transport toxic substances, such as amyloid-beta and tau, out of the brain. Together, autophagy and the glymphatic system form a coordinated defense against neurodegenerative diseases (NDDs). Autophagy handles intracellular waste, cleaning up and recycling damaged components within cells, while the glymphatic system manages extracellular waste, clearing it from the brain. This partnership is essential for maintaining a clean and healthy brain environment.
Studies have demonstrated how these systems work in tandem to clear harmful proteins. For example, research on Parkinson's disease (PD) has shown that autophagy breaks down toxic forms of alpha-synuclein (α-syn) within neurons, while the glymphatic system removes extracellular α-syn from the brain. Disruptions in either system can lead to the toxic buildup of these proteins, contributing to the development of diseases like Alzheimer’s and Parkinson’s.
As we age, both autophagy and glymphatic function decline, leading to an accumulation of waste inside and around neurons. Damaged proteins, dysfunctional mitochondria, and toxic extracellular substances such as amyloid-beta and tau begin to accumulate. This overload of waste exacerbates cellular damage, creating a vicious cycle: impaired autophagy increases the burden on the glymphatic system, while a struggling glymphatic system puts more pressure on autophagy. This failure to efficiently clear waste accelerates brain degeneration and contributes to the onset and progression of NDDs.
In addition to waste buildup, the failure of these systems triggers chronic inflammation. Toxic proteins and damaged cellular components activate the brain’s immune response, leading to sustained inflammation, which further damages neurons. This inflammatory response becomes a major driver of neurodegeneration, making it even more crucial to maintain the effective functioning of both autophagy and the glymphatic system.
Challenges in Glymphatic Research
The glymphatic system offers promising insights into brain health, aging, and neurodegenerative diseases, but research in this area faces several challenges and controversies. Many studies rely on postmortem (after-death) data to estimate fluid flow dynamics in the brain, using chemical tracers and imaging techniques. However, sudden changes in cerebrospinal fluid flow, such as those caused by cardiac arrest or stroke, can lead to misleading results in postmortem samples. This makes it difficult to capture the true function of the glymphatic system in living brains. [11, 12]
Another challenge involves the role of AQP4 channels, which are thought to be essential for moving CSF into brain tissue for waste clearance. While many studies support this, a 2017 study claimed that removing AQP4 did not significantly affect how CSF moved through the brain. In contrast, other research groups found that removing AQP4 reduced CSF flow. These conflicting results may be due to differences in experimental methods or the age of the test animals, as older brains manage fluid less effectively. [13]
Applying findings from animal studies to humans adds another layer of complexity. Much of what we know about the glymphatic system comes from animal research, but the system's mechanisms in humans still need to be fully understood. Structural and functional differences between species, such as brain architecture, blood flow, and metabolism, may significantly affect how the glymphatic system operates. Moreover, studying glymphatic function in living humans is challenging, as current imaging techniques may not adequately capture the system's dynamic processes. [14]
Additionally, the interaction between autophagy (the process of breaking down and recycling damaged cellular components) and glymphatic function still needs to be explored. Dysregulation of autophagy may worsen glymphatic dysfunction, particularly in the context of neurodegenerative diseases. Research that bridges these two systems is necessary to understand their combined role in brain health fully.
Despite these challenges, the glymphatic system holds great potential for improving our understanding of brain health and developing new treatments for neurodegenerative conditions. Resolving these controversies through refined research methods and interdisciplinary collaboration will be key to unlocking the full therapeutic potential of glymphatic function.
Therapeutic Implications and Future Directions
Researchers are actively exploring various interventions to enhance glymphatic function and promote overall brain health. Key focus areas include improving sleep quality, enhancing AQP4 function, optimizing vascular health, and encouraging beneficial lifestyle changes such as regular exercise and proper hydration. These approaches hold promise for improving glymphatic efficiency, which plays a crucial role in clearing waste from the brain and preventing neurodegenerative diseases.
Exercise is emerging as a particularly potent non-pharmacological intervention. Studies have shown that physical activity can significantly enhance glymphatic function. For example, He et al. (2017) examined the effects of exercise on glymphatic clearance in aging mice. They found that running on a wheel improved memory, increased AQP4 density in astrocytes, and reduced amyloid-beta (Aβ) buildup and inflammation. These changes were associated with protection against memory decline, highlighting the potential of exercise to enhance glymphatic function and reduce the risk of cognitive impairment. [15]
Similarly, Liu et al. (2022) studied the impact of exercise in mouse models of Alzheimer's disease and in mice lacking AQP4. They discovered that exercise improved the positioning and function of AQP4, facilitating more efficient waste clearance and reducing Aβ levels in normal mice's brains. However, mice that lacked AQP4 did not experience these benefits, underscoring the importance of AQP4 in the glymphatic system's ability to remove toxic proteins. These findings support the idea that exercise is a valuable intervention to promote glymphatic function, although further research on human participants is needed to understand better how exercise impacts glymphatic health in humans. Determining the most effective exercise routines based on age, health status, and other demographic factors is crucial for tailoring exercise as a therapeutic strategy. [16]
Lifestyle and pharmacological interventions targeting both autophagy and glymphatic function could offer synergistic benefits for brain health. Autophagy is the process by which cells break down and recycle damaged components, and promoting autophagy has been shown to enhance the brain's ability to clear toxic proteins like Aβ and tau. These proteins are key contributors to the development of neurodegenerative diseases. Strategies such as caloric restriction, intermittent fasting, and autophagy-inducing drugs—such as rapamycin, an mTOR inhibitor—can help induce autophagy and improve cellular cleanup.
Enhancing autophagy can complement efforts to optimize glymphatic function, creating a dual approach to waste clearance. Autophagy and glymphatic activity are closely linked, and promoting one system can relieve the burden on the other, resulting in more efficient removal of toxic proteins and waste products from the brain. [17]
Sleep optimization is another critical factor in enhancing glymphatic efficiency. The glymphatic system is most active during slow-wave sleep, a stage of deep sleep where cerebrospinal fluid flow increases, promoting the clearance of harmful substances like Aβ and tau. Research by Levendowski et al. (2019) and Lee et al. (2015) demonstrated the importance of sleep posture, revealing that sleeping in a lateral (side) position improves glymphatic transport compared to sleeping in supine (back) or prone (stomach) positions. These findings suggest that something as simple as adjusting sleep posture can significantly enhance waste clearance in the brain, potentially reducing the risk of neurodegenerative diseases. This effect can be further amplified by combining sleep optimization with autophagy-enhancing therapies, such as rapamycin or caloric restriction, to create an integrated approach to brain health. [17]
Closing Remarks
The future of glymphatic research and therapeutic interventions lies in a combination of lifestyle modifications and pharmacological strategies that address both the glymphatic system and autophagy. Lifestyle interventions, such as regular exercise, sleep optimization, and fasting, have shown significant promise in enhancing glymphatic function by improving vascular health, increasing slow-wave sleep quality, and promoting the efficient clearance of toxic proteins. When paired with pharmacological therapies such as rapamycin, which stimulates autophagy, and oxytocin, which is linked to cellular repair and stress reduction, these approaches create a comprehensive framework for improving brain health.
By targeting both glymphatic clearance and cellular autophagy, these strategies offer a dual approach to mitigating the toxic buildup of proteins like amyloid-beta, tau, and alpha-synuclein, which are implicated in neurodegenerative diseases (NDDs) such as Alzheimer's and Parkinson's. This holistic approach addresses the symptoms and potentially slows the progression of these conditions by enhancing the brain's natural waste removal systems.
However, more research is essential to fully understand how these interventions interact and how they can be tailored to individual needs, particularly in aging populations or those with a genetic predisposition to NDDs. The interplay between lifestyle modifications and pharmacological agents presents an exciting area of study with the potential for creating personalized therapeutic protocols. As we continue to learn more about the mechanisms of the glymphatic system and autophagy, these insights could pave the way for novel treatments that prevent cognitive decline and promote long-term brain health and longevity.
- Benveniste, H., Liu, X., Koundal, S., Sanggaard, S., Lee, H., & Wardlaw, J. (2019). The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology, 65(2), 106–119. https://doi.org/10.1159/000490349
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M: A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, includ ing amyloid beta. Sci Transl Med 2012; 4: 147ra111.
- Nedergaard M: Neuroscience. Garbage truck of the brain. Science 2013; 340: 1529–1530.
- Null M, Arbor TC, Agarwal M. Anatomy, Lymphatic System. [Updated 2023 Mar 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513247/
- Webb, J. L., Ravikumar, B., Atkins, J., Skepper, J. N., & Rubinsztein, D. C. (2003). Alpha-Synuclein is degraded by both autophagy and the proteasome. The Journal of biological chemistry, 278(27), 25009–25013. https://doi.org/10.1074/jbc.M300227200
- Hoshi, A., Tsunoda, A., Tada, M., Nishizawa, M., Ugawa, Y., & Kakita, A. (2017). Expression of Aquaporin 1 and Aquaporin 4 in the Temporal Neocortex of Patients with Parkinson's Disease. Brain pathology (Zurich, Switzerland), 27(2), 160–168. https://doi.org/10.1111/bpa.12369
- Kress, B. T., Iliff, J. J., Xia, M., Wang, M., Wei, H. S., Zeppenfeld, D., Xie, L., Kang, H., Xu, Q., Liew, J. A., Plog, B. A., Ding, F., Deane, R., & Nedergaard, M. (2014). Impairment of paravascular clearance pathways in the aging brain. Annals of neurology, 76(6), 845–861.
- Cui, H., Wang, W., Zheng, X., Xia, D., Liu, H., Qin, C., Tian, H., & Teng, J. (2021). Decreased AQP4 Expression Aggravates ɑ-Synuclein Pathology in Parkinson's Disease Mice, Possibly via Impaired Glymphatic Clearance. Journal of molecular neuroscience : MN, 71(12), 2500–2513. https://doi.org/10.1007/s12031-021-01836-4
- Plog B.A., Dashnaw M.L., Hitomi E., Peng W., Liao Y., Lou N., Deane R., Nedergaard M. Biomarkers of Traumatic Injury Are Transported from Brain to Blood via the Glymphatic System. J. Neurosci. 2015;35:518. doi: 10.1523/JNEUROSCI.3742-14.2015.
- Mestre, H., Mori, Y., & Nedergaard, M. (2020). The Brain's Glymphatic System: Current Controversies. Trends in neurosciences, 43(7), 458–466.
- Ma Q et al. (2019) Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta Neuropathol 137 (1), 151–165.
- Mestre H et al. (2020) Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science 367 (6483).
- Smith, A. J., Yao, X., Dix, J. A., Jin, B. J., & Verkman, A. S. (2017). Test of the 'glymphatic' hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife, 6, e27679. https://doi.org/10.7554/eLife.27679
- Mestre, H., Hablitz, L. M., Xavier, A. L., Feng, W., Zou, W., Pu, T., Monai, H., Murlidharan, G., Castellanos Rivera, R. M., Simon, M. J., Pike, M. M., Plá, V., Du, T., Kress, B. T., Wang, X., Plog, B. A., Thrane, A. S., Lundgaard, I., Abe, Y., Yasui, M., … Nedergaard, M. (2018). Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife, 7, e40070. https://doi.org/10.7554/eLife.40070
- He X. F., Liu D. X., Zhang Q., Liang F. Y., Dai G. Y., Zeng J. S., et al.. (2017). Voluntary exercise promotes glymphatic clearance of amyloid beta and reduces the activation of astrocytes and microglia in aged mice. Front. Mol. Neurosci. 10:14. 10.3389/fnmol.2017.00144
- Liu Y., Hu P. P., Zhai S., Feng W. X., Zhang R., Li Q., et al.. (2022). Aquaporin 4 deficiency eliminates the beneficial effects of voluntary exercise in a mouse model of Alzheimer's disease. Neural Reg. Res. 17:18. 10.4103/1673-5374.335169
- Lee H, Xie L, Yu M, Kang H, Feng T, Deane R, Logan J, Nedergaard M, Benveniste H (2015) The effect of body posture on brain glymphatic transport. J Neurosci 35, 11034–11044.
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5(4):e9979.