The Role of Urolithin A in Enhancing Mitochondrial Biogenesis and Muscle Function: Mechanistic and Clinical Insights
Mitophagy Restoration: Urolithin A activates mitophagy, a critical process for clearing damaged mitochondria, thereby enhancing mitochondrial efficiency and supporting muscle endurance, strength, and recovery.
PGC-1α Activation: By stimulating the PGC-1α pathway, Urolithin A promotes mitochondrial biogenesis and optimizes existing mitochondria, ensuring robust ATP production to meet the high energy demands of skeletal muscle.
AMPK Pathway Engagement: Urolithin A activates AMPK, improving energy balance by enhancing fatty acid oxidation, promoting glucose uptake through GLUT4 translocation, and regulating glycogen storage, all of which support sustained muscle performance and recovery.
Reduction of Oxidative Stress: Urolithin A mitigates excessive reactive oxygen species (ROS), both directly and by upregulating endogenous antioxidants like glutathione, protecting mitochondria and muscle cells from damage during intense physical activity.
Anti-Inflammatory Effects: Urolithin A reduces systemic inflammation by downregulating pro-inflammatory cytokines and pathways like NF-κB, supporting muscle recovery and mitigating soreness after exercise.
Muscle Growth and Maintenance: Urolithin A enhances muscle hypertrophy and mass maintenance by promoting efficient protein synthesis and inhibiting protein degradation, creating an anabolic environment conducive to muscle repair and growth.
Introduction
Muscle health is a cornerstone of physical performance, mobility, and overall well-being. However, as individuals age, the natural decline in muscle mass and function, known as sarcopenia, becomes a significant concern. This gradual loss of muscle strength and endurance is a hallmark of aging and a critical factor in increased frailty, reduced independence, and higher risk of chronic diseases in older adults. Sarcopenia is driven by a combination of mitochondrial dysfunction, chronic inflammation, oxidative stress, and disrupted protein turnover, all of which progressively weaken the structural and functional integrity of muscles.
Addressing these issues is not just relevant to older populations. Athletes and physically active individuals also face unique challenges, including maintaining muscle resilience, recovering efficiently from intense training, and mitigating long-term damage caused by the cumulative effects of oxidative stress and inflammation. Muscle recovery and adaptation depend on robust cellular systems to repair damage, regulate energy production, and manage inflammation—all of which are influenced by mitochondrial health.
Recent discoveries have identified Urolithin A as a potentially novel approach to counteracting these challenges. Urolithin A, a naturally occurring metabolite formed from ellagitannins found in certain fruits and nuts, has demonstrated the ability to target the root causes of muscle decline. By promoting mitochondrial quality control, reducing oxidative stress, and modulating inflammation, Urolithin A can enhance muscle health and performance across diverse populations. Its mechanisms of action are especially promising for individuals seeking to maintain muscle function and resilience, whether due to aging, physical stress, or high levels of activity.
In this research narrative review, Shriya Bakhshi, Kristen Race, and Shrestha Jolly of the Healthspan Clinical Team will explore the scientifically supported effects of Urolithin A on muscle health and its potential applications for both aging individuals and athletes looking to optimize performance and recovery. The review provides a comprehensive analysis of current research on the impact of Urolithin A on muscle health and performance, with a focus on evidence-based applications. It consolidates and evaluates existing experimental findings, offering insights into the biological processes and molecular mechanisms underlying Urolithin A’s effects on muscle function and identifying key areas for future investigation.
Background
Urolithin A is a naturally occurring metabolite formed from ellagitannins, a type of polyphenolic compound found in various fruits and nuts. These polyphenols, known for their potent antioxidant properties, help protect cells from damage caused by free radicals. Foods such as pomegranates, walnuts, and almonds are among the richest dietary sources of ellagitannins [1].
The production of Urolithin A relies heavily on the metabolic activity of the gut microbiota. After ellagitannins are ingested, they travel to the colon, where specific bacterial species convert them into Urolithin A through enzymatic reactions. Bacteria such as Clostridium, Bifidobacterium, Eubacterium, and Enterococcus faecium play a key role in this process [1]. However, the efficiency of Urolithin A production varies between individuals, depending on the diversity and composition of their gut microbiota. Maintaining a healthy gut ecosystem may optimize Urolithin A production and amplify its potential benefits for muscle health and performance [2].
Urolithin A and Aging
Urolithin A has garnered significant attention for its potential to counteract cellular aging through several key mechanisms. In this review, we will focus on Urolithin A’s role as a mitochondrial quality control molecule and its downstream effects on metabolic health and inflammation. To fully comprehend how Urolithin A enhances mitochondrial health, it is essential to first explore the concept of mitophagy.
Mitophagy: The Cellular Engine Maintenance System
The term "autophagy" is becoming increasingly prominent in the health, longevity, and anti-aging community. However, mitochondrial-specific autophagy, known as "mitophagy," is less widely understood. While mitophagy is similar to traditional autophagy, it focuses exclusively on identifying and removing damaged, dysfunctional, and inefficient mitochondria. This targeted removal is critical for maintaining mitochondrial efficiency, particularly in high-energy-demand tissues like skeletal muscle, the heart, and the brain.
Mitochondria, often referred to as the "powerhouses" of the cell, act as metabolic engines, converting nutrients into adenosine triphosphate (ATP)—the fuel required for nearly all cellular processes, including muscle contraction. In skeletal muscle, which requires a constant and substantial energy supply, the efficiency of these mitochondrial "engines" directly determines physical performance and recovery. However, like any engine, mitochondria are subject to wear and tear over time, particularly during periods of high physical stress or metabolic demand.
When mitochondria malfunction, they produce less ATP and emit excessive reactive oxygen species (ROS), the cellular equivalent of "exhaust fumes." While moderate levels of ROS play essential roles in cell signaling and adaptation, excessive ROS leads to oxidative stress, which damages the cellular structures vital for muscle health. Left unchecked, failing mitochondrial engines can degrade cellular and tissue function, contributing to fatigue, impaired recovery, and the progressive decline in muscle performance.
Mitophagy and Aging: Why Our Cellular Engines Break Down
Mitophagy plays a central role in muscle health by identifying and removing these malfunctioning engines, replacing them with efficient mitochondria capable of sustaining energy output. However, as we age, the efficiency of mitophagy declines, creating a bottleneck in this critical maintenance system. Cells lose their ability to recognize and clear damaged mitochondria, allowing these dysfunctional components to accumulate within muscle tissue over time.
Returning to the metabolic engine analogy, as we age, our cellular engines become increasingly inefficient. Damaged mitochondria not only produce less ATP but also emit excessive ROS, compounding oxidative stress and further damaging surrounding cellular structures. This vicious cycle contributes to a decline in mitochondrial health, impairing the energy supply necessary for muscle endurance, strength, and recovery.
Adding to the challenge, mitochondria contain their own DNA (mtDNA), distinct from the nuclear DNA housed in the cell nucleus. Unlike nuclear DNA, mtDNA is poorly protected and more vulnerable to oxidative damage. Accumulating ROS can harm mtDNA, leading to mutations that impair mitochondrial replication and function. Over time, this damage exacerbates mitochondrial dysfunction, contributing to aging-related muscle weakness and conditions like sarcopenia (age-related muscle loss).
Understanding Mitophagy Mechanisms and Strategies to Enhance It with Age
Mitophagy is a finely tuned process that relies on molecular signals to distinguish between healthy and damaged mitochondria. The cellular "pit crew" includes proteins such as PINK1 (PTEN-induced kinase 1) and Parkin, which act as sensors and markers for defective mitochondria. When a mitochondrion becomes dysfunctional, PINK1 accumulates on its outer membrane, signaling damage. This acts as a distress flag, recruiting Parkin, which tags the mitochondrion with ubiquitin molecules—essentially a "recycle me" label. Once tagged, these mitochondria are encapsulated in autophagosomes and delivered to lysosomes, where they are broken down into their basic components for reuse.
When mitophagy is compromised with age, the accumulation of defective mitochondria contributes to progressive declines in muscle function and increases the risk of age-related diseases. The failure to clear these "metabolic clunkers" results in persistent oxidative stress, which damages muscle fibers, delays recovery, and diminishes the capacity for physical activity. In tissues with high energy demands, like skeletal muscle, this inefficiency manifests as reduced endurance, strength, and resilience.
The benefits of mitophagy for muscle health are therefore critical. By clearing out damaged mitochondria, mitophagy ensures a pool of efficient "engines" capable of producing the ATP required for sustained physical activity and rapid recovery. Efficient mitochondrial turnover contributes to the maintenance of muscle mass and strength by preserving the cell's energy-producing capacity and preventing chronic inflammation triggered by dysfunctional mitochondria. Enhanced mitophagy also reduces the buildup of ROS, mitigating oxidative stress that can impair muscle function.
For athletes or individuals seeking to maintain muscle health with age, interventions that enhance mitophagy could be important for stemming this age-related decline in muscle function. Research has shown that Urolithin A enhances mitophagy, particularly in skeletal muscle, promoting mitochondrial renewal and improving endurance, strength, and recovery.
Following a 24hr exposure to Urolithin A in muscle cells, mitophagy was increased by ~104% compared to control [3]. In mice [3], middle-aged adults [4], and older adults [5], Urolithin A has been observed to increase the gene & protein expression of proteins related to mitophagy. By acting on the cellular "engine maintenance" system, Urolithin A supports the high energy demands of muscle cells, ultimately enhancing both performance and resilience. Let’s review these studies in greater detail.
Scientific Evidence Supporting Urolithin A and Mitophagy
Urolithin A Induced Mitophagy and Improves Muscle Function Pre-clinically
A growing body of evidence supports the connection between Urolithin A and its ability to stimulate mitophagy. For instance, a study by Luan et al. (2021), titled “Urolithin A improves muscle function by inducing mitophagy in muscular dystrophy,” investigated Urolithin A’s potential as a therapeutic approach for Duchenne muscular dystrophy (DMD), a severe genetic disorder primarily affecting boys. DMD leads to progressive muscle weakness and degeneration due to mutations in the dystrophin gene, which is crucial for maintaining muscle cell structure and function. Dystrophin acts as a stabilizing protein, linking the muscle cell membrane to the extracellular matrix and enabling muscles to withstand repeated contractions. In the absence of dystrophin, muscle cells become fragile, weakening over time and progressively deteriorating.
One of the key challenges in DMD is mitochondrial dysfunction, which significantly contributes to the disease’s progression. Mitochondria in muscle cells affected by DMD exhibit reduced functionality, lower energy production, and increased oxidative stress. Furthermore, research has shown that genes involved in mitophagy are often underexpressed in DMD, reducing the muscle cells' ability to clear out damaged mitochondria effectively. Studies on DMD mouse models have consistently revealed decreased levels of mitophagy markers, indicating impaired mitophagy in this disease context [6].
The researchers in this study explored whether Urolithin A could activate the mitophagy process to address this issue. They tested the effects of Urolithin A on both mice and human muscle cells. In the mice, Urolithin A was added to their food at a dose of 50 mg per kg per day. In human trials, muscle cells were obtained from three healthy boys and three boys with DMD, all aged 4 to 7 years. These cells were treated with 25 μM of Urolithin A for varying durations (2, 6, and 24 hours). The study found that Urolithin A enhanced the clearance of defective mitochondria in both the mice and the DMD-affected human muscle cells. This improvement led to better mitochondrial function and increased the muscle's ability to use oxygen, suggesting that Urolithin A may reduce symptoms of DMD by promoting mitophagy [6].
In addition to preclinical animal studies, research on Urolithin A has been extended to human trials.
Urolithin A in Human Randomized Clinical Trials
Published in Nature, the study titled "The Mitophagy Activator Urolithin A is Safe and Induces a Molecular Signature of Improved Mitochondrial and Cellular Health in Humans" is the first human clinical trial to evaluate the safety and efficacy of Urolithin A. The study specifically examined how Urolithin A impacts mitochondrial function and skeletal muscle health in older adults, a population particularly vulnerable to mitochondrial decline and muscle degeneration associated with aging.
Study Design
This double-blind, randomized, placebo-controlled clinical trial enrolled healthy older adults aged 61–85 years (mean age ~70), a population vulnerable to mitochondrial dysfunction and muscle decline associated with aging. The primary objectives were to assess Urolithin A’s safety, pharmacokinetics, and its impact on mitochondrial and cellular health.
Participants in the clinical trial were randomly assigned to receive either a placebo or Urolithin A at doses of 500 mg or 1000 mg. The study design included two dosing regimens: single ascending dose (SAD) and multiple ascending dose (MAD) arms. In the SAD arm, participants received a one-time dose to evaluate the immediate pharmacokinetics, safety, and tolerability of Urolithin A at varying dose levels. In the MAD arm, participants were administered UA daily for a period of four weeks to assess the compound’s pharmacokinetic profile under sustained exposure, as well as its safety and biological effects over time. This approach allowed researchers to comprehensively evaluate Urolithin A’s short-term and cumulative effects, including any potential dose-dependent responses [7].
Safety and Pharmacokinetics Assessment
- Safety: Urolithin A demonstrated an excellent safety profile across all doses, with no reported adverse effects.
- Pharmacokinetics: Urolithin A showed a dose-dependent increase in bioavailability up to 1000 mg, with peak plasma levels occurring 6–8 hours after intake and a long half-life of 17–22 hours. Importantly, no plasma accumulation was observed, and food intake did not affect bioavailability, making Urolithin A suitable for routine use.
Mitochondrial Function Assessment
Muscle biopsies from the vastus lateralis and plasma samples were collected at baseline and after four weeks of supplementation. These samples were analyzed for:
- Gene expression changes: Focused on mitochondrial function and mitophagy-related pathways.
- Metabolic markers: Plasma acylcarnitines were used to assess mitochondrial fatty acid oxidation.
Muscle-Specific Findings
In the vastus lateralis skeletal muscle, Urolithin A supplementation led to a dose-dependent enhancement in mitochondrial function:
- Gene Expression: Urolithin A increased the expression of key genes related to mitophagy, such as PARK2, GABARAPL1, and ULK1. These genes are integral to the mitophagy process, which identifies and removes dysfunctional mitochondria, ensuring a healthier mitochondrial network [7].
- Comparison to Pre-Frail Adults: These gene sets, which were underexpressed in pre-frail older adults compared to healthy, active individuals, were positively modulated by Urolithin A. This suggests Urolithin A’s potential to restore mitochondrial activity to levels associated with better physical fitness and resilience [7].
Systemic Effects
In plasma, Urolithin A supplementation resulted in a measurable reduction in the levels of several acylcarnitines, which are metabolites that serve as biomarkers of incomplete mitochondrial fatty acid oxidation. Elevated acylcarnitine levels typically indicate inefficiencies in the mitochondrial oxidative process, where fatty acids are not fully metabolized into usable energy. The observed reduction in these markers suggests that Urolithin A enhances mitochondrial efficiency, promoting more complete fatty acid oxidation. This improvement reduces the accumulation of metabolic byproducts, decreases energy wastage, and optimizes ATP production, ultimately supporting better cellular energy metabolism and overall mitochondrial health [7].
Implications for Muscle Health
The findings of this study underscore UA’s potential as an intervention for age-related muscle decline:
- Enhanced Mitochondrial Function: By improving mitochondrial efficiency, Urolithin A supports ATP production, essential for muscle contraction and endurance. This can directly enhance physical performance in older adults.
- Mitophagy Activation: The upregulation of mitophagy-related genes suggests that Urolithin A helps clear damaged mitochondria, preventing the accumulation of dysfunctional organelles that can impair muscle recovery and energy metabolism.
- Improved Energy Metabolism: Reduced plasma acylcarnitines reflect better mitochondrial fatty acid oxidation, a crucial factor for sustained muscle energy during both activity and recovery.
- Restoration of Age-Related Declines: The trial demonstrated that Urolithin A can reverse declines in mitochondrial function typically observed in older adults, aligning their mitochondrial gene expression profiles more closely with those of healthier, active individuals.
This first-in-human trial provided compelling evidence for Urolithin A’s safety, bioavailability, and efficacy in enhancing mitochondrial function. For muscle health, these findings are particularly significant. By restoring mitophagy and improving energy metabolism, Urolithin A addresses fundamental drivers of muscle fatigue, reduced endurance, and slower recovery in older adults. The study lays a strong foundation for further research on Urolithin A as a therapeutic intervention to preserve muscle function and promote physical resilience during aging.
Building on the foundational findings from the first-in-human trial, further research has explored Urolithin A’s effects on muscle health in different populations and over longer durations. One such study, conducted by Singh et al. (2022), titled "UA Improves Muscle Strength, Exercise Performance, and Biomarkers of Mitochondrial Health in a Randomized Trial in Middle-Aged Adults", examined the impact of Urolithin A on mitophagy and its functional outcomes in middle-aged adults over a four-month period [4].
The Study Design
The study sought to determine the most responsive functional outcomes, specifically focusing on markers linked to muscle strength, exercise tolerance, and overall physical performance. The goal was to identify outcomes that could guide the design and power calculations of future confirmatory clinical trials involving Urolithin A.
The study population included 88 untrained adults between the ages of 40 and 64 who were overweight and demonstrated low physical endurance, defined as a maximum oxygen consumption (VO2max) below 35 mL/kg/min. Participants were selected from an initial screening of 253 individuals based on inclusion and exclusion criteria, which required that subjects be healthy, as determined by their vital signs, anthropometric measures, and absence of chronic medical conditions. Participants were randomized into three groups: a 500 mg Urolithin A group, a 1,000 mg Urolithin A group, and a placebo group. The intervention spanned a 4-month period, chosen as the minimum duration expected to reveal impacts on physical performance and muscle function according to guidelines from expert groups on muscle-function clinical trials [4].
The ATLAS study was a double-blind, placebo-controlled trial with well-matched baseline characteristics across groups. Participants in the 500 mg UA, 1,000 mg Urolithin A, and placebo groups had similar average ages (around 51–54 years), BMIs (approximately 29 kg/m²), and baseline endurance (VO2max around 23 mL/kg/min). The study cohort included a higher proportion of female participants (2:1 female-to-male ratio) and was primarily of Western European ethnicity.
Throughout the study, plasma samples were collected to assess Urolithin A’s impact on metabolites and cytokines associated with cellular health, while skeletal muscle biopsies were performed to evaluate UA’s effects on the muscle transcriptome and proteome, with a specific focus on proteins related to mitochondrial health and cellular energy pathways. This comprehensive approach was designed to yield insights into UA’s potential to influence cellular health markers, muscle function, and overall physical performance.
Results
The study showed that participants who received Urolithin A exhibited enhanced mitophagy, significantly improving muscle strength (around 12%) and aerobic endurance. Participants receiving 500 mg and 1,000 mg Urolithin A exhibited notable increases in hamstring muscle strength, measured via isokinetic Biodex dynamometer testing. The average peak torque for hamstring muscles increased significantly in both groups, with a 12% increase in the 500 mg group (p = 0.027) and a 9.8% increase in the 1,000 mg group (p = 0.029) compared to placebo. Maximum torque during knee flexion also improved significantly in both Urolithin A groups, with increases of 10.6% in the 500 mg group (p = 0.017) and 10.5% in the 1,000 mg group (p = 0.022) compared to placebo [4].
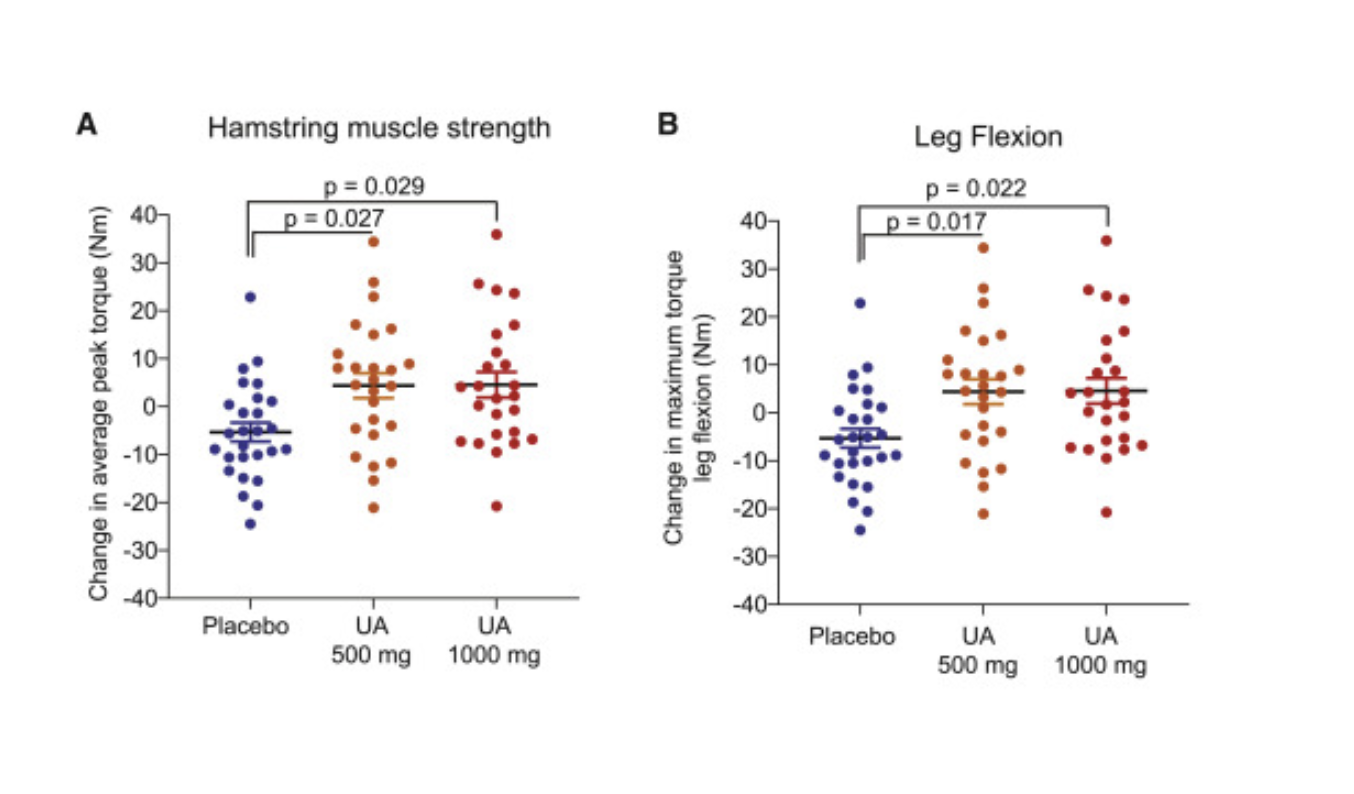
Aerobic endurance was measured by peak oxygen consumption (VO₂), which indicates how efficiently the body uses oxygen during exercise, and the 6-minute walk test (6MWT), a simple measure of physical endurance. [4]
PPO was the study’s primary pre-specified endpoint. Although no significant differences were observed between the Urolithin A-supplemented groups and the placebo group, both UA groups demonstrated non-significant increases in PPO of approximately 4% from baseline, suggesting a trend towards improved power output. The placebo group, in contrast, showed no change in PPO, indicating that UA supplementation may support small gains in exercise performance that require further investigation to confirm statistical significance.
Participants in the 1,000 mg Urolithin A group exhibited a 15% increase in total cycling distance from baseline to the end of the study (p = 0.03), along with a significant increase in time to fatigue during the exercise test. This finding indicates that UA at a higher dose may contribute to improved endurance, allowing participants to cycle further and sustain activity longer before fatigue.
The Urolithin A 1,000 mg dose group showed significant within-group increases in peak VO₂ and estimated VO₂max at both the 2-month midpoint and 4-month endpoint (p < 0.01), demonstrating a measurable improvement in aerobic endurance. Although these changes were not statistically significant compared to the placebo, they showed a strong trend in favor of the Urolithin A intervention (p = 0.058), particularly in the 1,000 mg dose group.
Interestingly, no significant changes in acylcarnitine levels were observed in the 1,000 mg Urolithin A group. This absence of change suggests that the downregulation of acylcarnitines may be dose- or duration-dependent, with the lower dose potentially providing an optimal level of Urolithin A for impacting this specific biomarker. It is also possible that the observed reductions in the 500 mg group reflect a response that emerges over time and may require a balance in dosing to affect certain plasma biomarkers [4].
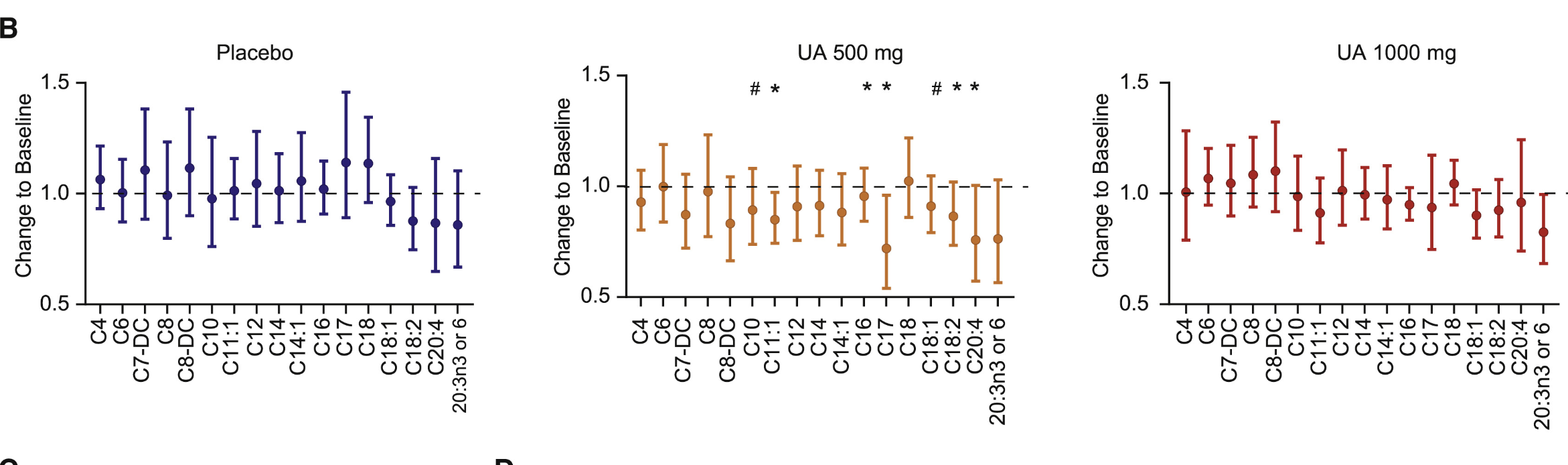
The study then analyzed the overall effects on inflammation and mitochondrial function. To determine if the improvements in muscle function observed with Urolithin A supplementation were also reflected in surrogate plasma biomarkers of metabolic health, researchers analyzed acylcarnitine levels in participants’ plasma. As we discussed, acylcarnitines are lipid molecules involved in fatty acid metabolism, and reductions in acylcarnitine levels, particularly in medium- and long-chain species, are often associated with enhanced fatty acid oxidation and metabolic efficiency [4].
In the Urolithin A 500 mg group, plasma acylcarnitine levels were reduced, with the most significant decreases seen in medium- and long-chain acylcarnitines. This downregulation suggests an improvement in fatty acid oxidation, as lower levels of circulating acylcarnitines indicate that fatty acids are being more effectively broken down and utilized as energy within cells. This finding aligns with prior clinical studies that have linked acylcarnitine reduction to improved mitochondrial function and metabolic health
In addition to mitochondrial efficiency, the study also analyzed markers of inflammation. Inflammation is a key factor in aging and metabolic dysfunction, making it a critical area of focus for assessing the broader benefits of Urolithin A supplementation. This study evaluated plasma levels of C-reactive protein (CRP) and several pro-inflammatory cytokines to determine whether UA’s effects on muscle function and mitochondrial health are accompanied by systemic anti-inflammatory benefits [4].
CRP is a well-established biomarker of systemic inflammation and a predictor of age-related chronic diseases. Elevated CRP levels are common in overweight individuals and are strongly associated with higher BMI. At baseline, the study population of middle-aged, overweight participants had average CRP concentrations of approximately 3 mg/L, placing them in a moderate- to high-risk category for chronic disease [4].
Urolithin A supplementation reduced plasma CRP levels at both doses, with the reduction reaching statistical significance in the 1,000 mg group. This suggests that Urolithin A at higher doses may effectively lower systemic inflammation, potentially mitigating risks associated with chronic inflammatory states [4].
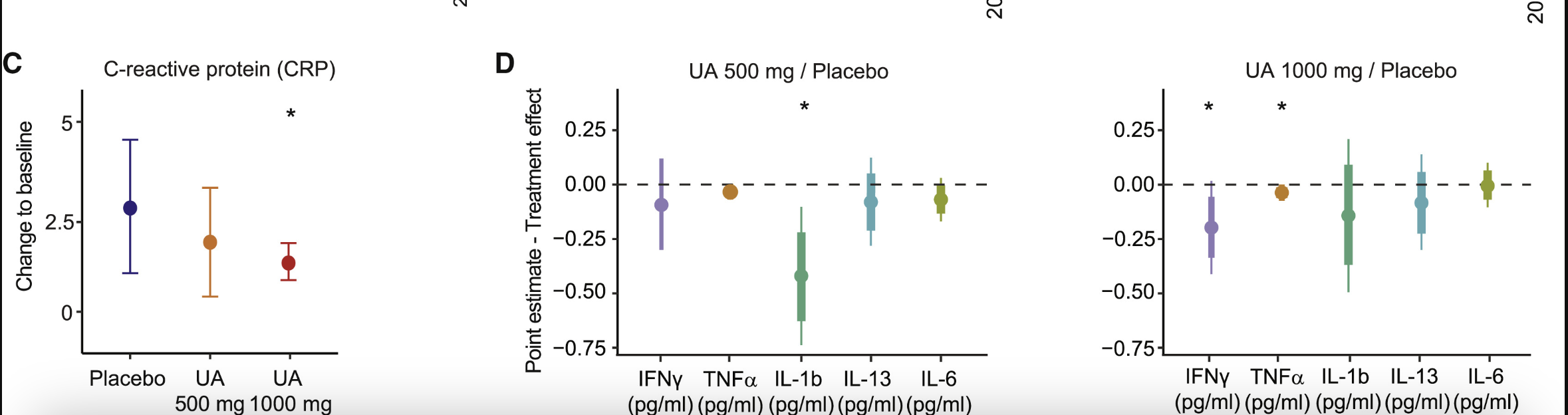
In addition to CRP, Urolithin A supplementation led to reductions in key pro-inflammatory cytokines, including interferon gamma (IFN-γ), interleukin-1 beta (IL-1β), and tumor necrosis factor alpha (TNF-α). These cytokines are major mediators of inflammatory processes and are often elevated in chronic inflammatory and age-related diseases. Although baseline cytokine levels in this study population were relatively low, reductions were observed following Urolithin A supplementation, suggesting a mild systemic anti-inflammatory effect [4].
The decrease in CRP suggests that Urolithin A also helped reduce systemic inflammation, likely due to its enhancement of mitophagy and mitochondrial metabolism in skeletal muscle. [4]
These findings suggest that Urolithin A contributes to better mitochondrial function, improved aerobic capacity, and reduced inflammation in middle-aged adults, making it a promising intervention for combating muscle decline with age.
This first-in-human trial established robust evidence for Urolithin A’s safety, bioavailability, and its ability to enhance mitochondrial function. These findings are particularly relevant for muscle health, highlighting Urolithin A’s potential to address core challenges associated with aging, such as muscle fatigue, diminished endurance, and delayed recovery. By restoring mitophagy and optimizing energy metabolism, Urolithin A supports the foundational processes required for maintaining muscle strength and resilience. This study not only validates preclinical observations but also provides a critical basis for future research into Urolithin A as a therapeutic strategy to preserve muscle function, improve physical performance, and promote healthy aging.
Mitochondrial Function and Energy Output
Having explored how Urolithin A activates mitophagy to clear dysfunctional mitochondria, we now shift our focus to the mechanisms and evidence supporting its ability to stimulate mitochondrial networks and mitochondrial biogenesis. These processes are critical for enhancing mitochondrial function, which serves as the foundation of energy production and muscle health.
For skeletal muscle—a tissue with exceptionally high energy demands—maintaining a robust and efficient mitochondrial network is essential. The ability to optimize mitochondrial biogenesis and functionality directly influences key aspects of muscle performance, including strength, endurance, and recovery capacity.
Mitochondrial Biogenesis
Urolithin A enhances mitochondrial function by activating the PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha) pathway, a master regulator of mitochondrial health and cellular energy metabolism. Often described as a molecular "switchboard," PGC-1α integrates various cellular signals to orchestrate the response to energy demands during periods of increased stress, such as intense exercise, caloric restriction, or metabolic challenges [8, 9]. Its activation is particularly critical in skeletal muscle, a tissue with exceptionally high energy demands, where sustained ATP production is essential to support muscle contraction, endurance, and recovery.
A key function of PGC-1α is driving mitochondrial biogenesis—the generation of new mitochondria. This process replenishes mitochondria lost to wear and tear, oxidative damage, or aging, while simultaneously bolstering the mitochondrial pool to meet increased energy demands during prolonged physical activity. PGC-1α achieves this by coactivating nuclear transcription factors such as NRF1 (nuclear respiratory factor 1) and NRF2, which regulate the expression of genes involved in mitochondrial replication and protein synthesis. This coordination ensures that the mitochondrial network is both quantitatively and qualitatively prepared to meet cellular energy requirements [8, 9].
Beyond increasing mitochondrial quantity, PGC-1α improves the functionality of existing mitochondria. It enhances the efficiency of the electron transport chain (ETC)—a series of protein complexes in the inner mitochondrial membrane responsible for generating ATP through oxidative phosphorylation. By optimizing ETC activity, PGC-1α reduces energy loss and minimizes the production of reactive oxygen species (ROS), harmful byproducts that can impair mitochondrial efficiency and cellular health [8, 9].
Impact on Muscle Performance
PGC-1α-mediated enhancements in mitochondrial biogenesis and function are particularly impactful for skeletal muscle, where energy demands fluctuate significantly during activity. By ensuring a steady and reliable supply of ATP, PGC-1α activation supports:
- Sustained Muscle Contractions: Meeting the high energy requirements of prolonged or high-intensity exercise.
- Endurance and Fatigue Resistance: Delaying fatigue through efficient energy production and utilization.
- Recovery and Adaptation: Facilitating the rapid restoration of energy reserves and adaptation to repeated physical stress.
These benefits make Urolithin A particularly effective for both aerobic and anaerobic activities, where energy demands vary widely but remain consistently high. Additionally, the fortification of mitochondrial networks helps preserve muscle health over the long term, mitigating the effects of age-related mitochondrial decline.
AMPK Activation: Enhancing Energy Regulation and Mitochondrial Biogenesis
In addition to activating PGC-1α, Urolithin A targets another key pathway for energy regulation: AMPK (adenosine monophosphate-activated protein kinase). AMPK serves as a master regulator of cellular energy balance, ensuring that energy production meets cellular demands during periods of metabolic stress. This pathway is particularly critical in skeletal muscle, where energy demands can spike dramatically during intense physical activity [10, 11].
AMPK is activated in response to low cellular energy, a condition marked by an increased AMP-to-ATP ratio that signals a shortfall in energy availability. This situation arises during activities such as high-intensity exercise, prolonged endurance training, or metabolic stressors like fasting. One essential function of AMPK in skeletal muscle is its ability to enhance glucose uptake by promoting the translocation of GLUT4 transporters to the cell membrane. GLUT4 is a glucose transporter responsible for moving glucose from the bloodstream into muscle cells. This ensures an immediate supply of glucose, which can be metabolized for ATP production to sustain muscle contractions during exercise.
Another critical function of AMPK is its inhibition of the mechanistic target of rapamycin (mTOR), a pathway that governs cell growth and protein synthesis. While mTOR activity is vital for anabolic processes like muscle building, it is energy-intensive. During periods of low energy availability, such as intense exercise, AMPK suppresses mTOR activity to conserve energy and prioritize processes like mitochondrial biogenesis and glucose metabolism.
When Urolithin A activates AMPK, it initiates a cascade of molecular events that stimulate mitochondrial biogenesis, complementing the effects of PGC-1α. Upon activation by Urolithin A, AMPK regulates mitochondrial biogenesis through the upregulation of transcriptional coactivators such as PGC-1α and transcription factors like NRF1 (nuclear respiratory factor 1) and TFAM (mitochondrial transcription factor A).
NRF1 and TFAM are critical for the replication and transcription of mitochondrial DNA (mtDNA), ensuring that newly formed mitochondria are equipped with the necessary genetic material and proteins for optimal function. This coordinated regulation enables cells to expand their mitochondrial network, compensating for energy deficits and supporting increased energy demands during physical activity or metabolic stress.
In addition to generating new mitochondria, AMPK activation enhances the functionality of existing mitochondria. It promotes the repair of damaged mitochondria by improving the efficiency of the electron transport chain (ETC), the machinery responsible for the breakdown of nutrients like glucose through a process called oxidative phosphorylation and ATP production. By optimizing ETC activity, AMPK reduces energy loss and minimizes the production of reactive oxygen species (ROS), protecting cells from oxidative damage and further enhancing mitochondrial resilience. [10, 11]
The result of these processes is a fortified mitochondrial network with improved energy-producing capacity. This is particularly beneficial in energy-intensive tissues like skeletal muscle, where mitochondrial biogenesis and enhanced functionality are critical for maintaining endurance, reducing fatigue, and supporting recovery. By stimulating AMPK, Urolithin A ensures that muscle cells have the energy reserves necessary for sustained performance and adaptation to repeated physical demands.
AMPK activation by Urolithin A has a direct impact on key metabolic pathways that fuel muscle performance:
- Fatty Acid Oxidation: AMPK promotes the breakdown of stored fatty acids for ATP production, a vital energy source during prolonged aerobic exercise. This process reduces reliance on glycogen stores, delaying fatigue and supporting endurance.
- Glycogen Synthesis and Storage: By enhancing glycogen synthesis, AMPK ensures that muscle cells maintain an adequate reserve of rapidly accessible energy for anaerobic activities. This dual role of utilizing fats and preserving glycogen creates an efficient energy balance tailored to varying physical demands.
The activation of AMPK by Urolithin A translates into measurable benefits for muscle health and performance:
- Prolonged Activity: By improving the efficiency of energy metabolism, AMPK enables muscles to sustain activity over extended periods without premature fatigue.
- Improved Recovery: The enhanced energy production supports faster replenishment of ATP and glycogen stores post-exercise, accelerating recovery.
- Adaptive Capacity: AMPK activation promotes cellular resilience, allowing muscles to better adapt to repeated physical stressors, such as progressive overload during training.
Through its activation of AMPK, Urolithin A addresses both immediate and long-term energy needs in skeletal muscle. The improved mitochondrial function and metabolic efficiency ensure that muscles can meet the high energy demands of physical activity while maintaining reserves for recovery and adaptation.
Reducing Oxidative Stress: Cleaner Metabolic Engine
During intense exercise, muscle cells produce substantial amounts of reactive oxygen species (ROS) as byproducts of increased energy demands and mitochondrial activity. While moderate levels of ROS serve signaling roles in muscle adaptation, excessive ROS accumulation can overwhelm the cell’s antioxidant defenses, leading to oxidative stress. This imbalance can result in cellular damage, muscle fatigue, and delayed recovery.
Urolithin A mitigates these effects through its potent antioxidant properties. It directly scavenges ROS, reducing their accumulation and thereby alleviating oxidative stress in muscle cells. By minimizing ROS-induced damage, Urolithin A helps maintain the structural integrity of muscle fibers and supports faster recovery following strenuous activity [12].
Beyond its direct antioxidant activity, Urolithin A enhances the body’s endogenous antioxidant defenses. It activates key enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GPx), which neutralize superoxide radicals and hydrogen peroxide, respectively, preventing their harmful effects on muscle cells. Additionally, Urolithin A promotes the synthesis of glutathione (GSH), a critical intracellular antioxidant that serves as a primary line of defense against oxidative damage. GSH not only scavenges free radicals but also regenerates other antioxidants, amplifying its protective effects.
By protecting mitochondria from oxidative damage, Urolithin A helps maintain their ability to efficiently produce ATP, the primary energy molecule essential for muscle contraction and exercise performance.
Beyond its antioxidant properties, Urolithin A directly enhances ATP production in muscle cells by improving the efficiency of the mitochondrial respiratory chain—the cellular machinery responsible for energy generation. The respiratory chain, located in the inner mitochondrial membrane, consists of a series of protein complexes (I to V) that work together like components of an intricate assembly line, each performing a specific task to ensure the efficient production of ATP.
Imagine the respiratory chain complexes as stations in a high-tech factory, where raw materials (electrons) are passed along a conveyor belt (the electron transport chain). At each station, these electrons are processed to drive the production of ATP, much like refined products are assembled from raw components in a factory. The final station, Complex V, acts as the powerhouse of this assembly line, using the energy generated by the preceding stations to synthesize ATP from ADP and inorganic phosphate through a process known as oxidative phosphorylation.
Research has shown that Urolithin A enhances both the formation and activity of these respiratory chain complexes. By improving the structural integrity and efficiency of the assembly line, Urolithin A ensures that electrons are transferred smoothly between complexes, minimizing energy loss and reducing the production of harmful byproducts like reactive oxygen species (ROS). This optimization allows mitochondria to produce ATP at an accelerated rate, providing a steady and robust energy supply to muscle cells during periods of heightened demand, such as exercise and recovery [12].
By improving respiratory chain function, Urolithin A directly supports the energy-intensive processes required for muscle contraction, endurance, and recovery.
By supporting mitochondrial function and optimizing muscle energy metabolism, Urolithin A has a multifaceted positive impact on muscle performance and recovery. Enhanced ATP production improves muscle endurance and strength, while the reduction of oxidative stress alleviates exercise-induced fatigue. Additionally, Urolithin A facilitates faster muscle recovery, ensuring that muscle cells regain their energy capacity and structural integrity more efficiently after physical activity.
The combination of a more robust mitochondrial network and reduced oxidative stress ensures a steady supply of ATP, even during prolonged or intense exercise. This translates to reduced muscle fatigue, faster recovery, and sustained physical performance. By targeting mitochondrial biogenesis, oxidative stress, and inflammation simultaneously, Urolithin A emerges as a promising compound for supporting muscle health, endurance, and overall physical performance.
Inflammation Reduction
Oxidative stress and inflammation are intricately linked processes, with reactive oxygen species (ROS) serving as key triggers for inflammatory responses. Excessive ROS levels can activate pro-inflammatory signaling pathways, amplifying inflammation and contributing to muscle soreness, tissue breakdown, and impaired recovery. By reducing oxidative stress, Urolithin A indirectly helps attenuate this cycle of ROS-induced inflammation.
Beyond its antioxidant properties, Urolithin A exerts direct anti-inflammatory effects that are critical for muscle health. Exercise-induced muscle damage naturally initiates an inflammatory response, which plays an essential role in tissue repair and adaptation [13]. However, when inflammation becomes excessive or prolonged, it can hinder recovery, contribute to muscle tissue damage, and impair growth.
Urolithin A modulates inflammation by inhibiting the release of pro-inflammatory mediators such as cytokines and chemokines. It also dampens key inflammatory signaling pathways, including the NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathway, which is a central regulator of inflammation. By controlling the intensity and duration of the inflammatory response, Urolithin A helps reduce muscle soreness and accelerate recovery, particularly after high-intensity workouts. [14]
This balanced regulation of inflammation allows Urolithin A to support the natural repair and adaptation process while preventing the detrimental effects of excessive inflammation. [13]
Muscle Growth and Maintenance
Building on Urolithin A’s role in enhancing mitochondrial function, reducing oxidative stress, and managing inflammation, its cellular-level benefits extend to promoting muscle hypertrophy and maintaining muscle mass. These effects are particularly relevant for individuals seeking to optimize muscle strength, endurance, and recovery.
Urolithin A supports muscle hypertrophy and the maintenance of muscle mass through multiple interconnected pathways. These include enhancing muscle cell repair and growth, improving mitochondrial function and energy metabolism, and regulating protein synthesis and degradation processes [9, 15].
One of Urolithin A’s key mechanisms is its ability to promote muscle cell repair and growth, essential for muscle hypertrophy. During exercise, muscle fibers experience microdamage that requires repair and growth to increase muscle mass and strength. By optimizing mitochondrial function and increasing ATP synthesis, Urolithin A creates an environment conducive to efficient muscle recovery and growth. This process contributes to an increase in the cross-sectional area of muscle fibers, enhanced muscle protein content, and improved muscle strength [16].
Additionally, Urolithin A regulates protein turnover within muscle cells, a critical factor for hypertrophy and the preservation of muscle mass. It stimulates protein synthesis, enabling muscle cells to produce new structural and functional proteins, while simultaneously inhibiting protein degradation pathways. This balance ensures the retention and growth of muscle tissue over time [15].
Together, these multifaceted effects position Urolithin A as a promising compound for individuals aiming to enhance muscle growth, preserve muscle mass, and optimize performance. While current findings are encouraging, further research is needed to fully elucidate its role in human muscle physiology and identify the most effective dosing strategies.
Metabolism and Bioavailability of Urolithin A
The metabolism and bioavailability of Urolithin A depend on the interplay between diet, gut microbiota, and individual metabolic processes. When foods rich in ellagitannins—such as pomegranates, walnuts, and berries—are consumed, these polyphenolic compounds undergo complex biochemical transformations, primarily in the colon.
The journey of Urolithin A begins in the gut, where specific bacterial species facilitate the conversion of ellagitannins into bioavailable metabolites. Microbes such as Proteobacteria, Clostridium, Bifidobacterium, and Eubacterium possess specialized enzymes that catalyze this conversion [17, 18, 19]. These transformations occur primarily in the large intestine, highlighting the critical role of gut flora in Urolithin A production.
Once Urolithin A is formed, it is absorbed across the intestinal lining and enters the bloodstream. Factors like gut health, intestinal permeability, and microbiome composition influence this absorption process [17, 18, 19. Once in circulation, Urolithin A travels to various tissues in its free form, allowing it to enter cells throughout the body. Urolithin A undergoes further metabolism inside cells, often in the liver, where enzymes convert it into mercaptan and sulfate conjugates. These modifications increase solubility, aiding in excretion through urine and bile [17, 18, 19].
Interindividual Variability in Urolithin A Bioavailability
The extent to which Urolithin A is absorbed and utilized varies widely among individuals due to differences in diet, microbiome diversity, and genetic factors. For instance, polyphenol-rich foods such as pomegranates, nuts, and berries supply ellagitannins but compete with other molecules for absorption sites in the gut, impacting the effective dosage of Urolithin A entering systemic circulation. Genetic variations affecting enzyme activity further contribute to individual differences in Urolithin A metabolism, influencing its breakdown and bioavailability.
The gut microbiome plays a pivotal role in determining Urolithin A production efficiency. Individuals with microbiomes rich in Urolithin A-producing bacteria are more likely to convert ellagitannins into bioavailable Urolithin A, resulting in higher systemic exposure. Conversely, those lacking these bacterial species may produce less Urolithin A, even with adequate dietary intake. This variability underscores the importance of gut microbiome health in optimizing Urolithin A production.
Future Research Directions
Research into Urolithin A (Urolithin A) continues to hold immense potential for uncovering its role in muscle health, exercise performance, and recovery. While current studies are promising, long-term investigations are essential to evaluate Urolithin A's safety, efficacy, and sustained effects on muscle function over extended periods. These trials can help determine optimal dosages, timing, and duration of Urolithin A supplementation. Additionally, exploring its effects on diverse populations, from athletes to the elderly, is crucial for tailoring its use across various contexts, including specific exercise types and recovery scenarios.
An important area for future study involves Urolithin A's interactions with other nutrients and supplements. Research could examine whether synergistic effects arise from combining Urolithin A with proteins, amino acids, or common exercise supplements, potentially maximizing muscle adaptation and recovery. Investigating how Urolithin A influences muscle metabolism, mitochondrial function, and protein synthesis at a molecular level will provide deeper mechanistic insights, enabling the development of optimized strategies for endurance enhancement and recovery. These findings could be particularly impactful for athletes and those undergoing rehabilitation for muscle injuries or disorders.
Urolithin A also holds promise as a therapeutic agent for addressing muscle pathologies. Its ability to enhance mitochondrial function, reduce inflammation, and promote muscle recovery positions it as a potential intervention for conditions such as age-related muscle degeneration and other muscle-related diseases. Exploring Urolithin A’s impact on gene regulation through genetic and gene expression studies could shed light on its ability to promote muscle health at a genetic level. Such research may pave the way for personalized interventions to improve exercise performance and prevent or manage muscle-related conditions.
Closing Thoughts:
Muscle health is essential for physical performance, mobility, and overall quality of life, yet it is often one of the first areas affected by aging and physical stress. Sarcopenia, the gradual loss of muscle mass and function, highlights the urgent need for effective strategies to combat muscle decline in aging individuals. At the same time, athletes and physically active individuals face their own challenges, including muscle resilience, recovery, and performance optimization. Both populations share a critical dependency on mitochondrial health, inflammation control, and effective repair processes to maintain muscle integrity and function.
Urolithin A (Urolithin A) emerges as a promising, science-backed solution to these challenges. By addressing key contributors to muscle decline—mitochondrial dysfunction, oxidative stress, and chronic inflammation—Urolithin A offers a novel approach to muscle health at the cellular level. Its ability to enhance mitochondrial quality control, regulate inflammatory responses, and support protein synthesis positions Urolithin A as a valuable tool for improving muscle performance and recovery, regardless of age or activity level.
The potential of Urolithin A lies not only in its applications for muscle health but also in its broader implications for aging, metabolic health, and overall cellular function. While more research is needed to refine its use and uncover additional therapeutic applications, the existing evidence highlights Urolithin A’s capacity to effectively target the root causes of muscle decline. For aging individuals, it offers a pathway to sustained mobility and independence. For athletes, it provides a means to optimize performance and recovery.
As our understanding of Urolithin A deepens, its role in advancing both healthspan and physical resilience becomes increasingly evident - whether as a means to address the challenges of aging or as a tool to elevate athletic performance.
- Gómez-Guzmán, M., Jiménez, R., & López-Sepúlveda, R. (2019). Pomegranate-derived polyphenols and urolithin A in the treatment of cardiovascular diseases. Journal of Nutritional Biochemistry, 70, 27-39. https://doi.org/10.1016/j.jnutbio.2019.04.007
- Williams, E. R., & Raghavan, R. (2018). Polyphenolic compounds in walnuts: Mechanisms and health effects. Food and Chemical Toxicology, 113, 135-144. https://doi.org/10.1016/j.fct.2018.01.001
- Ryu, D., Mouchiroud, L., Andreux, P. A., Katsyuba, E., Moullan, N., Nicolet-dit-Félix, A. A., ... & Auwerx, J. (2016). Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nature Medicine, 22(8), 879–888. https://doi.org/10.1038/nm.4132
- Singh A., D’Amico D., Andreux P.A., Fouassier A.M., Blanco-Bose W., Evans M., Aebischer P., Auwerx J., Rinsch C. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep. Med. 2022;3:100633. doi: 10.1016/j.xcrm.2022.100633. https://doi.org/10.1016/j.xcrm.2022.100633
- Liu S, D’Amico D, Shankland E, Bhayana S, Garcia JM, Aebischer P et al. Effect of Urolithin A Supplementation on Muscle Endurance and Mitochondrial Health in Older Adults: A Randomized Clinical Trial. JAMA Netw Open 2022; 5: e2144279–e2144279.
- Peiling Luan et al. ,Urolithin A improves muscle function by inducing mitophagy in muscular dystrophy.Sci. Transl. Med.13,eabb0319(2021).DOI:10.1126/scitranslmed.abb0319
- Andreux PA, Blanco-Bose W, Ryu D, Burdet F, Ibberson M, Aebischer P, Auwerx J, Singh A, Rinsch C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat Metab. 2019 Jun;1(6):595-603. doi: 10.1038/s42255-019-0073-4. Epub 2019 Jun 14. PMID: 32694802. https://europepmc.org/article/med/32694802
- Halling J.F., Pilegaard H. PGC-1alpha-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020;45:927–936. doi: 10.1139/apnm-2020-0005. https://doi.org/10.1139/apnm-2020-0005
- Singh A., D’Amico D., Andreux P.A., Fouassier A.M., Blanco-Bose W., Evans M., Aebischer P., Auwerx J., Rinsch C. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep. Med. 2022;3:100633. doi: 10.1016/j.xcrm.2022.100633. https://doi.org/10.1016/j.xcrm.2022.100633
- Lin S.C., Hardie D.G. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018;27:299–313. doi: 10.1016/j.cmet.2017.10.009. https://doi.org/10.1016/j.cmet.2017.10.009
- Tow W.K., Chee P.Y., Sundralingam U., Palanisamy U.D. The Therapeutic Relevance of Urolithins, Intestinal Metabolites of Ellagitannin-Rich Food: A Systematic Review of In Vivo Studies. Nutrients. 2022;14:3494. doi: 10.3390/nu14173494 https://doi.org/10.3390/nu14173494
- Boakye Y.D., Groyer L., Heiss E.H. An increased autophagic flux contributes to the anti-inflammatory potential of urolithin A in macrophages. BBA Gen. Subj. 2018;1862:61–70. doi: 10.1016/j.bbagen.2017.10.006 https://doi.org/10.1016/j.bbagen.2017.10.006
- Powers S.K., Deminice R., Ozdemir M., Yoshihara T., Bomkamp M.P., Hyatt H. Exercise-induced oxidative stress: Friend or foe? J. Sport. Health Sci. 2020;9:415–425. doi: 10.1016/j.jshs.2020.04.001 https://doi.org/10.1016/j.jshs.2020.04.001
- Toney A.M., Fox D., Chaidez V., Ramer-Tait A.E., Chung S. Immunomodulatory Role of Urolithin A on Metabolic Diseases. Biomedicines. 2021;9:192. doi: 10.3390/biomedicines9020192. https://doi.org/10.3390/biomedicines9020192
- Han Q.A., Yan C., Wang L., Li G., Xu Y., Xia X. Urolithin A attenuates ox-LDL-induced endothelial dysfunction partly by modulating microRNA-27 and ERK/PPAR-gamma pathway. Mol. Nutr. Food Res. 2016;60:1933–1943. doi: 10.1002/mnfr.201500827. https://doi.org/10.1002/mnfr.201500827
- Jayatunga D., Hone E., Khaira H., Lunelli T., SinghH., Guillemin G.J., Fernando B., Garg M.L., Verdile G., Martins R.N. Therapeutic Potential of Mitophagy-Inducing Microflora Metabolite, Urolithin A for Alzheimer’s Disease. Nutrients. 2021;13:3744. doi: 10.3390/nu13113744.
- Selma M.V., Beltran D., Garcia-Villalba R., Espin J.C., Tomas-Barberan F.A. Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct. 2014;5:1779–1784. doi: 10.1039/C4FO00092G. https://doi.org/10.1039/C4FO00092G
- Selma M.V., Tomas-Barberan F.A., Beltran D., Garcia-Villalba R., Espin J.C. Gordonibacter urolithinfaciens sp. nov., a urolithin-producing bacterium isolated from the human gut. Int. J. Syst. Evol. Micr. 2014;64:2346–2352. doi: 10.1099/ijs.0.055095-0. https://doi.org/10.1099/ijs.0.055095-0
- Zhang X., Fang Y., Yang G., Hou X., Hai Y., Xia M., He F., Zhao Y., Liu S. Isolation and characterization of a novel human intestinal Enterococcus faecium FUA027 capable of producing urolithin A from ellagic acid. Front. Nutr. 2022;9:1039697. doi: 10.3389/fnut.2022.1039697. https://doi.org/10.3389/fnut.2022.1039697