Metformin is a safe, inexpensive, and effective medication used worldwide for the treatment of type 2 diabetes. It is considered an essential medicine by the World Health Organization.
Metformin has been shown to extend lifespan and healthspan in various animals by affecting shared aging pathways, such as insulin-like signaling and mTOR.
In humans, metformin demonstrated beneficial effects on a wide range of conditions and their complications, such as HIV, PCOS, cancer, cognitive dysfunction, autoimmune and heart diseases.
Aging is a biological process that drives chronic diseases such as heart disease, dementia, cancer, and diabetes. By targeting aging, we can delay the development of age-related diseases.
By mimicking calorie restriction and affecting different nutrient signaling pathways, metformin inhibits processes that cause aging and disease, such as inflammation, oxidative stress, and senescence. It also enhances processes that protect cells and tissues from damage.
The TAME study aims to establish aging as a medical indication for treatment and show that prevention of age-related diseases in tandem is possible.
Introduction
If you have diabetes or know someone who does, you are likely familiar with metformin - a first-line medication universally used in the treatment and prevention of type 2 diabetes (T2DM). In recent years, numerous studies have provided evidence that metformin’s benefits extend beyond blood sugar control; it can tame the detrimental effects of aging by promoting autophagy and reducing inflammation and oxidative stress. The positive health effects commonly observed in individuals with T2DM who use metformin, along with insights gained from studies in animals like worms and mice, have raised a compelling question: could metformin serve as an anti-aging medication capable of extending healthspan?
Metformin’s Botanical Origins
Before it was synthesized into the medication we know today, metformin originated in the French lilac plant. In medieval Europe, French lilac had been used for centuries in traditional healing practices to alleviate the distressing diabetes-like symptom—frequent urination.
In 1918, it was discovered that one of the plant's active ingredients, guanidine, could lower animal blood sugar levels. However, guanidine was too toxic for the liver to be used long-term.
In the 1920s, several synthetic guanidine derivatives were developed, called biguanides, which were less toxic and more effective than its predecessor. Metformin was one of the first biguanides to be synthesized in 1922. However, due to the increased availability of insulin, it was not widely used until the 1950s, when it received approval for treating diabetes in Great Britain and France under the name Glucophage - "glucose eater."
It wasn't until 1995 that the U.S. Food and Drug Administration (FDA) granted metformin its authorization after other biguanides - phenformin and buformin - were withdrawn from the market due to their association with a rare but severe complication called lactic acidosis [1].
Since then, metformin has become the world's most prescribed oral drug for T2DM, with over 150 million users globally, and in the United States, it ranks as the 3rd most prescribed medication [2,3].
What sets metformin apart from other T2DM therapies is that it does not cause hypoglycemia, a potentially dangerous condition where the blood sugar level drops too much. Additionally, metformin's potential is not limited to diabetes management, as it also exhibits beneficial effects on conditions such as polycystic ovary syndrome (PCOS), gestational diabetes, obesity, and prediabetes.
Apart from being inexpensive, accessible, and well-tolerated, metformin is widely recognized for its unparalleled safety profile. Its common side effects are mild and mainly concentrate on gastrointestinal discomfort, which can be reduced by taking the tablet with a meal or gradually increasing the dose to build tolerance. These symptoms usually go away after a week or two. Given all its benefits, it is not surprising that the World Health Organization considers it an essential medicine for humanity. But how did it become viewed as a promoter of longevity?
Metformin in the Context of Aging
Unlike specific diseases, aging happens to all species. Even though the mechanisms of aging are not entirely the same across all organisms, there are several shared cellular pathways involved in the aging of animals.
The first indications that metformin might influence longevity come from studies on other organisms, which consistently demonstrated that—by affecting some of the common cellular pathways, such as insulin-like signaling and mechanistic target of rapamycin (mTOR)—metformin can generate positive effects on health and survival, that may translate to humans [4].
Our understanding of metformin’s impact on aging goes beyond animal models. The landmark study, UKPDS (U.K. Prospective Diabetes Study), followed over 5,000 diabetic patients for 20 years and established that metformin was especially effective in reducing the risk of heart attack and death by a third in overweight patients with T2DM [5]. While the UKPDS showed that complications of T2DM can be prevented or delayed by improving glucose and blood pressure control, the influential 2014 study by Bannister et al. implied that metformin may have anti-aging effects beyond its glucose-lowering action.
The study looked at 180,000 people and analyzed three groups: diabetics taking metformin, diabetics taking another medication – sulfonylurea, and individuals without diabetes, not on any medication. The findings were intriguing: individuals with diabetes who received metformin treatment, a group initially characterized by higher obesity rates and greater susceptibility to other chronic ailments, not only outlived those who used sulfonylurea but also surpassed the lifespan of their healthy, non-diabetic counterparts [6]. The table below shows the key findings from several studies on metformin’s influence on lifespan.
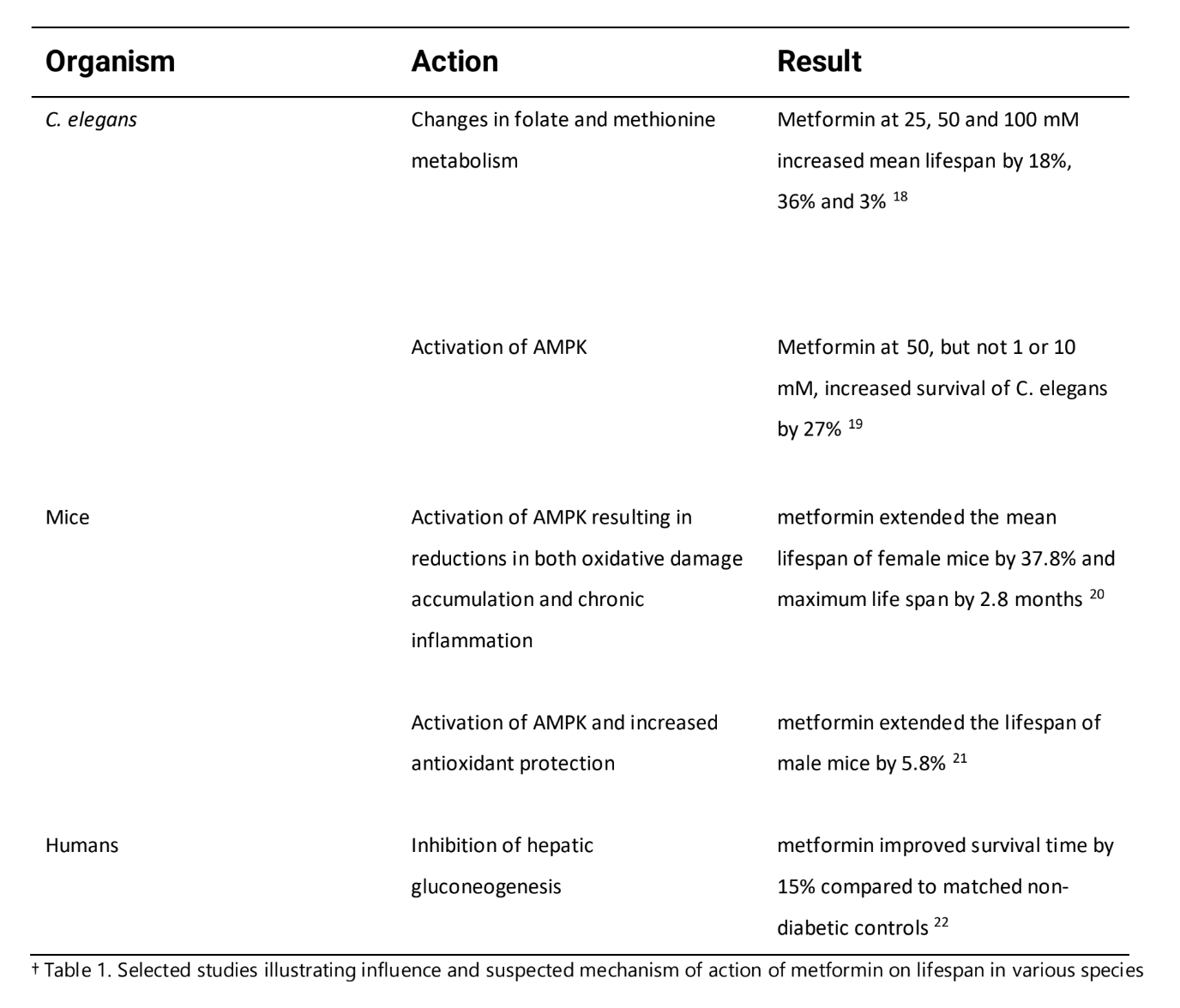
In addition to its role as a highly effective anti-hyperglycemic agent (lowering blood sugar), metformin consistently demonstrates its ability to improve a wide variety of conditions including HIV complications, PCOS, autoimmune diseases, and cognitive deterioration [7, 8, 9, 10, 11].
The topic of most attention, however, is metformin's potential role in cancer prevention and treatment. There is an ever-growing body of evidence that metformin can protect against different types of cancer, compared to both other diabetics and the general population, and that it can slow down tumor growth, particularly in colon, prostate, breast, and lung cancers [12, 13, 14, 15, 16]. Metformin is so promising that there are currently thirty ongoing trials investigating its impact on different cancers, either as a preventative agent or an addition to existing therapies [17].
How does metformin change the biology of aging?
Aging is, by far, the greatest and the most common risk factor for the development of chronic diseases like cancer, diabetes, cardiovascular disorders (stroke, heart failure, hypertension), and neurodegenerative disorders (dementia, Alzheimer's, and Parkinson's). In fact, the risk of death from these causes becomes 100 to 1000 times more likely between the ages of 35 and 85. These age-related conditions are rooted in a gradual build-up of damage in our cells over time, with worsening abilities of our bodies to repair that damage [23].
With its long reign as a drug of choice in diabetes, metformin's metabolic effects on people with T2DM are well understood. Metformin lowers blood sugar levels through three actions: it inhibits glucose production by the liver, decreases glucose absorption from the gastrointestinal tract, and improves cell response to insulin, a hormone that controls turning glucose into energy. Furthermore, it helps with weight loss by increasing the feeling of fullness, leading to lower mortality in obese patients.
But despite its six and a half decades of clinical use, scientists only recently started to uncover cellular mechanisms underlying metformin's mode of action and its influence on hallmarks of aging. We have discussed these hallmarks of aging in our previous articles. Recent research has found that metformin positively affects the whole catalog of hallmarks, primarily by impacting diet-linked cellular signaling pathways and mimicking calorie restriction, a lifestyle intervention known for promoting healthspan.
So how does metformin work at the cellular level?
When metformin enters the cell, it sticks to something called complex 1 (MC1) in the mitochondria, which changes how the cell senses its energy resources. Think of mitochondria as tiny power plants that make energy for the cell. Their role is to convert energy from food to ATP – a form of the body's energy currency. Metformin’s effect makes the cell think it has less energy than it does, and it sets in motion a cascade of actions:
Adenosine Monophosphate-Activated Protein Kinase (AMPK) Increase
AMPK is an enzyme that controls how the cell uses energy. It can tell how much energy the cell has and activates when cellular energy levels are low, such as during fasting, exercise, and calorie restriction. It then makes the cell use energy more efficiently - switching on processes that generate ATP, such as glycolysis and fatty acid oxidation, which help with weight control, and autophagy, which clears damaged cells. Simultaneously, AMPK blocks reactions that consume the energy to make new proteins and fats. In other words, cells shift their focus from growth to survival, preserving their health and becoming more resistant. Additionally, activating the AMPK pathway further inhibits glucose production in the liver and increases insulin sensitivity, reinforcing its beneficial effects on blood sugar control. AMPK activity generally slows down in aging leading to metabolic disorders like obesity and diabetes. Metformin may be a potential lever in activating AMPK’s positive cellular effects.
Metformin and Autophagy Activation
Autophagy is akin to a recycling system that breaks down cellular waste, such as senescent cells and converts these damaged components into new, useful resources. This maintenance process is essential for preventing cancer, as it stops cells from proliferating uncontrollably and removes defective cells that can become malignant. Similarly, autophagy also protects the brain from neurodegenerative diseases, such as Parkinson's and Alzheimer's, by removing toxic proteins that accumulate in the neurons [24].
Mammalian Target of Rapamycin (mTOR) Inhibition
Metformin uses AMPK to block the mTOR pathway. Picture mTOR as a growth switch in your cells - when it is on, it promotes protein, lipid, and DNA synthesis and self-preservation, allowing cells to grow and divide rapidly. However, if it stays on for too long, it can lead to uncontrolled cell growth, potentially leading to the development of tumors. In fact, mTOR is often overactivated in cancer cells, making them resistant to apoptosis and immune surveillance. In aging, mTOR also becomes more active, increasing the risk of cancer, inflammation, and neurodegenerative diseases [25].
Decreased Insulin-Like Growth Factor 1 (IGF-1) Signaling
IGF-1 is a hormone similar to insulin in structure but is more prominent in making tissues grow and develop. Together with insulin, they create a hormone system capable of sensing glucose levels, controlling how cells use energy and nutrients and how they grow and survive. During childhood and adolescence, IGF-1 is needed to facilitate body growth, however, as we mature, a shift of energy from growth to protection against breakdown needs to happen. Metformin favors this change by blocking IGF-1 signaling. When IGF-1 is inhibited, cells become more responsive and need less insulin to deliver glucose into the cell, lowering blood sugar levels more efficiently. Research has shown that people with decreased IGF-1 activity are likely to become centenarians and live past 100 years [26].
Decreased Production of Reactive Oxygen Species (ROS)
Oxidative stress happens when our body contains too many harmful molecules called ROS. These ROS can cause lasting damage to the important building block components of our body, including proteins, fats, sugars, and DNA. They can also interfere with our body's natural repair systems.
Mitochondria, the energy powerhouses of our cells, are considered the body's primary source of harmful ROS. Notably, they are also highly susceptible to oxidative damage, which reduces their effectiveness and causes a further rise in ROS production. Consequently, this creates a detrimental cycle that exacerbates cellular and tissue damage. As this damage gradually accumulates over time, it increases the risk of age-related diseases. Studies on skin cells suggest that metformin can help control ROS levels by employing an antioxidant called nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [27]. Keeping ROS in check improves DNA repair and metabolic function and reduces chronic inflammation.
Preventing Senescence
Senescent cells are dysfunctional, damaged cells that stop growing and dividing, releasing harmful substances that spark inflammation and damage nearby cells. Typically, our immune system is responsible for the removal of senescent cells. However, as we age, bodily defense responses become less efficient, leading to their pathological accumulation, which makes it harder for the body to heal and renew. This amassment can cause tissue and organ degeneration, primarily due to the senescence-associated secretory phenotype (SASP).
Think of SASP as a homeowner whose backyard is filled with weeds. These weeds can spread to neighboring lawns, damaging the plants and grass there as well. The growth of weeds represents the harmful molecules that can be released from senescent cells. Some studies indicate that metformin clears out senescent cells through the mTOR pathway and modulation of the immune system [28, 29, 30].
Metformin and Inflammation Reduction
Chronic low-level inflammation is like a fire burning in your body, damaging your cells and tissues over time and fueling the progression of age-related diseases. Metformin acts like a fire extinguisher, putting out the flames and preventing them from spreading. Its anti-inflammatory properties are long known, as in the 1940s, it was used to protect against flu and malaria [31]. Nowadays, we have more evidence from pre-clinical and clinical findings, which show that people who take metformin have lower levels of inflammation, are less likely to die during hospitalization and experience less inflammation and pain in arthritis. Metformin achieves that by decreasing the release of the inflammatory signaling molecules by modulating cellular pathways [32, 33].
TAME – Targeting Aging with Metformin
With much data already in existence about metformin’s benefits and impressive safety profile, researchers have been working to implement a large, controlled clinical trial to understand if and how metformin can delay the onset of multiple age-related diseases.
A recent trial, Targeting Aging with Metformin (TAME), is groundbreaking because it challenges the conventional approach of treating each disease separately, which does not guarantee that we will not develop another one later in life. Instead, TAME focuses on the underlying cause of aging and targets the diseases as a cluster. By doing so, TAME hopes to demonstrate that aging can be classified as a treatable medical condition by the FDA, which would open the door for more research, funding, and innovation in the field of aging and serve as a template for future drug approval and insurance coverage.
Led by Dr. Nir Barzilai - director of the Institute for Aging Research at the Albert Einstein College of Medicine - this study will enroll 3,000 non-diabetic participants aged 65-79 across the country and randomly assign them to either metformin or placebo. The trial will measure the effects of metformin on various health outcomes, such as cognitive function, physical function, and disease incidence, to see if they improve. TAME also seeks to identify biomarkers of aging. Amongst scientists, there are no universally approved ways to measure aging. TAME is aiming to change that and establish indicators of biological decline.
TAME is expected to last six years, with a five-year observation period. The trial has faced some delays due to the COVID-19 pandemic and the difficulty of securing funding, as metformin is a cheap and off-patent drug that offers little incentive for pharmaceutical companies to invest. However, TAME has received support from various sources, such as the American Federation for Aging Research, the National Institute on Aging, and private donors. The trial has a budget of $10 million per year and is currently in the process of recruiting participants [36]. TAME was inspired by the observation of centenarians, individuals who live to be over one hundred years. Dr. Barzilai, who has studied centenarians for decades, noticed that they often develop age-related diseases much later in life and spend less time sick before they die - a phenomenon known as compression of morbidity. TAME hopes to replicate this phenomenon in a more extensive and diverse group and show that aging can be modified and improved with a simple, safe intervention. The results from this study will provide valuable insights about metformin’s potential as an anti-aging protocol.
Conclusion
This article sheds light on the potential of metformin, a medication primarily known for its role in managing diabetes, to influence the science of aging and longevity. In the relentless pursuit of extending healthspan and lifespan, metformin's promise in the longevity space is not that of immortality but rather the delay of diseases that account for nearly thirty million deaths yearly.
Metformin's ability to positively impact multiple hallmarks of aging, from promoting autophagy to reducing inflammation and oxidative stress, offers hope for extending healthspan and preventing age-related diseases. Its inclusion of diverse populations, including those at higher risk of accelerated aging, underscores the potential societal benefits of this humble medication. As ongoing research, exemplified by the TAME trial, continues to explore metformin's role in aging, it is clear that this unassuming drug has the potential to redefine how we age and experience life in our later years.
- Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60(9):1566-1576. doi:10.1007/s00125-017-4318-z.
- Mohammed I, Hollenberg MD, Ding H, Triggle CR. A critical review of the evidence that metformin is a putative anti-aging drug that enhances healthspan and extends lifespan. Front Endocrinol (Lausanne). 2021;12:718942. doi:10.3389/fendo.2021.718942.
- Lewis S. The top 50 drugs prescribed in the United States. Healthgrades. Published September 29, 2022. Retrieved: November 3, 2023
- Pitt JN, Kaeberlein M. Why is aging conserved and what can we do about it? PLoS Biol. 2015;13(4):e1002131. doi:10.1371/journal.pbio.1002131.
- UK Prospective Diabetes Study: Overview.University of Oxford. Retrieved: October 29, 2023 from www2.dtu.ox.ac.uk/UKPDS/
- Bannister CA, Holden SE, Jenkins-Jones S, et al. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non diabetic controls. Diabetes Obes Metab. 2014;16(11):1165-73. doi: 10.1111/dom.12354.
- Hadigan C, Corcoran C, Basgoz N, Davis B, Sax P, Grinspoon S. Metformin in the treatment of HIV lipodystrophy syndrome: A randomized controlled trial. JAMA. 2000 Jul 26;284(4):472-7. doi: 10.1001/jama.284.4.472. PMID: 10904511.
- Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med. 2008;358(1):47-54. doi:10.1056/NEJMct0707092
- Moreira PI. Metformin in the diabetic brain: friend or foe? Ann Transl Med. 2014;2(6):54. doi:10.3978/j.issn.2305- 5839.2014.06.10.
- Madhu LN, Kodali M, Shetty AK. Promise of metformin for preventing age-related cognitive dysfunction. Neural Regen Res. 2022;17(3):503-507. doi: 10.4103/1673-5374.320971.
- Ursini F, Russo E, Pellino G, et al. Metformin and Autoimmunity: A "New Deal" of an Old Drug. Front Immunol. 2018;9:1236. doi: 10.3389/fimmu.2018.01236.
- Lv Z, Guo Y. Metformin and Its Benefits for Various Diseases. Front Endocrinol (Lausanne). 2020;11:191. doi:10.3389/fendo.2020.00191
- Andersson C, Vaag A, Selmer C, et al. Risk of cancer in patients using glucose-lowering agents: a nationwide cohort study of 3.6 million people. BMJ Open. 2012;2(3):e000433. doi: 10.1136/bmjopen-2011-000433.
- Morales DR, Morris AD. Metformin in cancer treatment and prevention. Annu Rev Med. 2015;66:17-29. doi:10.1146/annurev-med-062613-093128.
- Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, Ford LG. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia. 2017;60(9):1639-1647. doi: 10.1007/s00125-017-4372-6.
- Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: A systematic review and meta-analysis. Ageing Res Rev. 2017;40:31-44. doi: 10.1016/j.arr.2017.08.003.
- Retrieved: November 3, 2023 form clinicaltrials.gov
- Cabreiro F, Au C, Leung KY, et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153(1):228-39. doi: 10.1016/j.cell.2013.02.035.
- Onken B, Driscoll M. Metformin Induces a Dietary Restriction-Like State and the Oxidative Stress Response to Extend C. elegans Healthspan via AMPK, LKB1 and SKN-1. PloS One (2010) 5:8758. 10.1371/journal.pone.0008758
- Anisimov VN, Berstein LM, Egormin PA, et al. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7(17):2769-73. doi: 10.4161/cc.7.17.6625.
- Martin-Montalvo, A., Mercken, E., Mitchell, S, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192.
- Bannister CA, Holden SE, Jenkins-Jones S, et al. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non diabetic controls. Diabetes Obes Metab. 2014;16(11):1165-73. doi: 10.1111/dom.12354.
- Metformin in Longevity Study (MILES). Retrieved: October 29, 2023 from ClinicalTrials.gov classic.clinicaltrials.gov/ct2/show/NCT02432287
- Valencia M, Kim SR, Jang Y, Lee SH. Neuronal Autophagy: Characteristic Features and Roles in Neuronal Pathophysiology. Biomol Ther (Seoul). 2021;29(6):605-614. doi: 10.4062/biomolther.2021.012
- Mohammed I, Hollenberg MD, Ding H, Triggle CR. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front Endocrinol (Lausanne). 2021;12:718942. doi: 10.3389/fendo.2021.718942.
- Vitale G, Brugts MP, Ogliari G et al. Low circulating IGF-I bioactivity is associated with human longevity: findings in centenarians' offspring. Aging (Albany NY). 2012 Sep;4(9):580-9. doi: 10.18632/aging.100484.
- Ribeiro FM, Ratti BA, Dos Santos Rando F, Fernandez MA, Ueda-Nakamura T, de Oliveira Silva Lautenschlager S, Nakamura CV. Metformin effect on driving cell survival pathway through inhibition of UVB-induced ROS formation in human keratinocytes. Mech Ageing Dev. 2020;192:111387. doi: 10.1016/j.mad.2020.111387.
- Kulkarni AS, Gubbi S, Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 2020;32(1):15-30. doi:10.1016/j.cmet.2020.04.001
- Hu Q, Peng J, Jiang L, Li W, et al. Metformin as a senostatic drug enhances the anticancer efficacy of CDK4/6 inhibitor in head and neck squamous cell carcinoma. Cell Death Dis. 2020;11(10):925. doi: 10.1038/s41419-020- 03126-0.
- Moiseeva O, Deschênes-Simard X, St-Germain E, et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell. 2013;12(3):489-98. doi: 10.1111/acel.12075.
- Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60(9):1566-1576. doi: 10.1007/s00125-017-4318-z.
- Bai B, Chen H. Metformin: A Novel Weapon Against Inflammation. Front Pharmacol. 2021;12:622262. doi: 10.3389/fphar.2021.622262.
- Song Y, Wu Z, Zhao P. The effects of metformin in the treatment of osteoarthritis: Current perspectives. Front Pharmacol. 2022;13:952560. doi: 10.3389/fphar.2022.952560.
- Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernàndez-Real JM, Bäckhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017 Jul;23(7):850-858. doi: 10.1038/nm.4345. Epub 2017 May 22. PMID: 28530702.
- Vallianou NG, Stratigou T, Tsagarakis S. Metformin and gut microbiota: their interactions and their impact on diabetes. Hormones (Athens). 2019;18(2):141-144. doi: 10.1007/s42000-019-00093-w.
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a Tool to Target Aging. Cell Metab. 2016 Jun 14;23(6):1060-1065. doi: 10.1016/j.cmet.2016.05.011. PMID: 27304507; PMCID: PMC5943638.
- Noncommunicable diseases: mortality. World Health Organization. Published: September 16, 2023. Retrieved: November 1, 2023 from www.who.int/data/gho/data/themes/topics/topic-details/GHO/ncd-mortality