SGLT2 Inhibitors as Metabolic Senolytics: Clearing Senescent Cells to Combat Pathological Aging
Importance of Senescence: Senescent cells play a central role in aging and age-related diseases by secreting inflammatory molecules that disrupt tissue function and drive chronic inflammation. Targeting and clearing these senescent cells represents a promising strategy to mitigate age-related pathologies and extend healthspan.
Novel Role of SGLT2 Inhibitors: Recent findings indicate that SGLT2 inhibitors—originally developed as diabetes treatments—may enhance the clearance of senescent cells through metabolic and immune-mediated mechanisms, rather than through direct cytotoxicity.
Unique Effects of SGLT2 Inhibitors: Metabolic improvements from SGLT2 inhibitor treatment, such as enhanced glucose metabolism and insulin sensitivity, persist even after treatment cessation. However, these metabolic effects alone are insufficient to reduce senescent cell burden, highlighting the unique senescence-modulating properties of SGLT2 inhibitors.
Central Role of AMPK Activation: The activation of AMP-activated protein kinase (AMPK) emerged as a critical mechanism by which SGLT2 inhibitors induce senescence clearance, as blocking AMPK activity completely abolished these beneficial effects.
Immune System Involvement: The senolytic effects of canagliflozin are heavily dependent on immune activation, specifically the activity of T cells. Blocking T-cell function significantly impaired senescent cell clearance, underscoring the immune-dependent nature of SGLT2 inhibition.
PD-L1 Immune Checkpoint Modulation: Canagliflozin treatment was found to reduce the expression of the immune checkpoint protein PD-L1 on senescent cells, thereby restoring immune surveillance by natural killer (NK) and CD8+ T cells, enabling more effective senescent cell clearance.
Potential Combination Therapies: The combination of SGLT2 inhibitors with existing longevity therapies, such as rapamycin, metformin, and caloric restriction, represents a promising strategy. In particular, rapamycin’s mTOR inhibition and SGLT2 inhibitors’ AMPK activation have complementary mechanisms, suggesting a synergistic potential to improve healthspan and longevity outcomes.
Introduction
Aging is often conceptualized as a progressive decline in the body’s capacity to maintain cellular and tissue integrity, opening the door to chronic disease and functional deterioration. However, recent advances have raised a compelling possibility: What if aging isn't an inevitable biological fate, but rather a modifiable process that can be slowed or partially reversed? A central player repeatedly implicated in this process is the accumulation of senescent cells, frequently referred to as "zombie cells." These cells, although no longer dividing, persistently linger in tissues and release pro-inflammatory factors known as the senescence-associated secretory phenotype (SASP). While senescence initially protects tissues by preventing damaged or potentially malignant cells from proliferating, the chronic accumulation of these inflammatory “zombies” gradually disrupts tissue function and accelerates aging.
Under healthy conditions, the immune system actively surveils and removes senescent cells, thereby limiting their harmful effects. Yet, as individuals age or experience chronic metabolic dysfunction, this immune surveillance weakens, resulting in increased senescent cell burden and sustained inflammation. In response, researchers have turned to senolytics, compounds that selectively clear senescent cells, to restore immune function, mitigate inflammation, and potentially slow or even reverse aspects of aging.
Among recent breakthroughs, a surprising class of candidate senolytics has emerged: SGLT2 (sodium–glucose cotransporter 2) inhibitors, drugs already widely prescribed to manage type 2 diabetes. Originally developed to control blood glucose levels by increasing urinary glucose excretion, these drugs have unexpectedly demonstrated profound benefits beyond their intended metabolic effects—including protection against cardiovascular and kidney diseases and extended lifespan in animal models. Now, according to a landmark study recently published in Nature Aging, SGLT2 inhibitors appear capable of actively enhancing the immune system's clearance of senescent cells, suggesting that their benefits extend far beyond metabolic regulation alone. These findings are particularly significant as they illuminate a novel, indirect mechanism of senescence clearance and position SGLT2 inhibitors as a potentially transformative therapy to address multiple dimensions of aging.
In this review, we will critically analyze the recent Nature Aging study, dissect the underlying mechanisms by which SGLT2 inhibitors enhance senescent cell clearance, and discuss their broader implications for optimizing tissue function, mitigating age-related pathologies, and ultimately extending both longevity and healthspan.
Why Target Senescence? A Central Driver of Aging and Disease
Every tissue in the human body relies on its cells to perform specialized tasks, from repairing wounds to circulating oxygen. When cells become critically damaged or sense oncogenic threats, many enter a state known as senescence. This effectively locks the cell in place—still alive, yet no longer dividing—in order to prevent damaged cells from multiplying and leading to tumor formation. From an evolutionary perspective, senescence is a checkpoint to stop the proliferation and development of malignant cells.
The flip side is that senescent cells accelerate the loss of tissue function and lead to the progression of aging. Because the damaged cell is in a state of arrested development it is allowed to linger in a damaged state. While these non-dividing cells are metabolically active, they no longer contribute to tissue function and, more problematically, secrete a pro-inflammatory cocktail of cytokines, chemokines, and proteases—collectively termed the senescence-associated secretory phenotype (SASP). Over time, these factors can harm neighboring cells or trigger them to become senescent as well, amplifying damage.
Under ideal conditions, the immune system continuously monitors and clears senescent cells, preventing their accumulation. However, as aging progresses—or in individuals with metabolic disorders such as obesity and type 2 diabetes—this clearance mechanism becomes increasingly compromised. Excess glucose and fatty acids exacerbate senescence, particularly in adipose tissue, the liver, and vasculature, overwhelming the immune system's ability to remove these damaged cells. The result is a self-perpetuating cycle of chronic inflammation, which accelerates aging and increases susceptibility to conditions such as cardiovascular disease, neurodegeneration, and insulin resistance.
Given the detrimental role of senescent cells in aging and disease, researchers have turned to senolytic therapies—drugs that selectively eliminate senescent cells while sparing healthy ones. Early efforts have explored:
- Dasatinib + quercetin, a combination that induces apoptosis in senescent cells
- Fisetin, a flavonoid that reduces senescence burden and inflammation in aging mice
- BCL-2 inhibitors, which disrupt survival pathways in senescent cells
These preclinical studies have demonstrated improvements in physical function, reduced inflammation, and enhanced metabolic health—suggesting that targeting senescence may be a viable strategy to mitigate age-related pathologies and extend healthspan.
However, most senolytic therapies act by directly triggering apoptosis in senescent cells, a mechanism that may not fully address the systemic factors contributing to senescence accumulation—particularly metabolic dysfunction and immune decline. This led the researchers behind the Nature Aging study to explore a different hypothesis:
Could SGLT2 inhibitors, a widely used class of diabetes drugs, provide an alternative route to senescent cell clearance—not through direct apoptosis, but by modulating metabolic and immune pathways that restore the body’s natural ability to eliminate these dysfunctional cells?
Why SGLT2 inhibitors? A Drug Class with Unexpected Healthspan Benefits
SGLT2 inhibitors were originally approved by the FDA for the treatment of Type II Diabetes. SGLT2 inhibitors work via a completely different mechanism to other Diabetes drugs such as Metformin and Acarbose, which are biguanides and Alpha-glucosidase inhibitors, respectively. In short, these traditional drugs either (1) inhibit liver glucose production, (2) increase insulin sensitivity, or (3) slow down the digestion of carbohydrates, which all result in drastically improved blood sugar regulation. However, SGLT2 inhibitors are sodium-glucose cotransporter-2 inhibitors, which work by blocking the SGLT2 protein in the kidneys responsible for reabsorbing glucose from the urine back into the bloodstream [2]. By inhibiting SGLT2, these medications increase the excretion of glucose through the urine, effectively lowering blood glucose levels – Notably, it has been estimated that SGLT2 inhibitors can result in up to 70-90 grams of glucose being excreted in your urine per day [3], which is equivalent to the sugar content of 24 oz of Coca Cola.
The National Institute on Aging (NIA) Interventions Testing Program (ITP) has tested >50 molecules over the last 20 years, with only 12 showing a significant increase in lifespan: Acarbose, Aspirin, Astaxanthin, Captopril, Glycine, Meclizine, Nordihydroguaiaretic acid, Protandim, Rapamycin, 16-Hydroxyestriol, 17α-Estradiol, and SGLT2 inhibitors [4]. The first study on SGLT2 inhibitors showed that placing 6-month-old mice (equivalent to a 30-year-old human) on the drug Canagliflozin at a dose of 180 parts per million/day, which equates to ~30mg/kg/day [5]. This dose resulted in a 14% increase in lifespan in male mice but no effect in female mice, which was quite surprising as females had distinctly higher levels of the medication in plasma, brain, and kidney, which likely suggests sex-specific pharmacokinetic differences in drug metabolism. On the contrary, female mice on SGLT2 inhibitors display significantly more weight loss than male mice (19% vs. 8%), which is largely driven by a greater reduction in fat mass, resulting in a much leaner, healthier body composition compared to the control group [5]. As this study was done in "young" mice, it's often tested as to whether these molecules can have an effect when started late in life, as a potential tool for older adults to still gain improvements in metabolic health and lifespan extension.
Since there are such robust increases in lifespan with SGLT2 inhibitors, there has been a large interest in deciphering the mechanism by which they alter any of the Hallmarks of Aging.
Due to the ability of SGLT2 inhibitors to activate several key longevity metabolic pathways, they have recently been described as calorie restriction mimetics, as they, to a certain degree, mimic many of the benefits of calorie restriction without having to restrict your caloric intake by 20-30%. Several recent advances have begun to shed light on these molecular mechanisms, and we'll provide an overview of how SGLT2 inhibitors slow your rate of aging at a molecular level.
Cardiovascular and Kidney Function
Prior to its FDA approval, there was a clinical trial called "The Canagliflozin Cardiovascular Assessment Study (CANVAS)," whose sole purpose was to determine the cardiovascular safety of the SGLT2 inhibitor Canagliflozin. In 2017, data from over 10,000 participants was published in the New England Journal of Medicine [6].
These data showed a ~14% reduced risk of a cardiovascular event, ~27% reduction in albuminuria risk, and a ~40% reduction in estimated glomerular filtration rate, overall showing marked improvements in cardiovascular and kidney health with Canagliflozin [6].
A more recent meta-analysis combining data from six studies totaling nearly 47,000 patients showed similar data, overall suggesting a robust effect of Canagliflozin on reducing cardiac mortality rate and improving kidney health [7].
Similarly, the impact of empagliflozin, an SGLT2 inhibitor, on cardiovascular outcomes and overall mortality in patients with type 2 diabetes at high cardiovascular risk was assessed in the EMPA-REG OUTCOME trial. This study aimed to determine whether empagliflozin, when added to standard care, could reduce cardiovascular morbidity and mortality in this vulnerable population.
A total of 7020 patients were randomly assigned to receive empagliflozin (10 mg or 25 mg) or placebo, administered once daily. The primary composite outcome included death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, evaluated for the pooled empagliflozin groups versus the placebo group. The key secondary composite outcome incorporated the primary outcomes plus hospitalization for unstable angina [8].
After a median follow-up of 3.1 years, the primary composite outcome occurred in 10.5% of patients in the pooled empagliflozin group, compared to 12.1% in the placebo group. Empagliflozin was associated with:
- A 38% reduction in cardiovascular mortality (3.7% vs. 5.9%; HR, 0.62).
- A 35% reduction in hospitalization for heart failure (2.7% vs. 4.1%; HR, 0.65).
- A 32% reduction in all-cause mortality (5.7% vs. 8.3%; HR, 0.68).
One of the primary mechanisms by which SGLT2 inhibitors improve cardiac health is their ability to minimize hypertrophy, decrease cardiovascular inflammation, and prevent the accumulation of fibrosis, thereby leading to improved cardiac structure [9].
These findings raised a compelling question: Could the metabolic and anti-inflammatory benefits of SGLT2 inhibitors extend to cellular senescence?
Since SGLT2 inhibitors have been shown to activate AMPK, shift metabolism toward ketone utilization, and mimic aspects of caloric restriction (CR)—a well-established longevity intervention—researchers began to hypothesize that these drugs might not only slow the accumulation of senescent cells but actively enhance their immune-mediated clearance.
At its core, the Nature Aging study sought to determine whether SGLT2 inhibition could serve as an indirect senolytic strategy, leveraging metabolic and immune reprogramming to reduce the burden of senescence, mitigate age-related inflammation, and optimize tissue function.
From Hypothesis to Experiment: Investigating SGLT2 Inhibitors as a Senescence-Modulating Therapy
The growing body of evidence suggesting that SGLT2 inhibitors confer healthspan-extending benefits raised a critical question: Are these effects mediated, at least in part, through the clearance of senescent cells?
While prior studies established that SGLT2 inhibitors improve cardiovascular and metabolic health, reduce inflammation, and extend lifespan in preclinical models, many of the mechanisms underlying these effects remained unclear. In terms of senescent cell accumulation, do these drugs merely slow the formation of senescent cells, or do they actively enhance their clearance? And if so, is this process driven by metabolic reprogramming, immune surveillance, or a combination of both?
To systematically address these questions, the researchers behind the Nature Aging study designed a series of experiments to:
- Determine whether SGLT2 inhibitors reduce senescent cell burden across multiple tissues
- Identify metabolic pathways, such as AMPK activation, that may mediate senescence clearance
- Investigate the role of immune modulation—particularly T cell and NK cell activation—in facilitating senescent cell removal
- Assess whether SGLT2 inhibition translates into functional improvements in aging-related phenotypes
By integrating genetic models of premature aging, diet-induced metabolic dysfunction, and vascular pathology, the researchers sought to construct a comprehensive framework to evaluate the impact of SGLT2 inhibition on cellular senescence and tissue function.
Methodology & Experimental Design: Investigating SGLT2 Inhibitors as a Senescence-Modulating Therapy
To determine whether SGLT2 inhibitors influence senescent cell burden and aging-related outcomes, researchers employed a multifaceted experimental strategy, incorporating three distinct preclinical models that collectively capture the metabolic, degenerative, and vascular aspects of senescence-driven pathology.
Experimental Models
1. Diet-Induced Obesity Model: Metabolic Stress as a Senescence Accelerator
To assess how metabolic dysfunction contributes to senescence accumulation, researchers employed a high-fat diet (HFD) model—a well-established approach for inducing obesity, insulin resistance, and chronic inflammation.
- Mice were fed HFD for 8–10 weeks, leading to metabolic dysregulation and increased senescent cell burden in adipose and liver tissues.
Following dietary induction of metabolic stress, mice were treated with the SGLT2 inhibitor canagliflozin for either:
- Short-term (7-day) treatment to examine immediate metabolic and immune responses
- Extended (4-week) treatment to evaluate long-term senescence modulation
Control groups included:
- Untreated HFD-fed mice to establish baseline metabolic dysfunction
- Mice receiving insulin therapy, allowing researchers to distinguish between the effects of glucose lowering and senescence modulation
- Key endpoints: Glucose metabolism assessments, inflammatory cytokine profiling, and senescence markers (SA-β-gal, p53, Cdkn2a) in adipose and liver tissue.
2. Premature Aging Model: Zmpste24 Knockout Mice as a Progeroid System
To determine whether SGLT2 inhibition mitigates aging-related degeneration independent of metabolic dysfunction, researchers utilized Zmpste24 knockout (KO) mice, a model of Hutchinson-Gilford Progeria Syndrome (HGPS)—a condition characterized by accelerated cellular senescence, growth retardation, osteoporosis, cardiovascular decline, and early mortality.
- Zmpste24 KO mice were treated with canagliflozin or a control vehicle starting at 12 weeks of age.
- Researchers monitored lifespan, physical function (mobility, grip strength, rotarod performance), and senescence burden across multiple tissues.
This model enabled assessment of whether SGLT2 inhibitors exert geroprotective effects even in the absence of metabolic stress.
3. Atherosclerosis-Prone Model: Senescence in Vascular Aging and Cardiovascular Disease
To evaluate the impact of SGLT2 inhibition on vascular senescence, researchers employed Apolipoprotein E-knockout (ApoE-KO) mice, which are genetically predisposed to atherosclerosis when fed a Western diet high in cholesterol.
- ApoE-KO mice were placed on a Western diet for 12 weeks, followed by a 2-week treatment with canagliflozin.
Researchers analyzed whether SGLT2 inhibition:
- Reduces senescent cell burden in aortic plaques
- Modulates inflammatory signaling in vascular tissues
- Improves functional markers of vascular health
These three complementary models provided a framework for examining how SGLT2 inhibitors impact both metabolic and non-metabolic drivers of senescence.
Treatment Conditions & Controls
SGLT2 Inhibitor Canagliflozin
- Administered via laboratory chow (typically at 0.03% w/w) over treatment durations ranging from one to multiple weeks.
- Glucose metabolism and body weight were closely monitored to confirm that the drug was exerting systemic metabolic effects.
Comparison with Insulin Therapy
- To assess whether glucose lowering alone was sufficient to reduce senescent cell burden, a subset of mice received exogenous insulin therapy.
- This allowed researchers to differentiate the effects of SGLT2 inhibition from those of insulin-driven glucose normalization.
Dietary Switch to Normal Chow
In some HFD-fed groups, the diet was replaced with standard chow to test whether improving metabolic status alone could replicate the senolytic effects observed with SGLT2 inhibition.
Endpoints Measured
To fully characterize the effects of SGLT2 inhibition on senescence, researchers assessed a range of cellular, molecular, and functional markers:
Metabolic Assessments
- Glucose tolerance tests (GTT) & insulin tolerance tests (ITT) to evaluate metabolic function.
- Plasma glucose, insulin, and lipid profiles to determine systemic metabolic adaptations.
Senescence Markers
- SA-β-gal activity, a widely used histological marker of senescence.
- Tumor suppressor proteins (p53, p21) and Cdkn2a, which regulate the senescence-associated cell cycle arrest.
Inflammatory Cytokines
- TNF and CCL2 levels as indicators of senescence-associated inflammation.
Immune Modulation (PD-L1 Expression & T Cell Activity)
- Flow cytometry analysis of PD-L1 expression on senescent cells, as PD-L1 upregulation is known to protect senescent cells from immune clearance.
- T cell and NK cell activity to assess whether SGLT2 inhibitors reawaken immune surveillance of senescent cells.
Lifespan and Physical Health
- Survival curves, mobility, grip strength, and rotarod endurance to evaluate whether senescent cell clearance translates into functional benefits.
Additional Tools and Techniques
Matrigel Transplantation Assays
- Researchers embedded senescent cells in a gel matrix and transplanted them into mice, allowing them to observe how well the immune system cleared senescent cells under different treatment conditions.
Luciferase-Based Reporter Systems
- Some genetically engineered mice carried reporters that glow in the presence of senescent cells, providing a noninvasive method to track senescence accumulation or clearance over time.
Flow Cytometry for Immune Profiling
- Researchers used fluorescence-activated cell sorting (FACS) to examine immune cell populations, measuring changes in T cell and NK cell activity following SGLT2 inhibition.
By integrating multiple preclinical models, varied treatment durations, and comprehensive cellular and functional assessments, this study provided a robust framework to investigate whether SGLT2 inhibitors function as senescence-targeting therapies.
The findings from this research have significant implications for understanding how metabolic interventions influence aging and raise intriguing questions about whether SGLT2 inhibitors might be repurposed as geroprotective agents.
In the following section, we will examine the key findings from these experiments, uncovering how SGLT2 inhibition impacts senescent cell dynamics, immune function, and systemic aging phenotypes.
The Results
Reduction in Senescent Cells
Despite no significant differences in body weight, fat mass, or food intake, mice treated with canagliflozin exhibited substantial improvements in glucose metabolism and insulin sensitivity compared to untreated controls. Notably, these metabolic benefits persisted even after the drug had been fully cleared from their system, suggesting that SGLT2 inhibition induces lasting physiological adaptations beyond acute glucose lowering.
However, the most striking effects were observed at the cellular level. Tissue analysis revealed a significant reduction in senescent cell burden, particularly in visceral adipose tissue and liver—two metabolically active sites prone to senescence accumulation in the context of aging and metabolic dysfunction.
Further examination confirmed that mice receiving canagliflozin displayed markedly lower levels of senescence-associated β-galactosidase (SA-β-gal) activity, a widely used histological marker of senescence. Additionally, expression of key cell cycle arrest regulators, including p53 and Cdkn2a, was significantly reduced in treated animals. These findings indicate a notable decline in the presence of senescent cells within these tissues.
Alongside the reduction in senescence markers, pro-inflammatory factors associated with the senescence-associated secretory phenotype (SASP) were also significantly diminished. Since SASP factors are known to drive chronic inflammation, immune dysfunction, and tissue degradation, their suppression further supports the idea that SGLT2 inhibition may act as a senescence-targeting intervention, reducing both cellular burden and the systemic inflammatory signaling that accompanies it.
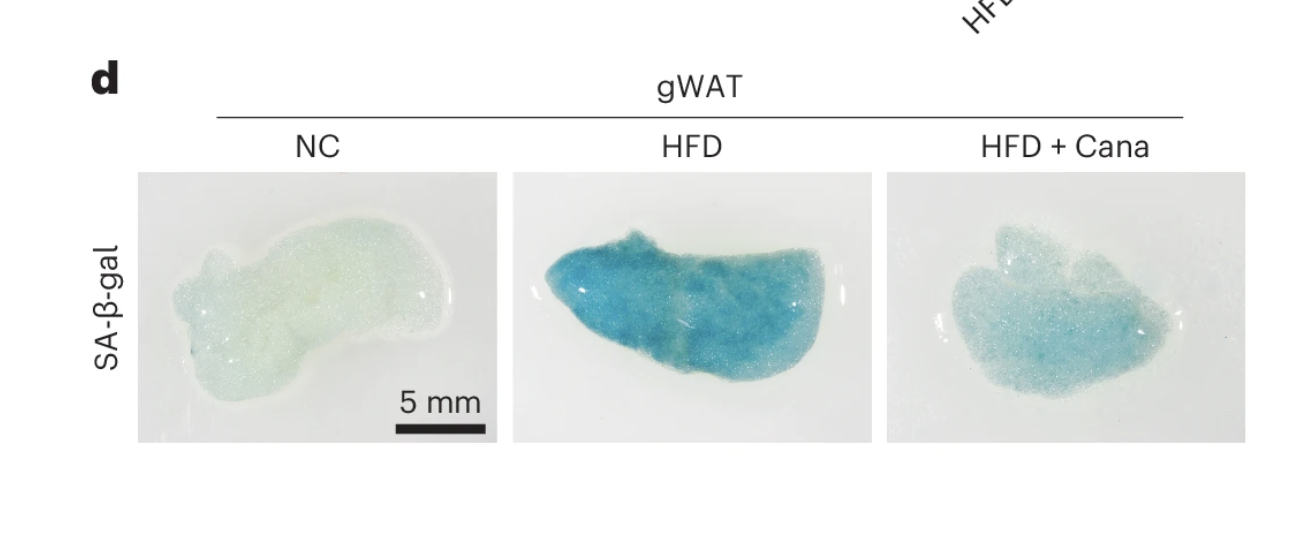
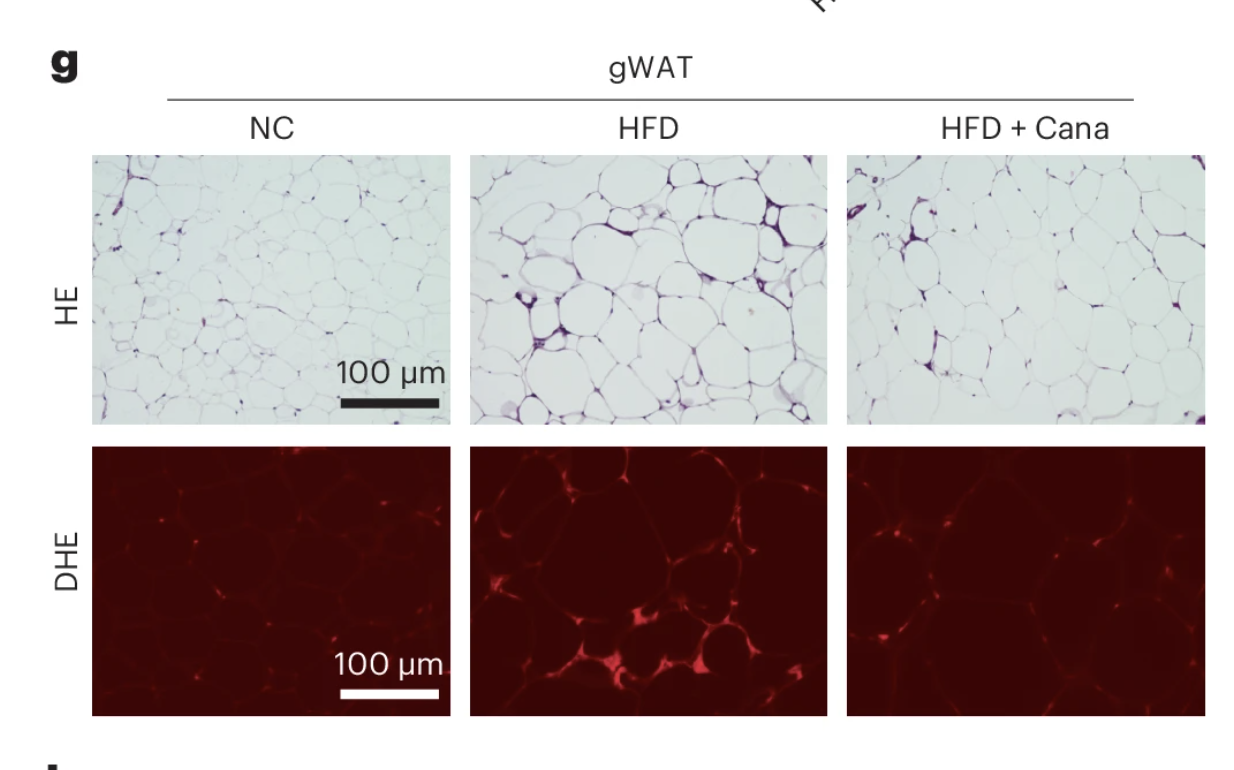
Reduction in Adipose Tissue Inflammation
In addition to lowering senescent cell burden, canagliflozin treatment led to a marked reduction in adipose tissue inflammation, a hallmark of metabolic dysfunction and aging. One of the most notable findings was the diminished presence of crown-like structures—clusters of immune cells that accumulate around dying or dysfunctional fat cells, serving as a key indicator of chronic inflammation in adipose tissue.
After four weeks of treatment, these anti-inflammatory effects became even more pronounced. Senescence markers remained significantly reduced, and the suppression of inflammatory signaling persisted, suggesting that SGLT2 inhibition induces a sustained shift in tissue homeostasis rather than a transient effect. Importantly, these improvements occurred without any measurable impact on body weight, reinforcing the idea that the benefits of canagliflozin extend beyond simple metabolic regulation and involve deeper cellular and immune-mediated mechanisms.
These findings provide further evidence that SGLT2 inhibitors not only regulate glucose metabolism but also exert direct effects on senescence-driven inflammation, which may have implications for targeting adipose tissue dysfunction in aging and metabolic disease.
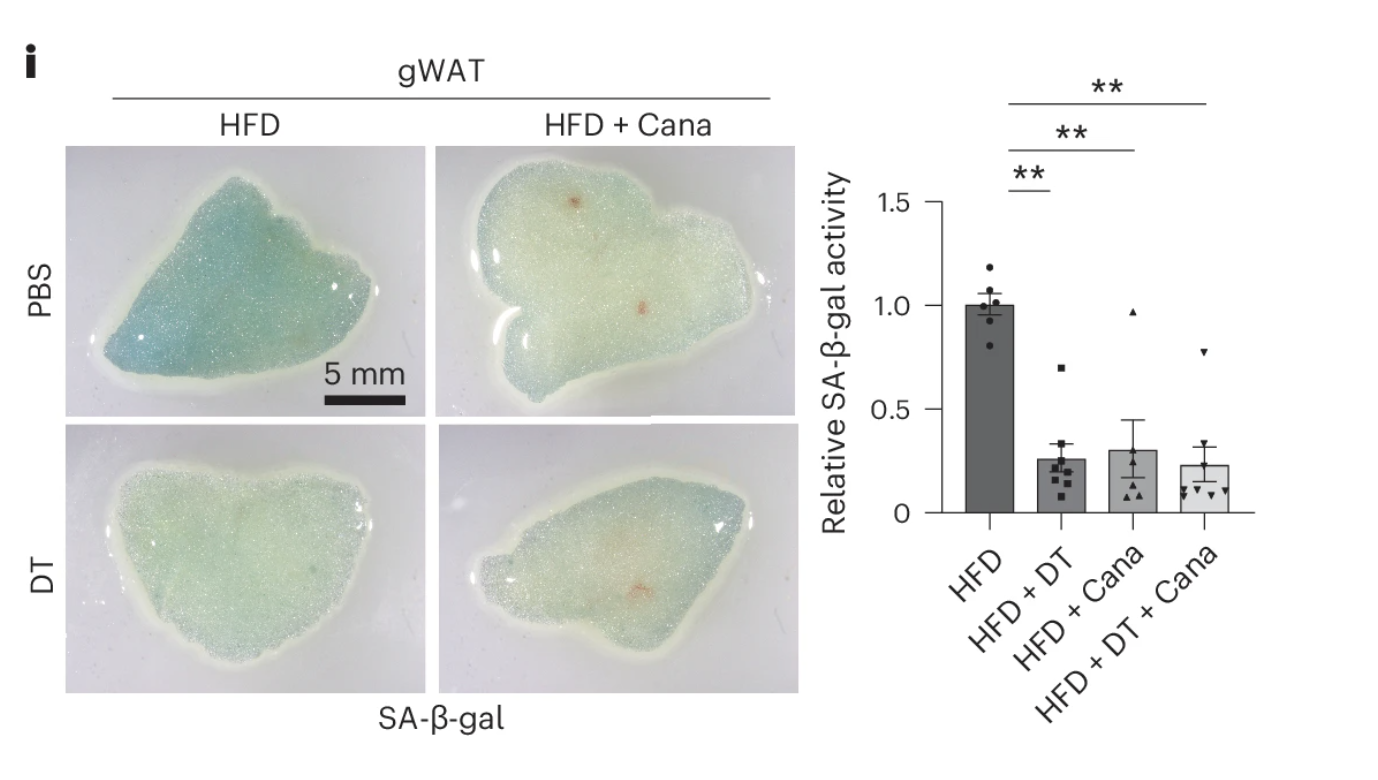
To determine whether canagliflozin actively eliminates senescent cells or merely alters their behavior, researchers employed a specialized transgenic mouse model engineered to selectively track and destroy senescent cells. These mice were genetically modified to express a diphtheria toxin (DT) receptor on senescent cells, allowing for their precise and controlled elimination upon DT administration.
To test the effects of SGLT2 inhibition on senescence burden, mice were first placed on a high-fat diet (HFD) to accelerate senescent cell accumulation. They were then divided into groups receiving either canagliflozin, DT, or a combination of both treatments.
The results were striking: mice treated with canagliflozin alone exhibited a reduction in senescent cell burden nearly identical to those receiving DT, indicating that the drug was not merely suppressing senescent cell activity but actively facilitating their clearance. Furthermore, adding DT to canagliflozin-treated mice provided no additional benefit, suggesting that the majority of senescent cells had already been eliminated by SGLT2 inhibition alone.
These findings provide compelling evidence that canagliflozin functions as a senolytic therapy, effectively reducing the accumulation of senescent cells without the need for direct pharmacological apoptosis inducers. The question that remains is how this clearance is achieved—whether through immune surveillance, metabolic stress responses, or a combination of mechanisms, a subject explored in subsequent analyses.
Insulin Lowers Blood Sugar—But Leaves Aging Cells Behind
At first glance, it might seem logical to assume that canagliflozin’s ability to clear senescent cells is simply a side effect of its ability to lower blood sugar. After all, chronically high blood glucose is known to accelerate cellular aging, so wouldn’t reducing it—whether through SGLT2 inhibition or insulin therapy—be enough to reverse these effects?
To put this idea to the test, researchers gave high-fat diet (HFD)-fed mice a week-long course of insulin treatment, which successfully improved their glucose metabolism, just as canagliflozin had. However, when scientists examined the fat tissue of these mice, a key difference emerged: insulin had no impact on senescent cell burden.
Despite better glucose control, markers of cellular senescence—like SA-β-gal activity and inflammatory signals associated with SASP—remained unchanged in the insulin-treated mice. Even extending the treatment for four weeks failed to make a difference. While insulin effectively restored glucose metabolism and insulin sensitivity, it did nothing to reduce the senescence-driven inflammation lurking in visceral fat.
To further explore whether glucose control alone could influence senescent cells, the researchers tried another experiment: instead of using insulin or canagliflozin, they simply switched the mice from a high-fat diet back to normal chow. This dietary shift lowered blood sugar and slightly reduced body weight, but once again, it failed to clear senescent cells. The stubborn markers of aging cells remained just as high as in the high-fat diet group.
These findings reveal a critical distinction: not all glucose-lowering interventions are created equal. While both insulin and dietary changes successfully normalized metabolism, neither had the senolytic effects seen with canagliflozin. This suggests that canagliflozin’s ability to clear aging cells isn’t simply due to better blood sugar control—but is instead driven by a distinct, glucose-independent mechanism.
The unanswered question now is what sets canagliflozin apart? Could it be activating cellular stress responses, modulating immune clearance, or reprogramming metabolic pathways to facilitate senescent cell elimination?
Understanding the Mechanisms
Digging deeper, the researchers uncovered a two-pronged mechanism of senolysis—one that goes beyond the usual glucose-lowering effect:
AMPK Activation
If SGLT2 inhibition helps remove senescent cells, how exactly does it work?
One possibility was that the drug directly kills senescent cells—but when researchers exposed cultured human cells to canagliflozin in the lab, nothing happened. Even at high concentrations, the drug failed to eliminate aging cells on its own. This suggested that canagliflozin's senolytic effects in living organisms must involve an indirect mechanism.
To uncover the mystery, scientists turned to metabolomics—a method of analyzing biochemical changes in the body. When they examined blood samples from high-fat diet (HFD)-fed mice treated with canagliflozin, they found a striking clue: elevated levels of a molecule called AICAR. This compound is known to activate AMPK (AMP-activated protein kinase)—a key energy sensor in cells that helps regulate metabolism and stress responses. Sure enough, AMPK activity was significantly increased in fat and liver tissues of canagliflozin-treated mice.
To test whether AICAR itself could help clear senescent cells, researchers injected mice with AICAR for a week. The results were remarkably similar to those seen with canagliflozin: senescent cell markers dropped significantly—even though body weight and fat mass remained unchanged.
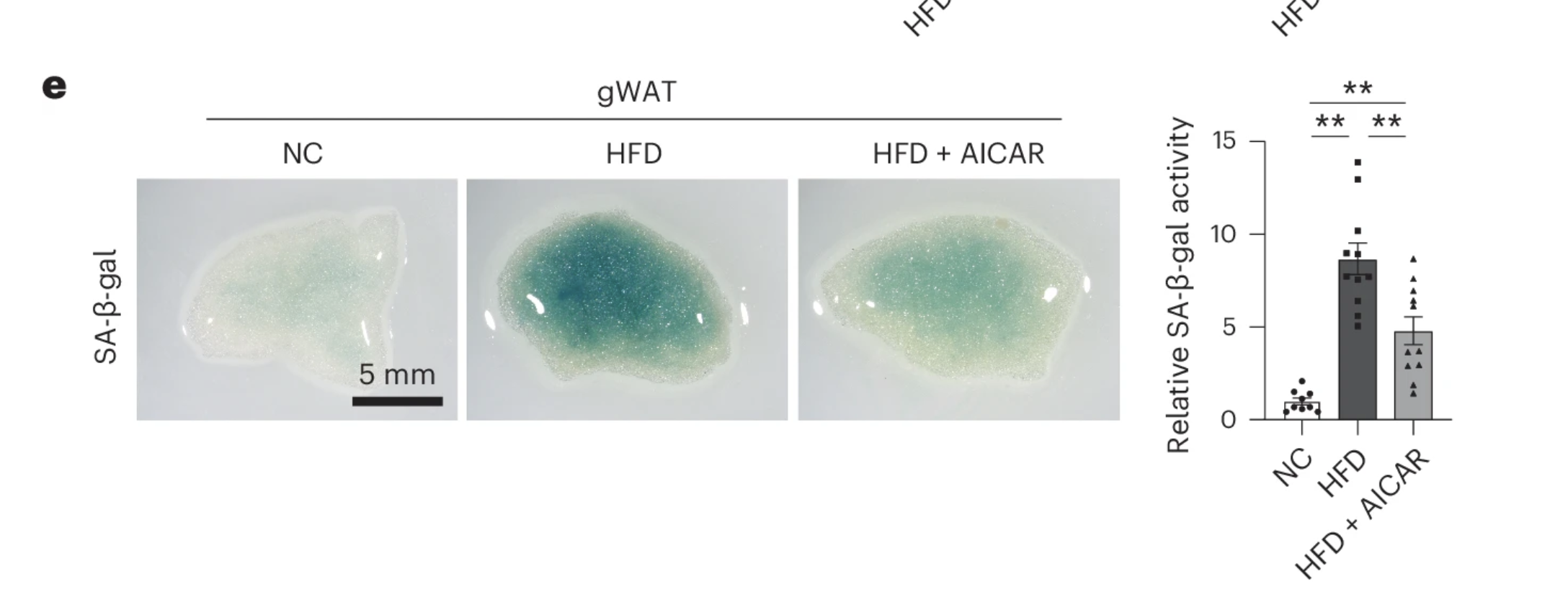
But was AMPK activation really the key to canagliflozin’s effects? To find out, the scientists blocked AMPK activity using a compound called Compound C in a group of canagliflozin-treated mice. The result? The senolytic benefits disappeared. Even though the mice continued to receive canagliflozin, their senescent cell burden shot back up—suggesting that AMPK activation is a critical link between SGLT2 inhibition and senescent cell clearance.
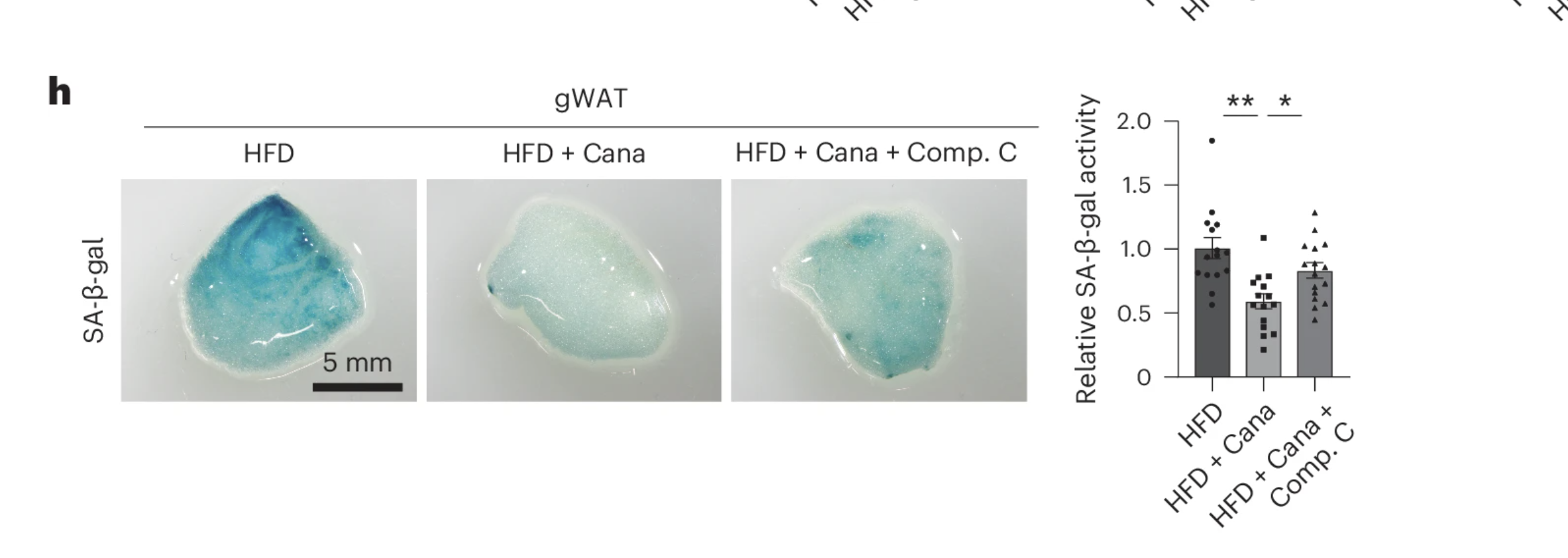
These findings point to an intriguing possibility: SGLT-2 inhibition doesn’t kill senescent cells directly—it kickstarts the body’s own metabolic defense mechanisms, helping tissues eliminate aging cells through an AMPK-dependent pathway. This indirect approach could have far-reaching implications, not just for treating metabolic disorders, but for slowing the accumulation of aging cells that drive chronic disease.
Yet, while AMPK activation clearly plays a central role, senescent cells are not passive targets. Many have evolved strategies to evade immune clearance, which raises another critical question: Does canagliflozin also enhance the immune system’s ability to recognize and eliminate these cells?
How Canagliflozin Helps the Immune System Target Aging Senescent Cells
Senescent cells don’t just sit idly in aging tissues—they actively disrupt the immune system’s ability to remove them. Normally, the body has built-in mechanisms to identify and clear out these aging cells before they accumulate and cause harm. But as we age, this system falters. Instead of being eliminated, senescent cells persist, releasing inflammatory molecules that fuel chronic disease. Scientists call this breakdown in immune surveillance anergy—a state where the immune system becomes blind to senescent cells.
One major contributor to this immune dysfunction is PD-L1 (programmed death-ligand 1), a well-known immune checkpoint molecule. Tumors frequently exploit PD-L1 to escape immune detection, and recent studies suggest that some senescent cells employ a similar strategy. By expressing PD-L1 on their surface, these cells inhibit T cell and natural killer (NK) cell activity, preventing immune-mediated clearance.
Even more concerning, the senescent cells with the highest PD-L1 expression also produce greater amounts of inflammatory SASP factors, making them even more damaging.
So, could SGLT2 inhibition help strip away this protective shield and restore immune surveillance?
To find out, researchers examined how canagliflozin affects PD-L1 expression in senescent cells. Their first clue came from an earlier finding: AMPK activation—triggered by AICAR—was already known to suppress PD-L1 expression. Since canagliflozin also activates AMPK, the researchers wondered if this might be part of its senolytic effect.
Their experiments confirmed this hypothesis. In high-fat diet (HFD)-fed mice, the number of PD-L1-expressing senescent cells increased dramatically.
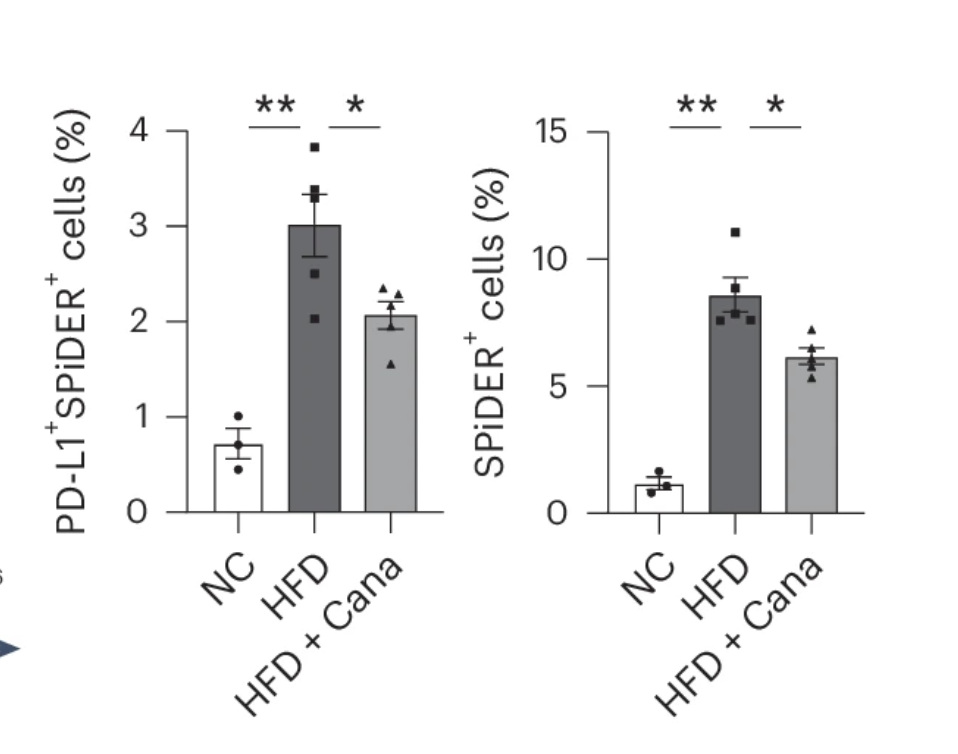
But after just a short course of canagliflozin treatment, those numbers dropped significantly. Meanwhile, a closer look at the immune system revealed another shift: the numbers of natural killer (NK) cells and CD8+ T cells—two key players in immune surveillance—were markedly higher in canagliflozin-treated mice. These immune cells had been depleted in the HFD-fed mice, suggesting that canagliflozin was restoring their presence, likely by removing PD-L1’s suppressive influence.
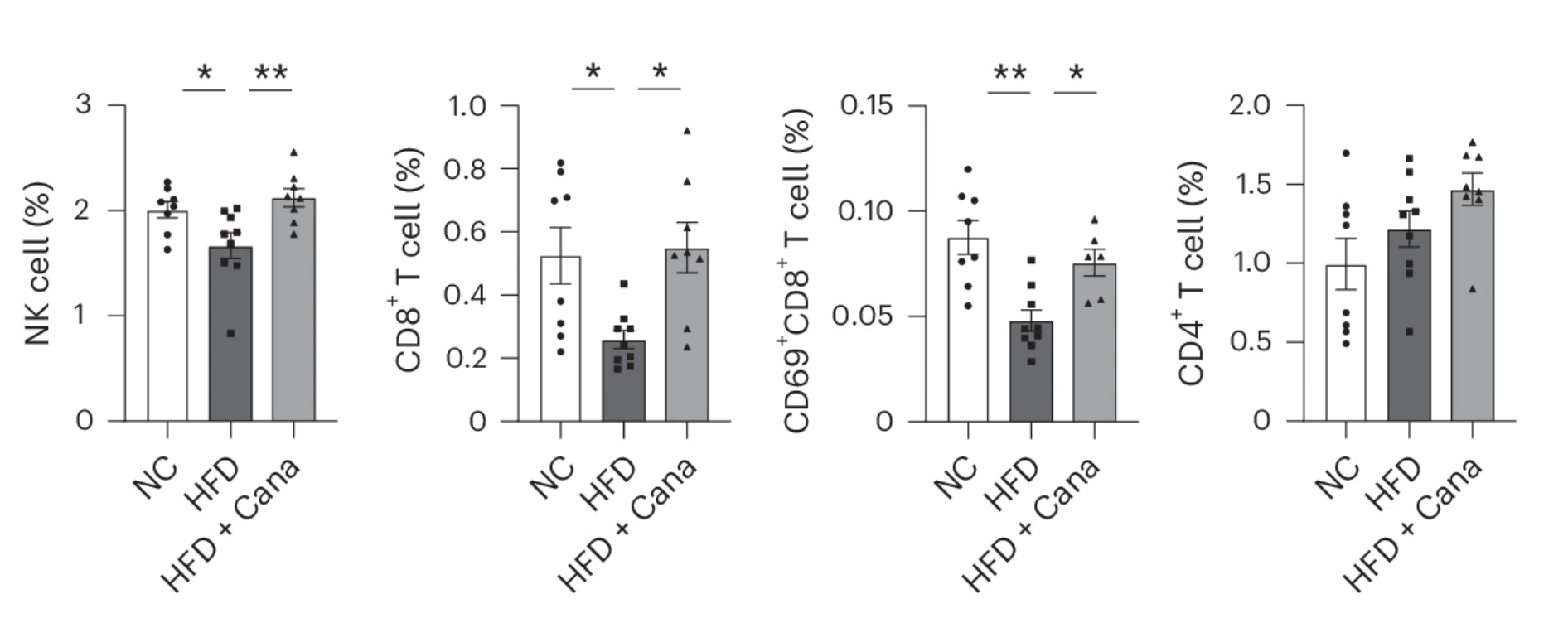
Further experiments confirmed that AMPK activation was driving this effect. When researchers treated mice with AICAR—an AMPK activator—PD-L1 expression on senescent cells dropped just as it did with canagliflozin. But when they blocked AMPK activity with Compound C, the effect vanished. Canagliflozin could no longer reduce PD-L1 expression or restore immune cell numbers.
These findings reveal an important new mechanism: canagliflozin doesn’t just help clear senescent cells—it also “reawakens” the immune system’s ability to recognize and remove them. By suppressing PD-L1 expression, the drug lifts the immune system’s blindfold, allowing it to do what it was meant to do: eliminate aging cells before they cause harm.
Canagliflozin’s Senolytic Effect Relies on the Immune System—Specifically, T Cells
While canagliflozin clearly facilitates senescent cell clearance, researchers sought to determine whether this effect was dependent on the immune system or occurred through an alternative mechanism. Given the emerging role of T cells in immune-mediated senescence surveillance, they hypothesized that these immune cells might be critical for canagliflozin’s senolytic activity.
To test this, they selectively suppressed T cell activity by administering a CD3-neutralizing antibody, which prevents T cells from functioning normally. The results were striking: once T cells were inhibited, the senolytic effects of canagliflozin were significantly blunted. The reduction in senescent cell burden was markedly diminished, suggesting that the drug does not act independently but relies on an active immune response to facilitate senescence clearance.
These findings further reinforce that canagliflozin does not directly kill senescent cells. Instead, it appears to enhance immune recognition and clearance, enabling T cells to efficiently target and remove senescent cells that would otherwise persist.
Tracking Senescent Cells in Real Time: Visualizing Immune Clearance
To directly observe how the immune system responds to senescent cells in the presence of canagliflozin, researchers employed a fluorescent tracking system. They used fibroblasts derived from genetically modified CAG-tdTomato reporter mice, which express a red fluorescent protein (tdTomato), allowing their fate to be tracked in vivo.
These fibroblasts were exposed to radiation to induce cellular senescence and then embedded in a Matrigel matrix, which was subsequently transplanted into wild-type mice. As a control, nonsenescent fluorescent fibroblasts were also implanted in the same mice.
The results provided direct evidence of selective senescent cell clearance:
- Senescent cells were eliminated, while healthy cells remained intact.
- Canagliflozin-treated mice exhibited more efficient clearance of senescent cells, suggesting that the drug enhances the immune system’s ability to recognize and remove these dysfunctional cells.
This experiment confirmed that the body possesses an intrinsic ability to remove senescent cells—but that this process can be enhanced through metabolic and immune modulation.
AMPK: The Key to Senescent Cell Clearance
Finally, to confirm whether AMPK activation was essential for canagliflozin’s senolytic effect, researchers introduced another layer to the experiment. They took the same fluorescently labeled senescent cells but genetically suppressed AMPK activity in them using a technique called shRNA knockdown. Then, they implanted these modified senescent cells into mice and treated them with canagliflozin.
The results were dramatic: when AMPK was suppressed, canagliflozin’s ability to clear senescent cells completely disappeared. Even though the drug was still circulating, the senescent cells remained untouched.
These findings offer an important insight: canagliflozin doesn’t just eliminate senescent cells—it recruits the immune system to do the job. By activating AMPK, it helps trigger T cells to recognize and remove aging cells. Without AMPK—or without functional T cells—the drug’s effects are dramatically reduced.
Canagliflozin and Aging Arteries: A Surprising Effect on Atherosclerosis
Aging takes a toll on the cardiovascular system, and atherosclerosis—the buildup of fatty plaques in arteries—is one of the most dangerous consequences. Over time, these plaques harden and narrow blood vessels, increasing the risk of heart attacks and strokes. But scientists are beginning to realize that these plaques don’t just accumulate fat—they also harbor senescent cells, which fuel inflammation and worsen disease progression.
To test whether canagliflozin could help clear senescent cells in atherosclerotic plaques, researchers turned to a well-established model: ApoE-knockout (ApoE-KO) mice, which lack a key protein involved in fat metabolism and rapidly develop arterial plaques when fed a high-fat western diet (WD).
After 12 weeks on the WD, the mice were given canagliflozin for just two weeks. Interestingly, despite its well-known metabolic effects, the drug did not alter body weight, blood glucose, or lipid levels. Cholesterol, triglycerides, and free fatty acids remained unchanged—suggesting that whatever was happening wasn’t due to improved metabolic function alone.
But when scientists examined the aortas of these mice, they found something striking:
- Markers of senescence, like SA-β-gal activity and Cdkn1a expression, were significantly lower.
- The plaques themselves were smaller, suggesting that canagliflozin helped slow or even reverse disease progression.
- Inflammatory signals were reduced, reinforcing the idea that senescent cells contribute to chronic vascular inflammation.
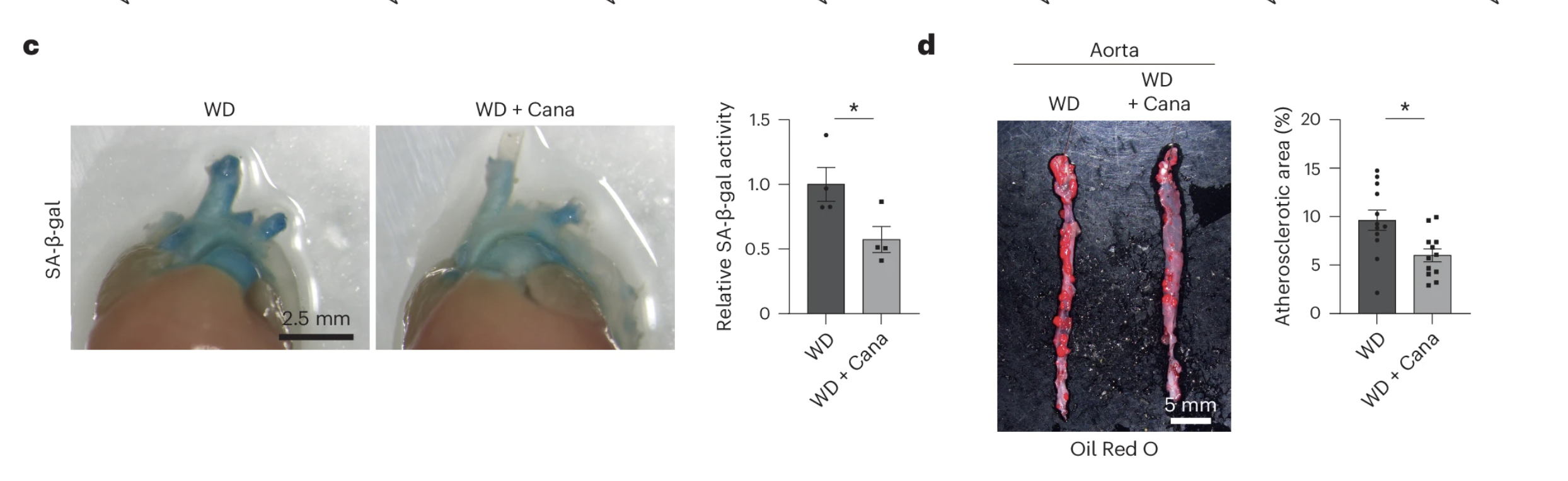
To confirm that these improvements were linked to senescent cell clearance, researchers used fluorescence-activated cell sorting (FACS) to identify senescent cells within the plaques. The results showed that canagliflozin-treated mice had significantly fewer senescent cells in their arterial walls—a clear indication that the drug was working beyond just metabolic regulation.
SGLT2 Inhibition and Premature Aging: A Lifespan-Extending Effect?
Aging is inevitable—but what if it could be slowed, or even reversed? Scientists exploring the biology of aging have long studied Hutchinson–Gilford progeria syndrome (HGPS), a rare genetic disorder that causes accelerated aging in children. Patients with HGPS develop early-onset muscle weakness, osteoporosis, and cardiovascular disease, and most succumb to complications in their teens. At the root of this disorder is a mutation in the LMNA gene, which disrupts the production of lamin A, a protein essential for nuclear stability.
To better understand premature aging, researchers use a mouse model of progeria, known as Zmpste24 knockout (KO) mice. These mice lack Zmpste24, an enzyme responsible for processing lamin A, and as a result, they develop hallmarks of aging—including growth defects, frailty, and a shortened lifespan.
Could canagliflozin—a drug already known to clear senescent cells—help extend the lives of these prematurely aging mice? To find out, researchers treated Zmpste24 KO mice with canagliflozin starting at 12 weeks of age and tracked their survival. The results were compelling: treated mice lived significantly longer than untreated controls.
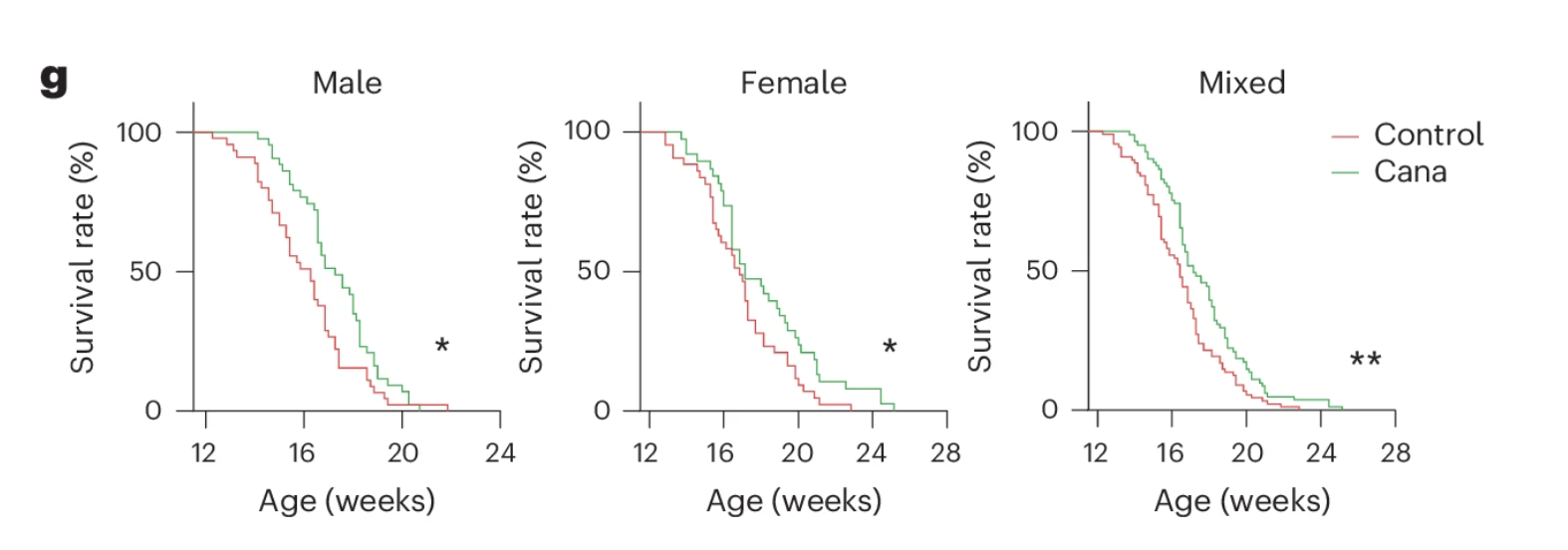
But what about aging in otherwise healthy individuals? To test whether canagliflozin could combat normal aging, the researchers administered the drug to middle-aged wild-type mice (50 weeks old) for 20 weeks and looked at key markers of functional decline. The findings were striking:
- Treated mice were stronger, showing improved grip strength and better performance on a rotarod test, a measure of motor coordination and endurance.
- Senescence markers in fat tissue (SA-β-gal activity) were significantly reduced, suggesting that canagliflozin was actively removing aging cells.
These results hint at a broader role for SGLT2 inhibitors beyond diabetes management. In both genetically accelerated aging and normal aging, canagliflozin extended lifespan, preserved physical function, and reduced the burden of senescent cells.
Strengths of the Study
- Multiple Mouse Models: One of the most commendable aspects of this research is its broad approach. By testing canagliflozin across a high-fat diet model, an atherosclerosis-prone model (ApoE-KO), and a progeria-like model (Zmpste24 KO), the investigators revealed how SGLT2 inhibition impacts senescence under several stress conditions. This multiplatform strategy boosts confidence that the drug’s effects aren’t limited to just one metabolic quirk.
- Mechanistic Clarity: Rather than merely stating that canagliflozin “worked” to diminish senescent cells, the team pinpointed a plausible mechanism. By tying the improvement to AMPK activation and a reduction in immune checkpoint molecules like PD-L1, they strengthened the argument for SGLT2 inhibitors acting as indirect senolytics.
- Direct Comparison With Insulin: The side-by-side testing of insulin allowed the authors to distinguish the unique value of canagliflozin. If the benefits simply boiled down to lowering blood sugar, insulin would have mirrored the effect. The fact that it didn’t underscores the central role of metabolic reprogramming and improved immune clearance, rather than mere glucose reduction.
Potential Limitations
- Sex Differences: Most experiments involved male mice, leaving open the question of whether female physiology might respond differently to SGLT2 inhibition. We know from other studies that hormonal differences can affect both metabolism and immune processes. Future work that includes balanced cohorts or focuses specifically on sex-based comparisons could refine the therapy’s relevance.
- Translational Hurdles: While SGLT2 inhibitors are already used in humans for diabetes, it remains uncertain whether the specific “senolytic” or immune-activating benefits documented in mice will translate directly to people—especially older adults. Age, pre-existing conditions, and genetic diversity might alter the treatment outcome.
- Narrow Focus on Certain Tissues: Although the team examined visceral fat, liver, and vascular tissue, other organs (like the brain or skeletal muscle) might harbor distinct patterns of senescence. Additional, organ-specific investigations could reveal new layers of complexity or highlight side effects not captured in this study.
The Potential for Combination Therapies: Synergizing Senolytics and Longevity Interventions
While the senolytic properties of SGLT2 inhibitors are promising on their own, an even more compelling prospect lies in their potential synergy with existing longevity interventions. Aging is a complex, multifactorial process, influenced by metabolic dysfunction, immune system decline, mitochondrial inefficiency, and cellular senescence. Given this complexity, it is unlikely that a single intervention will provide a comprehensive solution to aging. Instead, strategically combining multiple therapies that target distinct, yet interconnected, aging pathways may enhance overall efficacy while minimizing potential trade-offs.
Could SGLT2 Inhibitors Enhance Existing Senolytic Therapies?
Among the most well-characterized senolytic regimens is the combination of dasatinib (a tyrosine kinase inhibitor) and quercetin (a flavonoid with antioxidant properties), which has demonstrated significant efficacy in clearing senescent cells and improving physical function in both preclinical and early clinical studies. However, traditional senolytics such as dasatinib act by directly inducing apoptosis in senescent cells, often requiring intermittent, high-dose administration to minimize toxicity.
SGLT2 inhibitors, by contrast, appear to enhance immune-mediated senescence clearance rather than directly triggering apoptosis. This distinction raises an intriguing possibility: Could canagliflozin and other SGLT2 inhibitors work synergistically with existing senolytics, enabling lower, safer doses of cytotoxic agents like dasatinib while enhancing overall senescent cell clearance?
Rather than directly forcing senescent cells into apoptosis, canagliflozin might prime the immune system to recognize and clear them more effectively by modulating PD-L1 expression and restoring immune surveillance mechanisms. If validated, this approach could shift the paradigm of senolytic therapy from direct pharmacological elimination to a more immune-centered strategy, reducing the risks associated with high-dose senolytic regimens.
Rapamycin, Metformin, and Caloric Restriction: A Metabolic Longevity Stack?
Beyond senolytics, SGLT2 inhibitors share mechanistic overlap with key metabolic interventions known to extend lifespan. This raises important questions about whether they could be effectively combined with well-known longevity-promoting drugs such as rapamycin and metformin—or even function as caloric restriction mimetics.
Rapamycin: Balancing mTOR and AMPK
One of the most extensively studied longevity-enhancing compounds, rapamycin inhibits the mechanistic target of rapamycin (mTOR), a central regulator of cellular growth and metabolism. mTOR inhibition has been shown to extend lifespan in multiple species by promoting autophagy, reducing protein synthesis, and suppressing pro-inflammatory signaling.
In contrast, SGLT2 inhibitors activate AMPK (AMP-activated protein kinase), a key sensor of cellular energy balance. While mTOR and AMPK are often counter-regulatory, they also play complementary roles—mTOR inhibition slows anabolic growth pathways, while AMPK activation enhances energy conservation and stress adaptation.
Given these complementary mechanisms, combining low doses of rapamycin and an SGLT2 inhibitor may create an ideal balance, simultaneously optimizing metabolic health, enhancing autophagy, and boosting immune surveillance against senescent cells. Healthspan is currently analyzing detailed metabolic data to evaluate whether co-administration of these agents yields synergistic effects beyond either drug alone, potentially establishing a powerful, integrated approach to extending longevity and improving healthspan. This ongoing analysis aims to determine precisely how the unique metabolic pathways targeted by rapamycin and SGLT2 inhibitors interact, laying the foundation for novel combination therapies that could provide greater healthspan and longevity benefits than either intervention alone.
Metformin: AMPK Activation and Metabolic Synergy
Metformin, another widely used diabetes drug, has been proposed as a longevity-enhancing agent due to its AMPK activation, anti-inflammatory effects, and potential ability to suppress age-related metabolic decline. Given that canagliflozin also activates AMPK, the question arises: Would combining the two offer additive benefits, or would their mechanisms overlap, leading to redundancy?
Some studies suggest that SGLT2 inhibitors may reduce the risk of lactic acidosis, a rare but serious side effect associated with metformin. If true, this raises the possibility that co-administration of metformin and SGLT2 inhibitors could improve tolerability, particularly in aging individuals prone to metabolic dysregulation.
Further, metformin’s effects on gut microbiota composition and mitochondrial function differ from those of SGLT2 inhibitors, suggesting that combining the two could engage multiple, non-redundant longevity pathways.
Caloric Restriction (CR): Mimicking the Longevity Benefits of Nutrient Restriction
One of the most well-established longevity interventions, caloric restriction (CR) extends lifespan across species by reducing metabolic stress, lowering systemic glucose levels, and enhancing autophagy. Interestingly, SGLT2 inhibitors appear to mimic some of these effects, shifting metabolism toward fat oxidation and ketone production while reducing overall glucose availability.
Could SGLT2 inhibitors function as CR mimetics for individuals unable or unwilling to adhere to chronic caloric restriction? While direct evidence remains limited, this idea presents an intriguing avenue for future research. If SGLT2 inhibitors can trigger some of the same cellular stress adaptations as CR—without the need for prolonged nutrient restriction—they may serve as a practical intervention for promoting metabolic resilience in aging populations.
Toward a Multi-Targeted Longevity Strategy
Stacking SGLT2 inhibitors with senolytics, rapamycin, metformin, or caloric restriction regimens could yield a powerful anti-aging protocol, engaging multiple protective pathways at once. Such an approach would target:
- Cellular senescence (via immune clearance and apoptosis induction)
- Metabolic dysfunction (through AMPK activation and glucose modulation)
- Chronic inflammation (by suppressing SASP signaling and restoring immune function)
- Mitochondrial health (through increased fatty acid oxidation and autophagy)
While the theoretical basis for these synergistic interactions is strong, further research is needed to determine optimal dosing strategies, safety considerations, and whether these combinations translate to improved longevity outcomes in humans.
As the field of geroscience advances, the prospect of integrating metabolic interventions, immune modulation, and senescence-targeting therapies represents an exciting step toward a comprehensive, multi-targeted approach to extending healthspan and delaying age-related disease.
Conclusion
The analysis presented in this review highlights a promising, novel mechanism by which SGLT2 inhibitors—originally developed as antidiabetic agents—may modulate aging and improve healthspan through the indirect clearance of senescent cells. By activating AMPK-mediated metabolic pathways and enhancing immune surveillance via suppression of PD-L1 expression, SGLT2 inhibitors facilitate the clearance of senescent cells, mitigating inflammation and preserving tissue integrity. The evidence indicates that the effects of these drugs extend beyond mere glucose-lowering, implicating a unique convergence of metabolic and immune mechanisms in the management of senescence-driven pathology.
These insights underscore the potential for repurposing SGLT2 inhibitors as part of broader anti-aging therapeutic regimens. Furthermore, the distinct and indirect senolytic mechanisms uncovered through this analysis suggest the exciting possibility of combining SGLT2 inhibitors with other longevity interventions, such as rapamycin or established senolytics, to synergistically enhance healthspan outcomes.
Although further translational studies are necessary to confirm these findings in human populations and establish clinical protocols, this research represents a significant step toward understanding how metabolic therapies might be harnessed to target cellular senescence, reduce age-associated inflammation, and ultimately improve human healthspan and longevity.
- Katsuumi, G., Shimizu, I., Suda, M. et al. SGLT2 inhibition eliminates senescent cells and alleviates pathological aging. Nat Aging 4, 926–938 (2024).
- Perkovic, V. et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. New England Journal of Medicine 380, 2295–2306 (2019).
- Ferrannini, E. et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 124, 499–508 (2014).
- Jiang, N., Gelfond, J., Liu, Q., Strong, R. & Nelson, J. F. The Gehan test identifies life-extending compounds overlooked by the log-rank test in the NIA Interventions Testing Program: Metformin, Enalapril, caffeic acid phenethyl ester, green tea extract, and 17-dimethylaminoethylamino-17-demethoxygeldanamycin hydrochloride. Geroscience 46, 4533–4541 (2024).
- Miller, R. A. et al. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight 5, (2020).
- Neal, B. et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. New England Journal of Medicine 377, 644–657 (2017).
- McGuire, D. K. et al. Association of SGLT2 Inhibitors With Cardiovascular and Kidney Outcomes in Patients With Type 2 Diabetes: A Meta-analysis. JAMA Cardiol 6, 148–158 (2021).
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015 Nov 26;373(22):2117-28. doi: 10.1056/NEJMoa1504720. Epub 2015 Sep 17. PMID: 26378978.
- Dasari, D., Goyal, S. G., Penmetsa, A., Sriram, D. & Dhar, A. Canagliflozin protects diabetic cardiomyopathy by mitigating fibrosis and preserving the myocardial integrity with improved mitochondrial function. Eur J Pharmacol 949, (2023).