The Science of Skin Senescence: Rapamycin's Role in Targeting the Root Causes of Dermal Aging
Prefer to listen? Hit play for a conversational, audio‑style summary of this article’s key points.
Dermal Aging: Skin aging (Dermal Aging) involves complex cellular interactions, including DNA damage, inflammation, and varied signaling pathways. It's accelerated by both intrinsic (natural aging) and extrinsic factors (environmental stressors).
Role of Fibroblasts in Skin Aging: Fibroblasts, a type of skin cell, are crucial for producing collagen and maintaining skin structure. With age, they decrease in number and functionality, leading to reduced collagen production and skin elasticity and contributing to visible aging.
Impact of Reactive Oxygen Species (ROS): ROS, naturally produced in the body, cause DNA damage and contribute to aging. Increased ROS in fibroblasts is linked to skin aging, causing these cells to enter a state of cellular senescence and release inflammatory signals.
Senescence-Associated Secretory Phenotype (SASP): Senescent fibroblast cells release SASP, a mix of molecules that cause inflammation and degrade the skin's extracellular matrix, further accelerating aging.
Influence of Cellular Pathways on Aging Skin: Key cellular pathways like Nrf2, TGF-β, IGF-1, and mTOR play significant roles in skin aging. Dysregulation of these pathways can lead to oxidative stress, reduced collagen production, and overactive cell growth.
Potential of Anti-Aging Therapies: Rapamycin, an mTOR inhibitor, emerges as a promising treatment for dermal aging. It helps in regulating cellular metabolism and autophagy, potentially reversing aging signs.
In a realm where the pursuit of health and longevity intersects with the cutting-edge of molecular biology, skin aging is not just a cosmetic concern but a bio-molecular challenge to be decoded and reversed. It is the visible clock of our biological age, ticking away in the form of fine lines, wrinkles, and elasticity loss, but its roots run deep into the cellular machinery of our body.
In this detailed review, Shreshtha Jolly from Johns Hopkins University's Department of Molecular Biology takes us on a deep dive into the cellular underpinnings of dermal aging. Here, we dissect the complex interplay of DNA damage, cellular senescence, oxidative stress, and the intricate communication between various skin cells that contribute to the aging phenotype of the skin. This analysis isn't just about understanding the 'why' of skin aging; it's a quest to uncover the 'how' – how can we intervene, manipulate, and potentially reverse these underlying processes?
For those who frequent the frontiers of longevity research and seek to apply the latest scientific findings to personal health optimization, this review offers a treasure trove of information. It highlights Rapamycin, not merely as a subject of academic fascination, but as a practical tool in the quest to combat skin aging. With its potential to modulate pivotal cellular pathways, Rapamycin stands out as a promising agent in the quest for not just slowing, but visibly reversing the signs of aging skin.
From understanding the intricate processes that drive skin aging to exploring interventions like Rapamycin, this review is for those who are passionate about harnessing scientific knowledge for health optimization and longevity.
Understanding Dermal Aging and the Role of Senescence
Aging is an intricate process that leads to a decline in the function of tissues and organs. [1] Several internal and external factors can influence the aging process. These include the accumulation of DNA damage, exposure to environmental stressors, and damage to mitochondria, which are cellular 'energy powerhouses,' break down nutrients to release energy.
Aging also affects telomeres. Telomeres are short stretches of DNA found at the very tip of chromosomes (structures inside our cells that carry DNA). They serve as protective buffers, ensuring the stability and integrity of our genetic information.
As cells divide over time, telomeres naturally shorten, acting as a kind of biological clock that influences the aging process and the lifespan of cells. When they become too short, cells enter a state of 'senescence,' where they can no longer divide. You can imagine the relationship between telomere shortening and cell division as that of a burning candle. As the candle burns (cells divide), the wax (telomeres) gradually gets used. Once the wax is gone (telomeres are too short), the candle cannot burn anymore (the cell cannot divide).
Cellular senescence is also associated with aging and certain age-related diseases. [2] Senescent cells are cells that have lost their capacity to divide. They are not 'dead' per se; they remain metabolically active and can release a combination of molecules referred to as senescence-associated secretory phenotype (SASP) [2]. SASP contributes to inflammation and signs of aging.
We’ve written about how cellular senescence leads to tissue deterioration in a number of contexts:
One of the parts of our body that is particularly vulnerable to the harm of cellular senescence is the skin. Dermal aging refers to the aging process of the skin, encompassing the natural changes and alterations that occur in the skin over time. To understand this process, it is first essential to familiarize ourselves with the basic structure and function of the skin.
Structure and Function of Skin
You can think of your skin as a protective shield, defending your body against external insults. It has two main layers: the epidermis and the dermis. The outer layer, the epidermis, is like the shield's surface. It comprises different types of cells, including keratinocytes, melanocytes, and Langerhans cells. [3] Keratinocytes are like the bricks of the outer layer, providing strength and durability. Melanocytes are like painters, responsible for giving your skin its color and protecting it from the harmful effects of the sun. Langerhans cells act as the security guards, helping your immune system defend against invaders like bacteria. These cells work together to keep your skin firm and healthy. [3]
Beneath the epidermis is the dermis, which you can think of as the support structure of the shield. It is responsible for the overall structure and function of your skin. The dermis has various components that help keep your skin elastic and functional. In particular, it is composed of the extracellular matrix (ECM), a complex network of proteins, carbohydrates, and other molecules that provide strength and support to the skin. [4] The structure is made by a specialized set of cells known as fibroblasts. They act like 'construction workers' producing and maintaining the extracellular matrix (ECM).
In total, our skin is like a strong shield with a tough outer layer (epidermis) and a supportive structure (dermis) underneath. The dermis is composed of the extracellular matrix produced and maintained by fibroblasts. Together, the epidermis (outer layer) and dermis (inner layer) create a skin shield to protect the body from external harm.
Biological Processes Underlying Dermal Aging
Understanding the Effects of Excessive ROS in the Skin Layer
One of the primary factors that triggers skin aging is damage to DNA. Much of this damage is propagated by reactive oxygen species (ROS). These are molecules rich in oxygen, naturally generated within our bodies as cells utilize oxygen for energy production. As we age, internal factors like impairment in mitochondrial function, UV radiation, and other stressors synergistically augment the production of ROS and prevent their clearance.
Normally, ROS are produced in small amounts during the cell's metabolic processes and play roles in cell signaling and homeostasis. However, excessive ROS can cause oxidative stress, damaging cells' proteins, lipids, and DNA.
As ROS accumulate, they inflict harm on DNA through different mechanisms. One way is oxidation, a process where electrons (negative charges) are pilfered from DNA molecules. The removal of electrons triggers breaks or lesions and compromises the DNA's structural integrity.
Beyond oxidation, ROS may directly engage with DNA, leading to alterations in its sequence or hindering its ability to replicate accurately. ROS can also instigate the formation of other detrimental molecules, such as free radicals, which, like ROS, are highly reactive and capable of causing DNA damage.
Interestingly, there is a vicious cycle between Reactive Oxygen Species (ROS) and inflammation. ROS act as signals that kickstart inflammation, and inflammation, in turn, produces more ROS. It is this bidirectional system that accelerates the aging process [2, 3]. Additionally, Senescent cells can produce increased levels of ROS, which can further contribute to the aging process and age-related diseases.
Scientists have found that as we age, there is a significant increase in ROS levels in fibroblasts, the architect cells of our skin [4]. Fibroblasts are crucial cells in our skin, known for their role in producing collagen and maintaining tissue structure. This increase in ROS has been observed in both human fibroblasts grown in laboratory settings [4] and in aged rat skin [5].
This rise in ROS is not just a bystander effect; it's actively contributing to skin aging. How? Excessive ROS can induce DNA damage, which in turn can trigger cellular senescence as a protective response against potential malignant transformation.
In the spotlight of recent aging research, senescence is a double-edged sword. On one hand, it's a natural brake system that prevents damaged cells from becoming cancerous by halting their division. On the other, senescent cells are not silent retirees. They start to secrete a mix of inflammatory and harmful substances, known collectively as the senescence-associated secretory phenotype (SASP). This heightened inflammation leads to the acceleration of aging skin.
The SASP turns these once helpful fibroblasts into sources of tissue damage, promoting inflammation, degrading the extracellular matrix, and, crucially, disrupting the delicate balance of skin renewal. This shift not only accelerates skin aging but also compromises skin integrity, leading to common age-related conditions like wrinkles, loss of elasticity, and slower wound healing. To understand this better, let's look at a specific study on mice that are unable to clear excess ROS. In this pivotal study, scientists crafted a genetically modified strain of mice. These mice were engineered to lack SOD2, but with a twist: the deficiency was restricted solely to their skin fibroblasts. This precise genetic modification allowed researchers to isolate the effects of SOD2 deficiency in a specific cell type within a particular tissue—the skin—without the confounding influences of systemic changes.
SOD2 is an important protein because it helps to keep free radicals under control. Free radicals are unstable molecules that can damage cells. SOD2 specifically targets and neutralizes a type of free radical known as superoxide anions, converting them into less harmful substances, namely oxygen and hydrogen peroxide. This process is vital in maintaining cellular health and preventing damage from oxidative stress.
In the mice lacking SOD2 in their fibroblasts' mitochondria, there was a build-up of these superoxide anions because they weren't being converted into harmless substances. This build-up was linked to signs of rapid skin aging, such as more DNA damage and an increase in senescent fibroblasts.
This study highlights the critical role of ROS, and specifically the importance of managing them with proteins like SOD2, in preventing the accelerated aging of the skin. [1]
In summary, the intricate interplay between Reactive Oxygen Species (ROS) and various biological processes underpins the complexity of skin aging. ROS, arising from both internal and external stressors, not only directly damages DNA through oxidation and interaction but also perpetuates a cycle of inflammation and senescence that further exacerbates aging. The study on mice with a deficiency in the SOD2 protein in their fibroblasts' mitochondria underscores the significance of effectively managing ROS levels to mitigate rapid skin aging, emphasizing the pivotal role of cellular mechanisms in maintaining skin health and integrity. [1]
Understanding the Effects of Dermal Fibroblasts and Other Skin Cells
As previously discussed, cellular senescence, a state where cells cease to divide and grow, can be triggered by various stressors including Reactive Oxygen Species (ROS), DNA damage, UV irradiation, telomere shortening, and mitochondrial dysfunction. This process, while initially a protective mechanism, has far-reaching consequences for the skin, particularly in the context of fibroblasts, the skin's foundational cells.
Fibroblasts are not mere bystanders in the skin; they are the craftsmen, actively involved in wound healing and the upkeep of skin structure. They are the primary producers of collagen and other extracellular matrix components, which are the scaffolding of the skin, ensuring its strength and elasticity. However, as we age, these vital cells fall victim to the relentless march of senescence.
A striking illustration of this is the significant decrease in fibroblast count with age. Studies have shown a 35% reduction in total fibroblast count when comparing young skin, aged 18-29 years, to that of older individuals over 80 years. This decline is more than just a numerical decrease; it represents a fundamental change in the skin's ability to repair and maintain itself.
The impact of this reduction is multi-fold. With fewer fibroblasts, the skin's capacity to produce collagen diminishes, leading to the telltale signs of aging: wrinkles, sagging, and a loss of elasticity. Moreover, the diminished fibroblast population affects wound healing, making the skin of older adults more susceptible to injuries and slower to recover.
This process is a vicious cycle. As we age, not only does the total number of fibroblasts in our skin diminish, but there's also an intriguing shift in their quality: a higher proportion of these remaining cells enter into a state of senescence. This phenomenon is not just a trivial change but a fundamental alteration that affects the very fabric of our skin.
The presence of senescent cells in aging skin is most notably marked by the increase in p16INK4a-positive cells. The p16INK4a protein is a widely recognized biomarker for cellular senescence. These p16INK4a-positive cells are also linked to the formation of wrinkles and changes in skin elasticity.
Like other senescent cells, senescent fibroblasts do not divide and multiply. While this may sound like good news, it does come at a price. Senescent fibroblasts are like stubborn stains on a kitchen stove—despite rigorous cleaning (attack by the immune system), they persist and wreak havoc. [7] An excessive accumulation of these senescent fibroblasts significantly contributes to skin aging. They release harmful signals in the form of SASP and disrupt the balanced environment of the ECM, further impacting skin health. [7]
The decrease in healthy fibroblast count due to senescence contributes significantly to the aging process of the skin, leading to reduced collagen production, diminished skin elasticity, and the overall appearance of aged skin. [6]
Fibroblasts can also interact with other skin cell types and work synergistically together to amplify skin aging and inflammation. A key example of this cellular interplay involves fibroblasts and keratinocytes, the primary cell types in the skin.
Fibroblasts, as we've seen, are the structural foundation of the skin, responsible for collagen production and tissue repair. Keratinocytes, on the other hand, are the predominant cells in the epidermis, the outermost layer of the skin, and play a crucial role in providing a barrier against environmental damage.
The interaction between these two cell types is a dynamic and reciprocal one, particularly in the context of aging and inflammation. One of the pivotal elements of this interaction is the release of cytokine IL-1 by keratinocytes. IL-1 is a type of signaling molecule that plays a significant role in the body's inflammatory response. When keratinocytes release IL-1, it acts as a signal to the fibroblasts.
In response to IL-1, fibroblasts are stimulated to produce a range of other cytokines and growth factors. These substances, in turn, have various effects. They modulate immune responses and influence the behavior of neighboring cells, including the keratinocytes themselves. This creates a feedback loop, where fibroblast signals return to keratinocytes, prompting them to send more signals back to the fibroblasts.
Over time, this continuous 'conversation' between fibroblasts and keratinocytes can amplify the effects of aging and inflammation in the skin. As these signals become stronger and more frequent, they lead to increased inflammation, thereby accelerating the aging process. This can manifest in various ways, such as a decrease in collagen production, impaired skin barrier function, and increased susceptibility to damage from external factors like UV radiation. [8]
Now, how does this contribute to skin aging?
- Cytokines and Inflammation: The increased production of cytokines by fibroblasts and keratinocytes can lead to chronic inflammation in the skin. Think of it as a constant buzz of activity that, over time, can contribute to aging-related changes.
- Growth Factors: While growth factors are essential for skin repair, an excessive amount over a prolonged period might lead to alterations in skin structure and compromise their functions.
So, this ongoing crosstalk between keratinocytes and fibroblasts can contribute to the aging process by promoting inflammation and influencing skin structure. [8]
Besides keratinocytes and fibroblasts, melanocytes are another cell type that contributes to skin aging. [9] They serve as crucial resources for aging signals. Melanocytes are best known for their role in skin pigmentation. These cells produce melanin, the pigment that gives skin, hair, and eyes their color. This melanin is not just cosmetic; it provides crucial protection against ultraviolet (UV) radiation from the sun. Melanocytes become senescent as a result of chronological aging or chronic exposure to UV radiation.
The senescence of melanocytes can disrupt the normal pigmentation process, leading to age-related pigmentary changes. This can manifest as uneven skin tone, age spots, and other pigmentation irregularities that are common signs of aging skin. Furthermore, these senescent melanocytes can influence their surrounding microenvironment, affecting neighboring cells and extracellular matrix components.
Senescent melanocytes are problematic as they also release SASP, a group of pro-inflammatory markers. The SASP released by these senescent melanocytes can induce telomere shortening in neighboring cells, such as keratinocytes and fibroblasts, effectively halting their multiplication and pushing them into a state of senescence. These factors can exacerbate the aging process not only in the melanocytes themselves but also in the surrounding fibroblasts and keratinocytes. This creates a complex interplay of cellular interactions that accelerates skin aging. Overall, senescence induced by various stressors leads to a 35% decline in total fibroblast count in aging skin. As the count decreases, the proportion of senescent fibroblasts increases, as indicated by a rise in p16INK4a-positive cells, which are linked to wrinkles and altered skin elasticity.
We see a chain reaction and compounding of inflammation and transformation of healthy cells into senescent cells driven by the inflammatory mix of SASP molecules. While senescent fibroblasts cease division, they persist and release harmful signals via SASP. This positive feedback loop between keratinocytes and fibroblasts amplifies inflammatory signals, potentially intensifying the aging process through increased cytokine production and growth factor activity. Melanocytes undergo senescence with chronological aging or prolonged UV exposure. As they become senescent, they release SASP, which triggers telomere shortening and cell division arrest in neighboring cells.
Impact of Senescent Cell Secretions on Skin Aging: The Role of Lysophosphatidylcholines
As we’ve established, the dermal SASP corresponds to a mix of molecules released by senescent cells, affecting immune regulation and influencing the behavior of nearby non-senescent cells. These molecules, including cytokines, growth factors, and others, play a role in breaking down the skin's extracellular matrix and promoting inflammation. [8]
Interestingly, studies have also revealed that senescent fibroblasts release specific lipid molecules that interfere with the viability of non-senescent cells.
A fascinating study has shed light on yet another facet of the complex process of skin aging, highlighting the role of certain fats known as lysophosphatidylcholines (LPCs) in this phenomenon. LPCs, which are found in aged senescent fibroblasts, have been discovered to play a dual role in influencing skin aging, particularly through their interaction with the immune system.
Lysophosphatidylcholines are a type of lipid molecule that, in the context of this research, are produced by senescent fibroblasts - cells that have ceased to divide and have undergone certain changes in function. The presence of these LPCs in aged skin cells is not a passive occurrence; rather, they actively influence the surrounding non-senescent cells and the immune system.
One key action of these LPCs is to stimulate non-senescent cells to release chemokines. Chemokines are a type of signaling molecule that plays a critical role in immune responses, particularly in attracting immune cells to sites of inflammation or injury. This release of chemokines can create an environment that encourages inflammation, a fundamental aspect of skin aging.
Moreover, LPCs from senescent fibroblasts also interfere with the activity of macrophages. Macrophages are a type of white blood cell that are essential for the immune system's response to pathogens and damaged cells. They are also involved in tissue repair and remodeling. By modulating macrophage activity, LPCs can further contribute to inflammatory processes in the skin.
This dual influence of LPCs on both chemokines and macrophages highlights a crucial link between senescent cells, the immune system, and skin aging. It suggests that as fibroblasts age and become senescent, they don't just lose their functional abilities; they actively contribute to an inflammatory microenvironment in the skin. This inflammation, in turn, accelerates the aging process, manifesting as decreased elasticity, increased wrinkling, and other common signs of aged skin.
The discovery of the role of LPCs in skin aging offers a new perspective on how cellular aging contributes to the overall aging of the skin. It underscores the interconnectedness of different cell types and systems within the skin, particularly the interplay between senescent cells and the immune system.
Furthermore, this research opens up potential new avenues for anti-aging interventions. Targeting the production or action of LPCs in aged senescent fibroblasts could potentially modulate the inflammatory response and slow the skin's aging process. [9]
Deciphering Key Cellular Pathways in Skin Aging: The Roles of Oxidative Stress, TGF-β, and IGF-1 Signaling
In addition to cellular changes, skin aging also impacts various cellular pathways, each with a specific role.
One of the known determinants of aging is oxidative stress. In simple terms, oxidative stress occurs when our bodies cannot effectively clear reactive oxygen species (ROS). As mentioned, ROS are oxygen-rich molecules that inflict DNA damage and other physiological insults in our bodies. There are certain players called antioxidants that help remove these harmful molecules. Oxidative stress occurs when the antioxidant pool is not 'strong' enough to remove the ROS effectively. Excessive accumulation of ROS, as discussed before, underlies skin aging.
In the battlefield of ROS vs antioxidants, a significant player exists called Nrf2. Nrf2 is a protein that 'allies' with antioxidants. Its activation enhances the production of antioxidants, which are crucial in defending against ROS. However, as we grow older, levels of active Nrf2 dwindle. This allows ROS to accumulate, triggering oxidative stress and accelerating premature dermal aging. [10]
Apart from Nrf2, the Transforming Growth Factor-Beta (TGF-β) pathway also plays a crucial role in skin aging. As discussed previously, in aging skin, the structural integrity of the extracellular matrix (ECM) decreases. The TGF-β pathway is like the manager of the ECM. Activation of the pathway boosts the production of critical components of the ECM, like collagen, while preventing their breakdown. This keeps the skin firm.
However, oxidative stress and UV rays can interfere with TGF-β signaling. This interference results in reduced collagen production and changes in skin structure, leading to decreased resilience. Hence, safeguarding your skin from stress and UV rays is like helping the manager keep everything in good shape. [11]
The Insulin-like Growth Factor-1 (IGF-1) signaling pathway is also implicated in skin aging. IGF-1 signaling controls processes like cell growth and protein production. However, as we age, there is a buildup of certain substances that can disrupt the pathway from performing its function correctly. This disruption can limit the growth of fibroblasts and the production of collagen, both of which are essential for maintaining skin firmness. Researchers are still figuring out how IGF-1 is involved in skin aging through more studies.
Exploring the mTOR Pathway in Skin Aging and the Therapeutic Potential of Rapamycin
One last pathway of note is the mechanistic Target of Rapamycin (mTOR) pathway. The mTOR (mammalian target of rapamycin) is a protein kinase that plays a crucial role in regulating cell growth. You can think of mTOR as the air-traffic controller for cellular growth. When cells receive signals indicating abundant nutrients or growth factors, mTOR becomes activated and stimulates cellular protein synthesis and cell growth. This activation of mTOR promotes cell growth and division, which is essential for tissue repair and growth in developing organisms.
Its contribution to dermal aging comes from its regulatory role in cellular metabolism and autophagy. Autophagy is a physiological process that identifies and cleans up damaged or old cell parts. It acts as an inner janitorial service, ensuring that everything stays in optimal condition at the microscopic level.
Previous studies have demonstrated that activation of autophagy reverses dermal aging, making it a candidate to capitalize on. Unfortunately, the anti-aging effects of autophagy can be compromised when the mTOR pathway gets overactivated.
Hyperactivation of mTOR can occur due to various factors, including nutrient abundance, stress, inflammation, genetics, and others. As we get older, mTOR may stay active all the time—opening the door to out-of-control cell growth.
The hyperactivation, in addition to reducing autophagy, can cause skin aging by causing cell overgrowth. This is because hyperactive mTOR signals can cause skin cells to multiply too quickly, leading to wrinkles and other signs of aging.
In senescent cells, mTOR amplifies its hypergrowth and functionality, resulting in excessive growth and output that is toxic to the tissue—it makes the zombie cells bigger, more active, and more pernicious in recruiting healthy cells into a dysfunctional state. This hyperactivation of mTOR leads to the cellular dysfunction that characterizes cellular senescence. mTOR-driven hyperfunction causes the acceleration of aging skin appearance.
Fortunately, scientists have developed unique treatments to shun mTOR overactivity. One such treatment involves the compound, Rapamycin. Rapamycin can yield visible improvements in the appearance of aging skin when used in appropriate dosage and frequency. [12]
If mTOR accelerates the aging of cells and tissues, then rapamycin is a potentially potent molecule to decelerate the aging process. Rapamycin binds and inhibits the activity of mTOR—slowing down the progression of senescence and cellular dysfunction. In a recent study published in the Journal of Geroscience, researchers set out to investigate the effects of applying topical rapamycin to the skin to see if it reduced the markers of senescence and aging in human skin [13].
Study Methodology: Evaluating the Efficacy of Topical Rapamycin in Skin Aging
The study was an exploratory, prospective, randomized trial that involved a total of 60 participants aged 40 and above. Participants enrolled in the study were provided with a container of rapamycin cream and a container of placebo in identical dispensers with labels for right or left hand and were instructed to apply the creams to the dorsal side of each hand every 24 to 48 h in the evening before bed. Before and after the treatment period, the researchers collected skin samples from the participants and analyzed them for markers of senescence and aging. They also assessed the participants' skin for signs of aging, such as wrinkles and sagging, using a standardized scoring system [13].
Study Findings: Impact of Topical Rapamycin on Skin Senescence Markers
The results of the study showed that topical rapamycin significantly reduced markers of senescence in the skin samples collected from the treatment group. The researchers evaluated the impact of rapamycin by analyzing skin biopsies for the expression of proteins related to senescence and aging.
Research Outcomes: Rapamycin's Effect on Senescence Marker p16 in Skin Aging
The researchers focused on a protein called p16INK4A (p16), which is involved in regulating the cell cycle and senescence. In the skin, senescence cells measured by increased expression of the p16 protein have been found to correlate with markers of aging such as elastic fiber morphology and wrinkling of the skin. In addition to its role in senescence, p16 has been shown to be involved in regulating inflammation in the skin. Inflammation is a driver of skin aging, and high levels of inflammation in the skin have been associated with the development of wrinkles and other signs of aging.
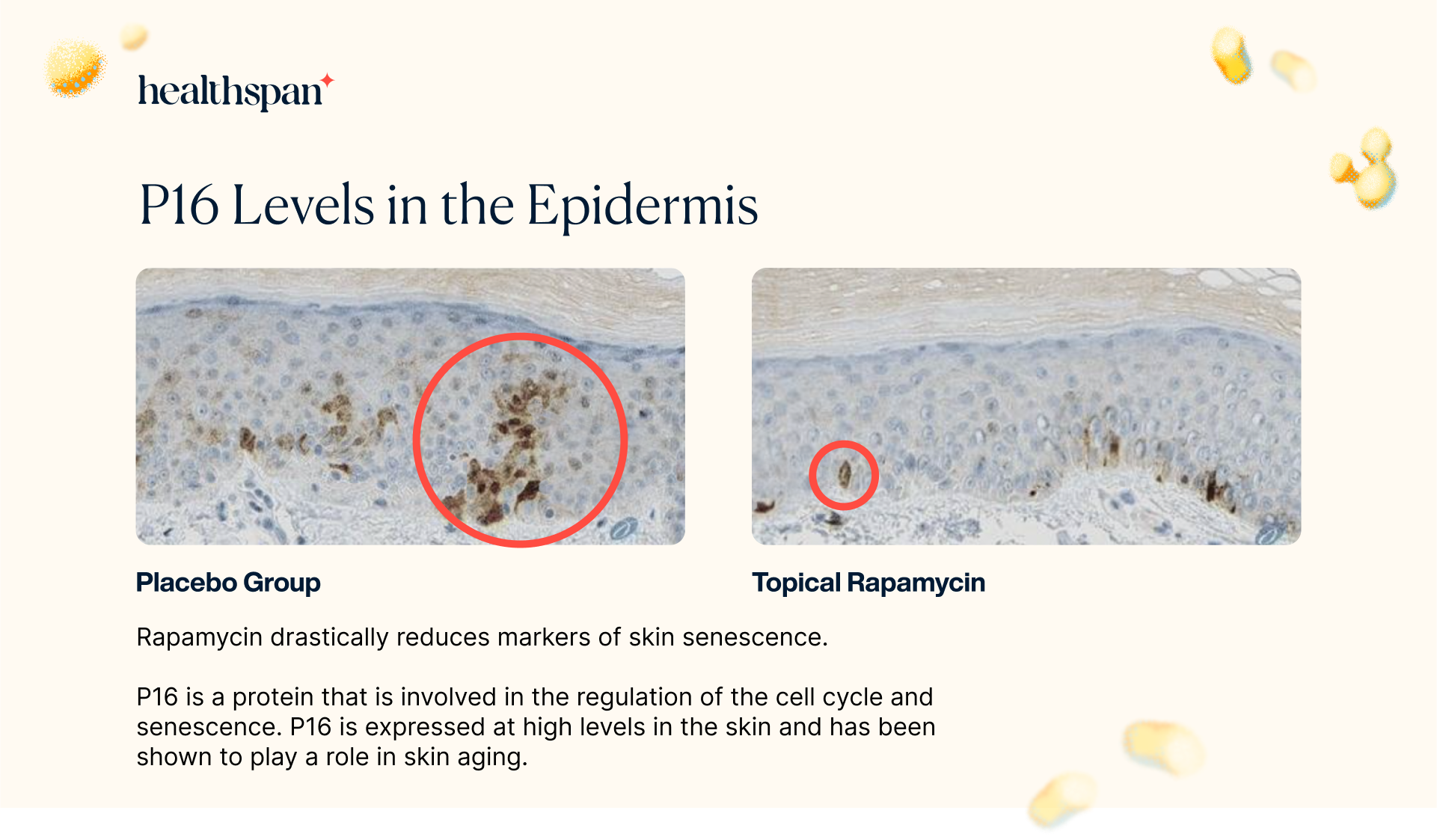
The study's findings revealed that the application of topical rapamycin led to a noteworthy 50% decrease in p16 expression in treated biopsies compared to the control group. This significant reduction in p16 expression strongly indicates that the absolute number of senescent cells in the epidermis was diminished. Unlike conventional treatments that simply modify senescent cells in the tissue, rapamycin treatment is either reducing the number of cells that enter senescence, or enhancing the clearance of existing senescent cells.
Topical Rapamycin Demonstrates Efficacy in Decreasing Solar Elastosis in Skin Samples
In the study, the researchers also assessed the levels of a skin condition known as solar elastosis. This condition is commonly seen in individuals who have experienced chronic sun exposure, particularly in the face, neck, and hands. Solar elastosis occurs when abnormal elastic fibers accumulate in the skin, resulting in a thickened and yellowed appearance. It is a key feature of actinic keratoses, which are pre-cancerous skin lesions caused by sun damage. By evaluating the levels of solar elastosis in the skin biopsies, the researchers aimed to determine whether topical rapamycin could prevent or reduce the development of this condition.
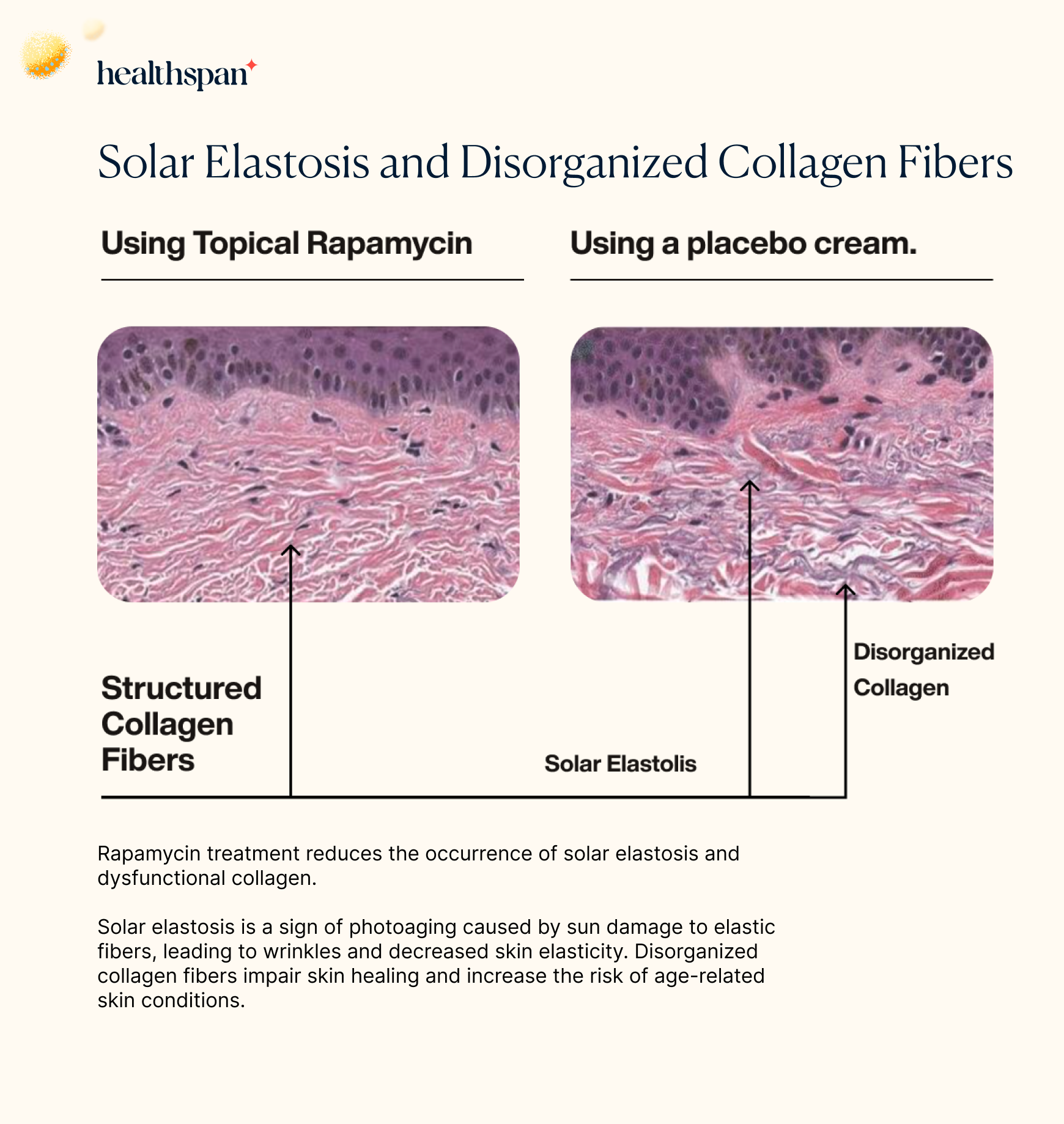
When analyzing the histological images, the researchers found that the placebo group had significantly higher levels of disorganized collagen fibers, which are associated with sun damage, as well as evidence of solar elastosis. This finding further highlights the potential of topical rapamycin as a preventative measure against the harmful effects of chronic sun exposure on the skin. Furthermore, it appears that rapamycin treatment induces autophagy—a crucial cellular process that helps to maintain cellular health and prevent the accumulation of damaged proteins and other molecules associated with age-related diseases and tissue dysfunction. By promoting autophagy, rapamycin treatment enhances the health and function of the skin layer by increasing the clearance of damaged cellular components and byproducts.
Improvement of Cytokeratin 5/6 Distribution in Basal Layer Observed with Rapamycin Treatment
The skin biopsies also revealed that rapamycin had a positive effect on the distribution of cytokeratin 5/6 in the basal layer. Cytokeratin 5 and cytokeratin 6 are crucial components for the development and maintenance of healthy skin. The proper distribution of cytokeratin 5 in the basal layer is vital as it provides structural support to the cells, ensuring their shape and function are maintained. Moreover, cytokeratin 5 is thought to regulate the process of mitosis, which is crucial in the replacement of skin cells that are shed from the surface of the skin.
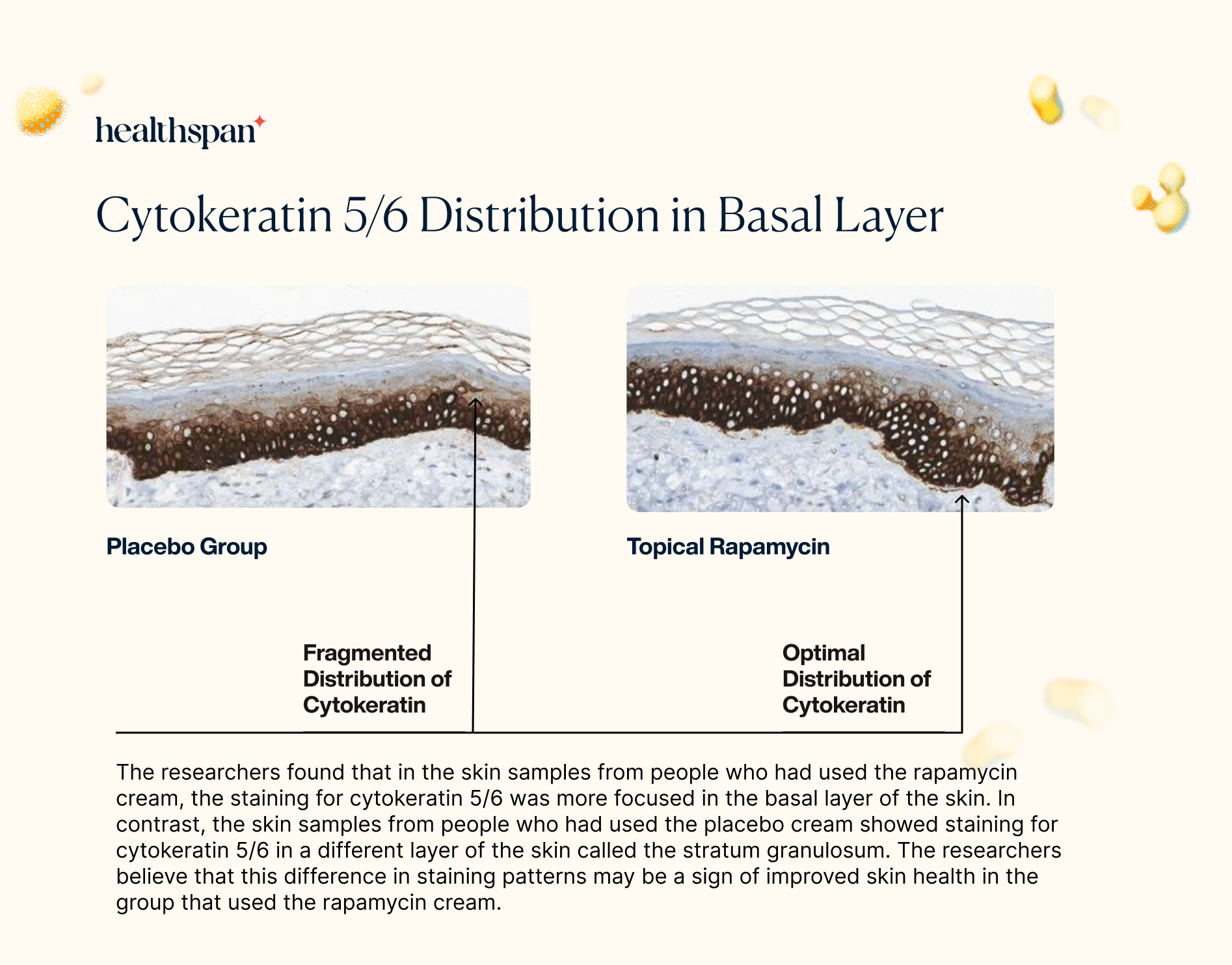
By improving the distribution of cytokeratin 5/6 in the basal layer through its stimulation of autophagy, rapamycin could have a significant impact on the health and vitality of the skin. These findings suggest that topical rapamycin may have a broader impact on the maintenance and repair of the skin, providing additional benefits beyond reducing markers of aging and preventing sun damage.
Rapamycin's Efficacy in Elevating Collagen VII Protein Levels in Skin
To explore the potential impact of rapamycin on collagen VII, the researchers utilized immunohistochemistry to assess the levels of this protein in skin samples. Collagen VII is an important structural component of the skin's extracellular matrix, which is responsible for maintaining the skin's strength and elasticity. The findings showed that the levels of collagen VII protein were significantly increased in the skin samples treated with rapamycin. This result is particularly noteworthy since the loss of collagen VII has been associated with the development of several skin disorders, including epidermolysis bullosa and skin aging.
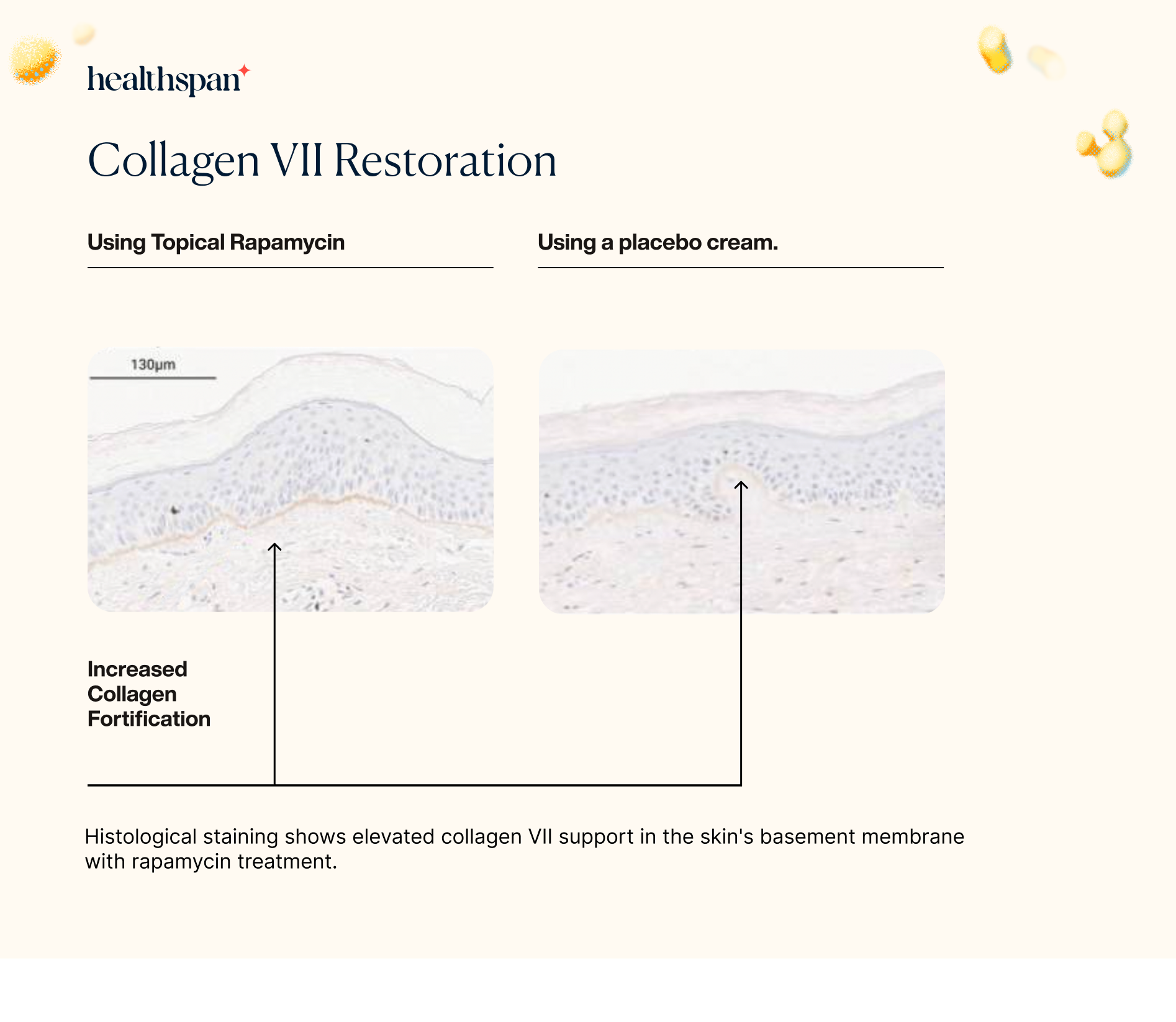
The increase in collagen VII protein levels observed in the rapamycin-treated skin samples suggests that the use of topical rapamycin could potentially help to prevent or treat these skin disorders by promoting the maintenance and repair of the skin's extracellular matrix. Overall, these findings highlight the potential of rapamycin as a promising new treatment option for various skin conditions associated with collagen loss.
The results of the study on topical rapamycin demonstrated notable improvements in several clinical signs of cutaneous aging. Observational assessments revealed a decrease in fine wrinkles, an increase in dermal volume, and a brighter, more even skin tone in the treated areas. Additionally, sagging of the skin was reduced, indicating a tightening effect. Among the study subjects, all but two exhibited improvements in the clinical signs of aging. These improvements included a decrease in the prominence of veins and tendons, finer wrinkling, and reduced dyspigmentation and dull appearance associated with photoaged skin. These changes were observed approximately four months after treatment began and continued to improve with subsequent visits.
Dr. Mikhail Blagosklonny's Advocacy for Topical Rapamycin in Skin Aging Management
Dr. Mikhail Blagosklonny, the creator of the theory of cellular hyperfunction and renowned for his research on rapamycin, has recommended using rapamycin-containing cream as a potential cosmetic solution for skin aging. Specifically, the cream may be applied to selected areas of the skin, such as the hands and face, that are affected by age-related spots and pathologies. However, Dr. Blagosklonny notes that the systemic use of rapamycin, such as through oral or intravenous administration, is likely a better option for treating the entire skin surface, as many manifestations of skin aging are related to systemic organismal aging and disease. While topical application of any drug is generally safer than systemic administration, Dr. Blagosklonny suggests that a combined approach of systemic and topical use of rapamycin in selected areas of the skin may be the best strategy for some cases, particularly in cases with noticeable signs of skin aging.
Summary of Signaling Pathways' Effects on Skin Aging
Skin aging is a complex process involving multiple pathways, each playing a unique role. Oxidative stress, a key determinant of aging, can be managed by activating the protective protein Nrf2, which boosts the production of antioxidants. The TGF-β pathway is crucial for maintaining the skin's structural support, but oxidative stress and UV exposure can disrupt its signaling, leading to decreased collagen production. The IGF-1 pathway, responsible for cell growth and protein production, can also contribute to aging when disrupted. Additionally, the mTOR pathway, regulating cellular processes like metabolism and autophagy, plays a role in skin aging. Hyperactivation of mTOR can cause skin cell overgrowth, contributing to wrinkles and aging.
Fortunately, treatments like rapamycin have shown promise in reversing these aging processes, presenting opportunities for visibly improving the appearance of aging skin. Understanding and targeting these pathways provide potential avenues for effective anti-aging interventions.
Conclusion
As we gracefully age, the concern of skin aging becomes a common theme, and one key player in this process is fibroblast senescence. Fibroblasts are the backstage crew, working behind the scenes to keep our skin in top shape. However, internal and external factors can hinder their performance - leading to a ripple effect of inflammation beyond just the skin.
Addressing skin aging, explicitly by targeting fibroblast senescence, could be a game-changer for maintaining a youthful look and supporting certain health conditions in older adults. Therapeutic candidates like Rapamycin that control mTOR overactivity reflect a beacon of hope in this quest to discover effective anti-dermal aging strategies. The future holds more discoveries, helping us unlock more innovative and effective ways to age healthily and stay vibrant.
- Zhang, J., Yu, H., Man, M. Q., & Hu, L. (2023). Aging in the dermis: Fibroblast senescence and its significance. Aging cell, e14054. Advance online publication.
- Shammas M. A. (2011). Telomeres, lifestyle, cancer, and aging. Current opinion in clinical nutrition and metabolic care, 14(1), 28–34.
- Liu, X., Zhu, R., Luo, Y., Wang, S., Zhao, Y., Qiu, Z., Zhang, Y., Liu, X., Yao, X., Li, X., & Li, W. (2021). Distinct human Langerhans cell subsets orchestrate reciprocal functions and require different developmental regulation. Immunity, 54(10), 2305–2320.e11.
- Hosseini, M., Koehler, K. R., & Shafiee, A. (2022). Biofabrication of Human Skin with Its Appendages. Advanced healthcare materials, 11(22), e2201626.
- Tahara, S., Matsuo, M., & Kaneko, T. (2001). Age-related changes in oxidative damage to lipids and DNA in rat skin. Mechanisms of ageing and development, 122(4), 415–426.
- Varani, J., Warner, R. L., Gharaee-Kermani, M., Phan, S. H., Kang, S., Chung, J. H., Wang, Z. Q., Datta, S. C., Fisher, G. J., & Voorhees, J. J. (2000). Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. The Journal of investigative dermatology, 114(3), 480–486.
- Solé-Boldo, L., Raddatz, G., Schütz, S., Mallm, J. P., Rippe, K., Lonsdorf, A. S., Rodríguez-Paredes, M., & Lyko, F. (2020). Single-cell transcriptomes of the human skin reveal age-related loss of fibroblast priming. Communications biology, 3(1), 188.
- Waldera Lupa, D. M., Kalfalah, F., Safferling, K., Boukamp, P., Poschmann, G., Volpi, E., Götz-Rösch, C., Bernerd, F., Haag, L., Huebenthal, U., Fritsche, E., Boege, F., Grabe, N., Tigges, J., Stühler, K., & Krutmann, J. (2015). Characterization of Skin Aging-Associated Secreted Proteins (SAASP) Produced by Dermal Fibroblasts Isolated from Intrinsically Aged Human Skin. The Journal of investigative dermatology, 135(8), 1954–1968.
- Narzt, M. S., Pils, V., Kremslehner, C., Nagelreiter, I. M., Schosserer, M., Bessonova, E., Bayer, A., Reifschneider, R., Terlecki-Zaniewicz, L., Waidhofer-Söllner, P., Mildner, M., Tschachler, E., Cavinato, M., Wedel, S., Jansen-Dürr, P., Nanic, L., Rubelj, I., El-Ghalbzouri, A., Zoratto, S., Marchetti-Deschmann, M., … Lämmermann, I. (2021). Epilipidomics of Senescent Dermal Fibroblasts Identify Lysophosphatidylcholines as Pleiotropic Senescence-Associated Secretory Phenotype (SASP) Factors. The Journal of investigative dermatology, 141(4S), 993–1006.e15.
- Kapeta, S., Chondrogianni, N., & Gonos, E. S. (2010). Nuclear erythroid factor 2-mediated proteasome activation delays senescence in human fibroblasts. The Journal of biological chemistry, 285(11), 8171–8184.
- de Bengy, A. F., Lamartine, J., Sigaudo-Roussel, D., & Fromy, B. (2022). Newborn and elderly skin: two fragile skins at higher risk of pressure injury. Biological reviews of the Cambridge Philosophical Society, 97(3), 874–895.
- Chen, Q., Zhang, H., Yang, Y., Zhang, S., Wang, J., Zhang, D., & Yu, H. (2022). Metformin Attenuates UVA-Induced Skin Photoaging by Suppressing Mitophagy and the PI3K/AKT/mTOR Pathway. International journal of molecular sciences, 23(13), 6960. https://doi.org/10.3390/ijms23136960
- Chung CL, Lawrence I, Hoffman M, Elgindi D, Nadhan K, Potnis M, Jin A, Sershon C, Binnebose R, Lorenzini A, Sell C. Topical rapamycin reduces markers of senescence and aging in human skin: an exploratory, prospective, randomized trial. Geroscience. 2019 Dec;41(6):861-869. doi: 10.1007/s11357-019-00113-y. Epub 2019 Nov 25. PMID: 31761958; PMCID: PMC6925069.
Related studies