Rapamycin's Potential: In recent years, rapamycin has shown promise as an anti-aging compound, potentially delaying age-related diseases and extending lifespan. However, questions about dosage, long-term impacts, and the full scope of benefits still need answers.
Rapamycin and mTOR: Rapamycin affects the mTOR pathway, a protein central to cell growth and survival. By inhibiting this pathway, it can mimic calorie restriction benefits known to extend lifespan in organisms, possibly translating to age-related health benefits for humans.
Rapamycin in Clinical Trials: Several clinical trials are currently underway to better ascertain rapamycin’s role in metabolic health, visceral fat development, neurological disorders, and many other age-associated diseases. Research is also ongoing to determine rapamycin’s interaction with exercise and optimal dosing for rapamycin.
The Need for Ongoing Research: Rapamycin's potential in redefining aging and longevity understanding is vast. Current research is gradually revealing its capabilities in age-related health interventions, but the entire narrative is yet to unfold.
Introduction
Over the past few years, a molecule named rapamycin has risen to prominence in the fields of gerontology and molecular biology, drawing significant attention for its potential anti-aging properties. Originally discovered on Easter Island and initially used as an immunosuppressant in organ transplantation, this compound has shown in various preclinical studies—spanning from simple organisms like yeast to mammals—to potentially delay age-associated diseases and possibly extend lifespan.
While the preliminary data on rapamycin are undoubtedly promising, its journey from laboratory benches to potential longevity clinics is paved with questions. Determining the ideal dosage for anti-aging benefits without inducing undesirable side effects, understanding its long-term implications on human health, and decoding its multifaceted therapeutic actions are all subjects of intense scientific inquiry. Moreover, as with any new therapeutic agent, there's a rigorous process of validation through well-structured clinical trials to ensure its efficacy and safety.
In this review, we will navigate the complex world of rapamycin, elucidating its mechanisms of action and spotlighting the latest clinical trials. These studies, designed to explore rapamycin's role in human aging, are critical stepping stones, gradually illuminating its potential to redefine our understanding of age-related health and longevity.
Rapamycin—A Brief Overview
Rapamycin has garnered significant attention for its potential anti-aging properties, primarily through its impact on the mTOR (mechanistic target of rapamycin) pathway. mTOR is a kinase, a type of protein, that plays a central role in cell growth and proliferation.
What is emerging from research on rapamycin and mTOR is a universal theory in which overactivity of the mTOR pathway acts as an accelerant of aging. Whether it is immunosenescence, cancer, neurodegeneration, or metabolic dysfunction, we can see a consistent theme of mTOR overactivity of mTOR accelerating tissue dysfunction and the onset of age-related pathologies. In this context, mTOR inhibition through the use of rapamycin decelerates this process.
By inhibiting the mTOR pathway, rapamycin can mimic certain aspects of calorie restriction, a condition known to extend lifespan in a variety of organisms. This inhibition has been shown to delay the onset of age-related diseases, improve cellular health, and potentially extend lifespan in several animal models [1].
The relationship between rapamycin, mTOR inhibition, and aging suggests a pivotal interaction that could have profound implications for age-related health and longevity in humans.
However, the full extent of rapamycin benefits, optimal dosage, and potential side effects are areas of ongoing research. Scientists across the world are currently attempting to answer lingering questions about rapamycin. In this review, we will look at these questions and highlight what current and future clinical trials aim to discover.
Is rapamycin capable of reversing the aging process?
In our previous research reviews, we presented data that demonstrate rapamycin's several anti-aging benefits including promoting autophagy (clearing out toxic debris), combatting cellular senescence, and reducing inflammation. By stimulating these cellular pathways, rapamycin seems to protect healthy tissue function over time. What remains unclear is the overall impact rapamycin has on healthspan and its potential impact on lifespan.
Identifying successful anti-aging interventions that have a discernible impact on lifespan is complicated. Aging is driven by multiple biological pathways. The complexity of the aging process and the lack of long-term studies, standard definitions, and reliable methods of measurement make it difficult to identify successful anti-aging interventions.
For this reason, the National Institute for Aging (NIA) decided to create one of the most comprehensive Interventional Testing Programs (ITP) for longevity drugs and to gain more clarity on how these biological pathways interact.
The National Institute on Aging Interventions Testing Program (ITP) was designed to be the most exhaustive testing framework and system to evaluate whether longevity molecules extend longevity in mice and understand the underlying mechanisms leading to those benefits.
The ITP involves the collaboration of three research labs running experiments in parallel. These labs conducted evaluations on a selection of promising longevity molecules, including rapamycin, metformin, nicotinamide riboside, and the SGLT-2 inhibitor canagliflozin, among others.
At the end of the study, only five of the molecules that the ITP studied were shown to increase longevity. The researchers evaluated the combined effect of rapamycin and acarbose when given to mice starting at either 9 or 16 months. The 9-month mice had the greatest results, increasing the median lifespan of female and male mice by 28% and 34%, respectively. The 16-month mice still increased the medium lifespan of both female and male mice by 13% [3].
While the ITP provides a solid foundation for the use of rapamycin to promote healthspan and possibly lifespan, there is much more to understand about how these findings translate into humans. How does a patient who takes rapamycin compare to an individual who doesn’t?
RAP-PROTECT Study at The University of Wisconsin
Dr. Dudley Lamming and his team at The University of Wisconsin are looking to evaluate these fundamental questions. In their RAP-PROTECT study, Dr. Lamming’s team is recruiting 100 individuals who will take varied doses of rapamycin or everolimus (a compound similar to rapamycin). This group will be contrasted with a control group of individuals who are not administered rapamycin or related compounds.
One of the primary metrics the researchers will evaluate is HOMA-IR. While a deeper discussion of the mechanism is beyond the scope of this overview, it is essential to note that HOMA-IR is an indicator of insulin resistance. Essentially, a higher HOMA-IR score suggests a decreased efficiency in sugar metabolism, which can have implications for metabolic health.
Dr. Lamming has hypothesized that individuals who are taking rapamycin will have a lower HOMA-IR score, suggesting that rapamycin can improve metabolic health. If this hypothesis turns out to be true, it will provide further evidence for rapamycin’s role in enhancing metabolic health and overall healthspan. The outcomes of this study will be invaluable for individuals and clinicians considering rapamycin, as it will shed light on potential differences in aging markers between those who use rapamycin and those who do not. [1]
What is the optimal dosage of rapamycin for therapeutic benefits?
Another critical aspect that is often scrutinized in longevity research pertains to the determination of optimal dosages. In prior discussions, we have examined the evidence suggesting that the potential benefits of rapamycin in relation to longevity may be dose-dependent.
Dr. Joan Mannick’s work on rapamycin and immune function showed for example that rapamycin, when taken in low doses, improved immune function, while at high doses, immune function was suppressed [4]. To understand these differential effects, a deeper understanding of the mTOR pathway is warranted.
As a recap, the mTOR pathway is divided into two main complexes: mTORC1 and mTORC2.
mTORC1: This complex, which is sensitive to rapamycin, oversees protein synthesis, autophagy, and nutrient sensing. To conceptualize, one could envision mTORC1 as the cellular unit responsible for nutrient assessment, machinery building, and intracellular waste management. Notably, even minimal doses of rapamycin can profoundly impact mTORC1.
mTORC2: Typically less affected by rapamycin during short-term exposure, mTORC2 regulates immune responses, cell survival, and lipid metabolism. Analogously, this complex ensures cellular structural integrity and resilience under adverse conditions.
At low doses, rapamycin primarily targets the mTORC1 complex, promoting autophagy and minimizing cellular senescence. However, as the dosage increases, there's a potential impact on mTORC2—a complex integral for immune function and metabolic health [4]. Hence, calibrating the correct dosage is paramount to optimize rapamycin's benefits while averting unintended consequences on essential cellular operations.
At the University of Wisconsin, Dr. Adam Konopka and his research team are initiating a systematic study to explore the question of optimal dosing for rapamycin. The team plans to enroll 72 participants, administering varying doses of rapamycin: 5 mg, 10 mg, or 15 mg on a weekly basis. After receiving rapamycin for 24 weeks (approximately 6 months) the team will measure various physiological parameters including insulin sensitivity, metabolic function, cognitive function, cardiac function, and physical function.
Moreover, in ensuring a holistic perspective, the study won't solely focus on potential therapeutic outcomes. Equally important will be the identification of any adverse reactions, with special attention to their correlation with dosage.
Should the outcomes of this study reveal improved metabolic and cognitive markers, without notable adverse reactions, it might provide empirical support to the ongoing discussion about rapamycin's potential in addressing age-related conditions. Additionally, for physicians and patients looking to optimize their dosing, this study will provide clearer guidelines on the appropriate dosage of rapamycin to maximize benefits while minimizing side effects. [1]
What is the relationship between rapamycin/mTOR inhibition and physical exercise?
Current research suggests that by inhibiting mTOR—whether via rapamycin or through prolonged fasting—there's potential to mitigate the cellular dysfunction that underlies many age-related diseases and perhaps the aging process itself.
Parallel to this, the role of physical exercise in bolstering health and enhancing longevity is well-documented. It contributes to a spectrum of health outcomes, encompassing improved cardiovascular function, cognitive resilience, and metabolic regulation. Intriguingly, the molecular mechanisms underpinning the benefits of exercise have been found to overlap with the mTOR pathway in certain respects.
With the established roles of both mTOR inhibition and physical exercise in promoting health, an intriguing question arises: How do rapamycin/mTOR inhibition and physical exercise interact at the cellular and physiological levels?
mTOR and Muscle
As we age, our bodies exhibit a reduced capacity to construct new muscle proteins, even in the presence of anabolic stimuli like resistance exercise or protein intake. This perplexing state has captivated scientists for decades, yet the precise molecular mechanisms behind anabolic resistance have remained shrouded in ambiguity.
Previous studies from the lab of Dr. David J. Glass, MD, shed light on the dysregulation of mTOR as we age as a reason why it becomes harder to grow muscle. Glass’s study found that heightened mTOR activity in aging individuals impedes their ability to further activate mTOR in response to anabolic stimuli—leading to what could be referred to as an mTOR insensitivity to stimuli [2].
The study administered rapamycin for a duration of six weeks. The outcomes were fascinating. The rapamycin treatment led to a restoration of mTOR signaling intermediates and a reversal of sarcopenia—the harmful loss of muscle mass, strength, and function that afflicts many older adults [2].
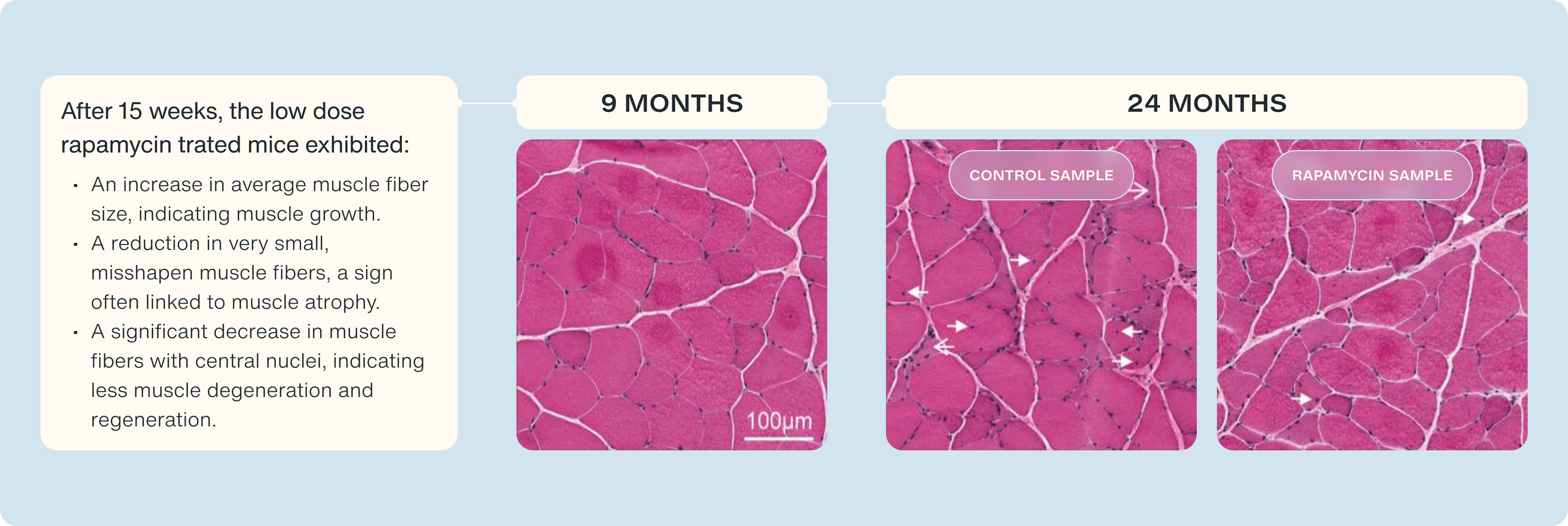
However, while the Glass studies provide us with an understanding of how rapamycin recalibrates mTOR activity to respond to anabolic stimuli there are a whole host of other metabolic pathways that we are interested in understanding rapamycin’s role in [2].
Dr. Jorgen Wojtaszewski and colleagues in Denmark are working to examine the relationship between mTOR inhibition, exercise, metabolic health, and muscle protein synthesis.
Wojtaszewski’s study will recruit young men who will take either rapamycin or a placebo before exercising. Since this is a "cross-over" study, at different times, the same participant will experience both conditions (taking the actual drug and the placebo) so comparisons can be made within the same person. After exercising, the researchers will use biological markers to measure how effectively each participant is responding to insulin, and how effectively their body is synthesizing protein into muscle.
This study will give us insights into how rapamycin might change the usual benefits of exercise on how our muscles use glucose as a fuel source. For individuals looking to take rapamycin, the results of this study can help guide how to effectively pair exercise and rapamycin to gain the most benefits. [1]
In what ways might rapamycin address the accumulation of visceral fat?
Visceral fat, distinct from the familiar subcutaneous fat that lies just below our skin, is situated deeper within our body. Rather than merely being an aesthetic concern, this type of fat encases vital organs such as the liver, pancreas, and intestines.
Its location alone is not what makes visceral fat worrisome. This "deep fat" is metabolically active and is known to secrete pro-inflammatory molecules, contributing to systemic inflammation. Chronic inflammation, in turn, has been linked to a host of health issues, including cardiovascular diseases, insulin resistance, and certain cancers.
The accumulation of visceral fat often signals an imbalance in metabolic health. It can result from chronic caloric excess where the body, having already filled its regular energy storage sites like adipose tissue and muscle glycogen, starts depositing these excess calories in less optimal locations. Think of visceral fat as a makeshift storage space—a place the body resorts to when other, healthier storage options are full. This is akin to storing hazardous materials in a residential area because industrial storage facilities are overloaded.
Contrary to subcutaneous fat, which primarily serves as an energy reservoir, visceral fat actively contributes to inflammation. Its inflammatory nature poses risks as it can trigger a cascade of reactions leading to tissue damage and exacerbation of various health conditions. Reducing visceral fat is therefore not just about improving physical appearance but, more importantly, about enhancing overall health and longevity.
Researchers are actively exploring potential interventions to address the build-up of visceral fat. Rapamycin has come under scrutiny as a candidate, with some evidence suggesting its potential role in reducing visceral fat accumulation. Notably, bloodwork from some patients on rapamycin has shown elevated triglyceride levels. There's a prevailing hypothesis within the scientific community that this triglyceride elevation could be indicative of the mobilization or breakdown of visceral fat in the presence of rapamycin. However, further investigations are warranted to conclusively establish this relationship.
Dr. James Watson of UCLA and Dr. Sajad Zalzala are currently leading the PEARL trial to better understand the intricacies of how rapamycin might address the buildup of visceral fat. While they hypothesize that visceral fat will decrease with rapamycin usage, results from their clinical trial will provide clear data to either support or refute their hypothesis.
The PEARL study will recruit 150 adult participants and will administer weekly rapamycin for one year. The rapamycin dose will vary at either 5 mg or 10 mg weekly. After one year, the researchers will measure visceral fat using a medical imaging technique known as DEXA.
The accumulation of visceral fat is directly linked to aging and therefore this trial’s outcomes will be crucial. If the study shows reduced visceral fat (a marker often associated with age-related health risks) with rapamycin, it might become a significant consideration for those wanting to take rapamycin for longevity and age-related health maintenance. [1]
Can rapamycin play a role in mitigating age-related neurological disorders like Alzheimer’s?
Aging encompasses more than just the external markers we commonly recognize; internally, a multitude of changes occur that can significantly impact the brain. Various age-related neurological disorders, from Alzheimer's disease to Parkinson's, are believed to have connections to mTOR pathway overactivity. The scientific community is tirelessly working to identify interventions that could potentially mitigate or even reverse these serious neurological conditions. It's worth noting that in conditions like Alzheimer’s Disease, many proposed pharmacological solutions haven't yielded significant advancements. Rapamycin’s potential influence on cognitive function and preventing/treating neurological disorders has been a topic of intense research.
The mTOR signaling pathway plays an intricate role in various cellular functions, and unsurprisingly, its dysregulation has been associated with several diseases, particularly neurodegenerative disorders. We’ve written about this in detail (mTOR Signaling and Neurodegenerative Disorders: Exploring the Therapeutic Potential of Rapamycin).
It's been observed that mTOR signaling can go awry in multiple neurodegenerative disorders [5]. Alzheimer's disease (AD), characterized by memory loss and cognitive decline, stands out prominently in this context.
Research has shown mTOR to be unusually active, or hyperactive, in both laboratory models (in vitro) and living organisms (in vivo) designed to mimic AD [6]. Furthermore, in post-mortem examinations of brain tissues from Alzheimer's patients, similar heightened mTOR activity has been detected [7].
Studies of mice, genetically engineered to exhibit AD-like symptoms, known as 3×Tg-AD mice are pivotal in our understanding of Alzheimer's progression. In groundbreaking experiments, 3×Tg-AD mice treated with rapamycin showed a retention of their cognitive abilities, contrasting sharply with their untreated counterparts who manifested cognitive decline [8]. Even more encouraging was the timing: rapamycin treatment was initiated post-symptom onset, implying that it could potentially halt, if not reverse, the progression of AD-like symptoms.
However, biology is rarely straightforward. Other studies using the 3×Tg mouse model painted a more complex picture. When treatment began post-symptom onset, around 15 months of age, rapamycin's effects were far less pronounced [9]. However, starting the treatment early in life showed marked benefits. These divergent results underline two things: first, the significance of maintaining a balanced mTOR activity for optimal brain health, and second, the existence of a 'golden window' for therapeutic intervention.
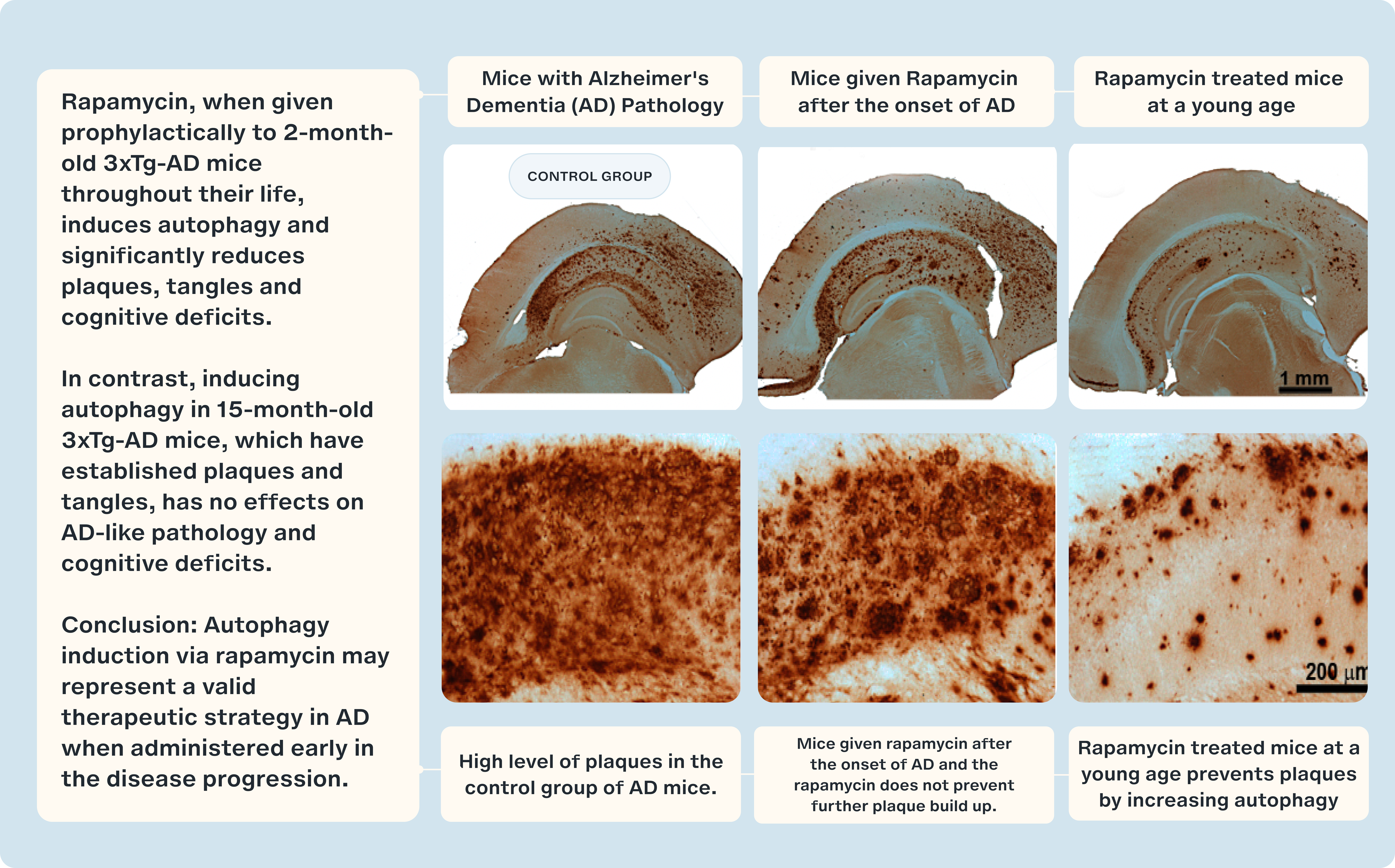
Now the challenge is to translate these findings in mice into humans.
Dr. Mitzi Gonzales and Dr. Sudha Seshardi, researchers at the University of Texas, have launched the REACH Trial to measure rapamycin’s effects on Alzheimer’s Disease and cognitive health. They are recruiting 40 patients with mild cognitive impairment or early Alzheimer’s Disease and administering rapamycin daily for one year. Throughout the study, they will track rapamycin levels in the patient’s bloodstream, adverse events, cognitive changes, and biomarkers specific to Alzheimer’s Disease.
Currently, rapamycin is not considered a treatment for Alzheimer’s. However, if rapamycin demonstrates efficacy in slowing cognitive decline or improving Alzheimer's biomarkers, it could become a first-line treatment for those at risk of AD or those in the early stages of cognitive impairment. [1]
The Broadening Scope of Rapamycin Research
In this review, we've delved into five pivotal clinical trials poised to offer invaluable insights to both medical practitioners and patients. Yet, these represent only a fraction of the ongoing research seeking to unravel the full promise of rapamycin. Numerous other studies are zeroing in on specific conditions, including respiratory tract infections, early ovarian failure, and muscle functionality in older adults. Together, these investigations will deepen our understanding of the potential anti-aging benefits of rapamycin and mTOR inhibition. [1]
Conclusion
Throughout our exploration of rapamycin, one thing remains clear: its potential to redefine our understanding of aging and longevity is both fascinating and complex. This review has served as a spotlight, highlighting the molecule's multifaceted role—from possibly preventing age-related diseases to potentially battling neurological decline. While we have unpacked significant strides from various clinical trials, the full story of rapamycin remains a work in progress. With every new study, another piece of the puzzle falls into place, helping us shape a more comprehensive view of its capabilities. As research continues to evolve, so does our optimism for the role rapamycin may play in the future of age-related health interventions.
- Konopka, A.R., Lamming, D.W., RAP PAC Investigators. et al. Blazing a trail for the clinical use of rapamycin as a geroprotecTOR. GeroScience (2023).
- Joseph GA, Wang SX, Jacobs CE, Zhou W, Kimble GC, Tse HW, Eash JK, Shavlakadze T, Glass DJ. Partial Inhibition of mTORC1 in Aged Rats Counteracts the Decline in Muscle Mass and Reverses Molecular Signaling Associated with Sarcopenia. Mol Cell Biol. 2019 Sep 11;39(19):e00141-19. doi: 10.1128/MCB.00141-19. PMID: 31308131; PMCID: PMC6751631.
- Harrison, DE, Strong, R, Alavez, S, et al. Acarbose improves health and lifespan in aging HET3 mice. Aging Cell. 2019; 18:e12898. https://doi.org/10.1111/acel.12898
- Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, Glass DJ, Klickstein LB. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014 Dec 24;6(268):268ra179. doi: 10.1126/scitranslmed.3009892. PMID: 25540326.
- Bove J, Martinez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosc. 2011;12(8):437–452.
- Caccamo A, Maldonado MA, Majumder S, Medina DX, Holbein W, Magri A, Oddo S. Naturally secreted amyloid-beta increases mammalian target of rapamycin (mTOR) activity via a PRAS40-mediated mechanism. J Biol Chem. 2011;286(11):8924–8932.
- Li X, Alafuzoff I, Soininen H, Winblad B, Pei JJ. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer's disease brain. FEBS J. 2005;272(16):4211–4220.
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5(4):e9979.
- Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PloS One. 2011;6(9):e25416.