Rapamycin
The most powerful tool to stop the acceleration of aging caused by mTOR dysfunction and cellular senescence.
A continuation of our Rapamycin for Longevity series exploring rapamycin and its effect on human longevity. Part 1 of 2.
rapamycin
17 mins
By: Daniel Tawfik
Rapamycin was unlucky enough to be initially discovered and labeled an immunosuppressant, a class of drugs that characteristically inhibit the immune system. From what we now know of rapamycin and its analogs is that they are capable immunomodulators, drugs which support and regulate the immune system, and anti-inflammatory compounds. But, we stand on the shoulders of giants in biology and with more than twenty years having passed since this initial “stifling” of rapamycin and its applications shoehorned it into treating renal transplant patients and painted a one-off and limited drug. Thankfully, our understanding of rapamycin has only improved since its discovery, with a long list of interactions within various models stemming from a wide array of experiments conducted since its discovery. At anti-aging doses, rapamycin “eliminates hyperimmunity rather than suppresses immunity” [2]. This ability to “rejuvenate” your immune system allows for rapamycin to act as a immunostimulator, thus increasing immune system activity, [4–6], improving the immune systems of cancer patients [7] and the elderly [8,9]. Rapamycin also reduces the risk of infection by Cytomegalovirus or CMV in organ transplant patients who are prone to infection due to post-transplant anti-rejection drugs' propensity to inhibit proper immune system functions [10–12]. Rapamycin has been shown to improve antipathogenic and anticancer immunity in mice [13–15]. Rapamycin also prolongs the lifespan of infection prone and cancer prone mouse models [16]. Rapamycin additionally protects against pneumonia in aged mice [17]. Rapamycin inhibits viral replication via the disruption of mTORC1 and mTORC2 activation by influenza during its late stages of replication. [18,19]. Rapamycin has been shown to inhibit the 1918 flu virus by 100-fold and helps protect against a lethal infection by influenza virus via the administration of rapamycin during vaccination [19,13].At one point in time it was believed that rapamycin treatment would increase the risk of cancer. But, as our understanding of rapamycin pharmacokinetics increased, so too did our understanding of how rapamycin actually prevents lymphoma and other cancers in transplant patients [20–27]. To lend more credence to its anti-cancer properties, rapamycin analogs, everolimus and temsirolimus are common and widely used in cancer therapies. Another key point to make is that rapamycin is the most effective known cancer-preventative agent for mice and extends the lifespan of cancer-prone mice [25,28–36]. A further study conducted by Ehninger et al suggests that rapamycin extends lifespans via the prevention of cancer [37].You may be wondering, “Why was rapamycin ever considered to cause cancer when it has been shown to be quite effective at treating it?” This confusion stems from an old, think twenty plus years, and a miscited FDA warning that all immunosuppressants “Increased susceptibility to infection and the possible development of malignancies such as lymphoma and skin cancer may result from immunosuppression.” This broad stroke of a statement does not specifically say that rapamycin or its analogs cause malignancies. Despite the overwhelming evidence that rapamycin nor its analogs having been a cause of cancer as well as the fact that these drugs are FDA approved for treating cancers and lymphomas, and that there is no study that has shown that mTOR inhibitors cause cancer, yet this baseless rumor that rapamycin causes cancer will persist.
When overactivated by nutrients and insulin, mTOR via S6K inhibits insulin signaling, thereby causing insulin resistance [39–44]. Acute rapamycin treatment nullifies insulin resistance in cells as well as animals including humans [45–51]. A study by Makki et al showed that chronic rapamycin treatment may prevent insulin resistance [52]. However, some mouse models suffer from glucose intolerance and even insulin resistance caused by chronic rapamycin treatment [53–56]. These findings were interpreted as a deleterious side effect or perhaps type 2 diabetes (T2D). However, these findings are in line with the metabolic features of starvation pseudo-diabetes (SPD)[57,58], in which starvation or prolonged fasting causes our bodies to compensate for the lack of viable glucose levels via decreases in insulin levels and increases insulin resistance in order to suppress utilization of glucose by non-brain tissues to ensure that our brains receive the appropriate amount of glucose. When given a meal, a patient who has fasted will have glucose appear in their urine, while yes, this is a symptom of diabetes, it is because prolonged fasting or starvation leads to decreases in insulin secretion and increases in insulin resistance. With subsequent re-feeding causing only transient hyperglycemia and transient amounts of glucose appearing in the patient's urine. Not only can SPD be caused by prolonged fasting, but can also be caused by severe restriction of calorie and carbohydrate intake, [38]. For example, some patients who undergo severe calorie restrictions can suffer from diabetes-like glucose intolerance [59]. In lieu of that possibility, low calorie diets are still one of the most effective treatments for type 2 diabetes [60–62].A main symptom of starvation is ketosis, the metabolic state in which ketones are used as a substitute source of energy for the brain in response to low levels of glucose availability. The ketogenic diet, a promising treatment for diabetes, can also cause symptoms of SPD in rodents as well as humans [64]. Please note that only one study by Lamont et al warns against the ketogenic diet and that it can favor type 2 diabetes [65]. This warning may not be justified, with multiple studies providing evidence that counters these claims [64,66–68].Rapamycin can also be characterized as a potential caloric restriction mimetic, a class which mimics the anti-aging effects of caloric restriction upon various laboratory animals as well as humans [69–71]. Thus, prolonged treatment of rapamycin will inevitably lead to the emergence of SPD in some patients [72]. This assertion has been confirmed with rapamycin-fed mice, which had developed insulin resistance, glucose intolerance, and mild hyperglycemia [54]. In spite of this, rapamycin-fed mice lived longer and were remarkably healthier than mice fed a standard diet [54]. Further ventures to completely understand why SPD was only observed in a few studies and not in others is needed as our current understanding is lacking [38,73].Most importantly, SPD is reversible and does not lead to complications. Furthermore, we have multiple studies providing evidence that rapamycin reduces the incidence of diabetic complications such as diabetic neuropathy in rodents [42,74–85]. We also have a few studies in which healthy elderly humans were given a chronic treatment of either rapamycin or everolimus with neither treatments causing hyperglycemia in the subjects [8,9,86].The study conducted by Mannick et al further contradicted the assertion that rapamycin treatment causes hyperglycemia with results in which the risk of hyperglycemia was lower in the mTOR inhibitor treatment group than in the placebo group [9].In a small number of cancer patients, high doses of rapamycin or everlominus can cause hyperglycemia, albeit most often in a mild form (grade 1-2) which is reversible and does not interrupt rapamycin treatment [87–89]. Something to quickly note, hyperglycemia is a common side effect of many oncotargeted drugs and is not a manifestation of diabetes. For example everolimus can cause hyperglycemia by decreasing insulin production [89].To be clear, chronic treatment of high doses of rapamycin may cause symptoms of reversible SPD. When such rapamycin-induced SPD occurs, it is at best a relatively rare side effect which could be negated by administering rapamycin intermittently or at lower doses, and if SPD does occur, discontinuation of rapamycin treatment will revert patients to a non-SPD state.In a number of studies conducted with transplant patients, rapamycin and everolimus did not increase the risk of diabetes [90–96]. In Veroux et al’s study not one patient out of twenty one patients treated with rapamycin or its respective analogs developed diabetes. While the incidence of diabetes in patients treated with either cyclosporine or tacrolimus, two immunosuppressant drugs used to stop the rejection of newly transplanted organs by the body, was 7% in comparison [96]. But, most importantly, cyclosporine- or tacrolimus- induced diabetes was resolved in 80% of patients after the conversion from cyclosporine/tacrolimus treatment to that of rapamycin [96].Conversely, a large retrospective study reported on the association between patients being billed by Medicare for diabetes treatment as well as patients undergoing rapamycin treatment. The study suggests a link between rapamycin treatment and an increased risk of diabetes [97]. However, this link is more accurately explained by the interaction between rapamycin and calcineurin inhibitors, which act in a synergistic fashion, increasing each other's levels [96,98,99]. Consequently, it remains unclear whether rapamycin directly increases the risk of diabetes in transplant patients [96]. Moreover, this problem is further complicated by the prevalence of transplant patients spontaneously developing type 2 diabetes without rapamycin treatment [100].
If used properly, rapamycin and its analogs are no more dangerous than over the counter aspirin. Yes, aspirin, whose list of side effects is long and concerning at a surface level. Possible side effects include but are not limited to life threatening gastric bleeding, ringing of the ears, confusion, hallucinations, seizures, severe nausea, vomiting, bloody stools, coughing up blood, fever, and swelling. Yet still, millions of people prophylactically take aspirin daily to prevent cardiovascular disease and cancer let alone the myriad of other reasons to take aspirin heedless to the possible side effects. It was actually calculated that the benefits of aspirin use are greater than their risks [101,102]. The study conducted by Cuzick et al determined that prophylactic aspirin use for a minimum of 5 years at doses between 75 and 325 mg/day appears to have a favorable benefit-harm profile, with longer use likely having a greater positive effect [101]. Thus, the benefits of aspirin use far outweighs the possible adverse effects of aspirin usage.With rapamycin and its respective analogs, the list of possible side effects is less impressive than aspirins, with its most concerning side effects lacking any direct confirmation to have originated directly from rapamycin treatment. At low doses, or when administered as a single high dose, no side effects have been detected thus far within the elderly demographic of rapamycin users [8,9,86,103]. Another possible side effect caused by the treatment of rapamycin and everolimus at high doses is the slowing of cell proliferation, which consequently decreases blood cell counts. This initial side effect cascades into the resulting “sub-side effects” of thrombocytopenia, anemia, and leukopenia. But as it turns out, a mild reduction of platelets could prove to be beneficial as one of the main features of aspirin is a decrease in platelet function with the aim to lower platelet aggregation, thus lowering the chances of fatal clot formations. Another rare side effect is skin and subcutaneous bacterial infections during rapamycin treatment, which is easily remedied with antibiotics.These are the most common side effects of rapamycin treatment, which are easily reversed if need be.There is one crucial reason why the results of many rapamycin studies seem to paint an exaggerated prevalence of adverse side effects. The frequency of rapamycin side effects has often and unfortunately been estimated in studies lacking a placebo group. In cancer and transplant patients, numerous effects erroneously ascribed to rapamycin, such as fatigue, are often caused by the disease itself. A study by Brattström et al actually utilized a placebo group of healthy human volunteers. The goal of the study was to understand the Pharmacokinetics, safety, and tolerability of rapamycin, a then newly christened immunosuppressant drug, with a group of 45 healthy men aged between 19 and 36 years of age.The volunteers were split into 5 groups of 9, with each group differing only in levels of rapamycin treatment received, with one group of 9 volunteers receiving a placebo. Subsequently, the placebo group reported more side effects such as fatigue than did the groups treated with rapamycin [104]. In 2018, two studies on the efficacy of rapamycin treatment within the same context of the study by Brattström et al conducted back in 2000 albeit with healthy elderly male and female humans replacing the more youthful group of men. The first study by Mannick et al determined that TORC1 inhibition via rapamycin enhances immune function and reduces infections in the elderly. The second study by Kraig et al was a randomized control trial in order to establish the feasibility and safety of rapamycin treatment in an elderly demographic ranging from 70-95 years of age as well the subsequent Immunological response, physical performance, and cognitive effects of rapamycin treatment. Both studies demonstrated a clear lack of noticeable side effects caused directly by rapamycin treatment in treatment groups when compared to the side effects felt by their placebo group counterparts. [9,86].While aspirin has the capacity to cause gastric ulceration and bleeding, rapamycin has the capacity to cause stomatitis, inflammation of the mouth and lips, and mucositis, inflammation and ulceration of the digestive tract lining, with a prevalence to also affect the mouth. These side effects arise only when administered at high doses or chronically. Another rare side effect of rapamycin is noninfectious interstitial pneumonitis, which itself is a lung disorder that can cause difficulty breathing, fatigue, and a dry cough to name a few symptoms. Thankfully noninfectious interstitial pneumonitis is easily and successfully treated with corticosteroids [105]. Due to rapamycin’s innate ability to inhibit neutrophil function, a possible increase in the severity of bacterial infections is also a rare side effect [106]. The discontinuation of rapamycin treatment is required for the proper management of these adverse effects. When it comes to anti-aging purposes, rapamycin treatment may be used in either an intermittent fashion or at low daily doses, with side effects easily negated with a discontinuation of treatment.
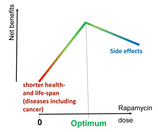
Although nearly all drugs, including nonprescription drugs such as aspirin, can be fatal at sufficiently high doses, there are no known fatal cases of acute rapamycin overdose [103]. For example, in a failed suicide attempt, an 18-year old woman ingested 103 tablets of 1 mg doses rapamycin, with the only detectable effect being an elevation on total blood cholesterol [103]. Additionally this example is the highest known acute rapamycin overdose recorded. In rats, rapamycin’s LD50, a measure of drug lethality determined by the amount of material, given all at once, causes the death of 50% of a group of subjects, could not be determined. This is because rapamycin’s LD50 is higher than 2500mg/kg, well beyond any amount of rapamycin patients could physically be prescribed, even if all rapamycin pills of the prescription were consumed. While a single dose of rapamycin poses no danger, it is still sufficient to extend life and decrease obesity in several rodent models [1,107]. Furthermore, transient treatment with rapamycin can be long lasting, extending the lifespan and preventing obesity long after discontinuation of rapamycin treatment [107–112]. The magnitude of life extension by rapamycin is largely dependent on reaching a high peak blood level [[112-113]. The study of Leontieva et al reached similar conclusions of rapamycin’s efficacy even within an obese mouse model [112]. In 2008 it was concluded that an intermittent treatment of rapamycin would improve regeneration of stem cells [114] without the secondary effect of mTORC2 inhibition, which is caused by an overabundance of available rapamycin. [54,115].Therefore, to avoid possible negative side effects while maximizing the efficacy of rapamycin, the most feasible treatment regimen would be to prolong intervals between rapamycin administrations while keeping the dosage constant. For example, instead of daily rapamycin treatments, a weekly administration of a higher dose can be suggested to achieve a high peak blood level, which is followed by a drug-free period to negate any negative effects. Still, it has been shown in elderly patients that an everyday treatment of rapamycin at 1 mg/day for several weeks produced no negative side effects and has been shown to be safe [86]. Similar results of lifespan extension, enhanced immune function, and a reduction in infections were achieved within an elderly subject pool with low doses of other mTOR inhibitors as well [9]. Another option is an alternating schedule; for example, a 3 month course of weekly rapamycin treatment alternating with a rapamycin treatment free month. Essentially, anti-aging schedules can be tailored to best fit each individual patient, thus optimizing each patient's rapamycin treatment. The optimal anti-aging dose is a personalized maximum dose that does not cause any side effects in each particular patient.
image
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6814615/#r57In conclusion, the side effects of rapamycin are well-known and reversible. When used on an anti-aging schedule, side effects may be absent but, if they do arise, they may be mitigated by combining rapamycin with other anti-aging drugs, such as metformin, or by temporarily discontinuing rapamycin treatment.
On the one hand, from the dawn of civilization humans have dreamed of immortality. On the other hand, from the dawn of civilization a myriad of anti-aging remedies turned out to be empty promises. Even worse, they often shorten lifespan. Three notable examples are antioxidants, human growth hormone, and mercury. The idea that free radicals, or reactive oxygen species (ROS), cause aging was based on a “wild guess,” as Harman, a father of the ROS theory, acknowledged when he titled his paper, “I thought, thought, thought for four months in vain and suddenly the idea came” [116]. The idea is simple and intuitive, and it was widely accepted based on circumstantial evidence. In fact, ROS are inevitable products of metabolism, and they do damage biomolecules. Moreover, excessive ROS can shorten lifespan. Similarly, the atomic bomb can shorten life span. Yet this does not mean that either atomic bombs or oxidants are the cause of normal aging as we know it.Numerous experiments support the ROS theory. However, key experiments ruled the ROS theory out [2,117–122]. To make a long story short, antioxidants could in theory prolong lifespan if mTOR-driven aging were suppressed and we lived long enough to die from ROS-induced post-aging syndrome. Indeed, ROS will kill any organism eventually. However, organisms normally die from mTOR-driven, age-related diseases and aging itself before ROS can kill them. As an analogy, consider most of the passengers on the Titanic. Would antioxidant treatment have been useful to them for life extension? The best way to extend life for members of that group would have been to carry more lifeboats. Only after their safe rescue could one expect antioxidants to potentially increase their life further. Similarly, only after rescue from the mTOR-driven process of aging may antioxidants potentially have an impact.Not surprisingly, antioxidants did not extend lifespan in any clinical trials and were detrimental in some [122–133]. As Ristow et al put it, they were “worse than useless” [119]. For example, in two very large randomized controlled trials, antioxidants increased the incidence of cancer, especially the incidence of lung cancer in smokers [131–133]. Antioxidants also increased all-cause mortality. The results were so disturbing that two trials were stopped earlier than planned [131–133]. Also disturbing is the finding that antioxidants accelerate cancer progression and promote metastasis [134–136]. But despite their biological uselessness, antioxidants continue to be a multibillion-dollar business. They are widely sold as natural products in the forms of nutritional supplements and in foods “rich in antioxidants.”Another example is human growth hormone (HGH), which is widely used for rejuvenation and longevity. Yet, it actually accelerates aging and shortens lifespan [137,138]. Growth hormone is a pro-aging hormone because it indirectly activates mTOR [139]. Notably, the hype around growth hormone is based on a single publication [140], which misinterpreted its acute effects [141].A quite unique example is mercury, which was believed to grant immortality during the Qin Dynasty. The First Emperor of Qin, Qin Shi Huang, in particular, was known to drink mercury in the hopes that it would grant him eternal life. You may not recognise the name, but it is the very same Qin Shi Huang that has the famous terracotta warriors protecting his tomb.Given that all previous anti-aging remedies have failed to meet expectations, it is not surprising that the discovery of the anti-aging effects of rapamycin are being met with skepticism too. But unlike HGH, the effects of rapamycin are not based on one single paper as were HGH, nor is it based on a wild guess as were ROS. When it comes to mercury though, there's no way that consuming mercury will grant any boon other than death, something that rapamycin is incapable of doing as mentioned before [103].
Citations
Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J, Wall VZ, Gagnidze A, Oh K, Wasko BM, Ramos FJ, et al.. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science. 2013; 342:1524–28. 10.1126/science.1244360 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle. 2006; 5:2087–102. 10.4161/cc.5.18.3288 [PubMed] [CrossRef] [Google Scholar]
Blagosklonny MV. An anti-aging drug today: from senescence-promoting genes to anti-aging pill.Drug Discov Today. 2007; 12:218–24. 10.1016/j.drudis.2007.01.004 [PubMed] [CrossRef] [Google Scholar]
Dao V, Liu Y, Pandeswara S, Svatek RS, Gelfond JA, Liu A, Hurez V, Curiel TJ. Immune-Stimulatory Effects of Rapamycin Are Mediated by Stimulation of Antitumor γδ T Cells. Cancer Res. 2016; 76:5970–82. 10.1158/0008-5472.CAN-16-0091 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Jung JW, Veitch M, Bridge JA, Overgaard NH, Cruz JL, Linedale R, Franklin ME, Saunders NA, Simpson F, Frazer IH, Steptoe RJ, Wells JW. Clinically-Relevant Rapamycin Treatment Regimens Enhance CD8+ Effector Memory T Cell Function In The Skin and Allow their Infiltration into Cutaneous Squamous Cell Carcinoma. OncoImmunology. 2018; 7:e1479627. 10.1080/2162402X.2018.1479627 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Bravo-San Pedro JM, Senovilla L. Immunostimulatory activity of lifespan-extending agents. Aging (Albany NY). 2013; 5:793–801. 10.18632/aging.100619 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Svatek RS, Ji N, de Leon E, Mukherjee NZ, Kabra A, Hurez V, Nicolas M, Michalek JE, Javors M, Wheeler K, Sharp ZD, Livi CB, Shu ZJ, et al.. Rapamycin Prevents Surgery-Induced Immune Dysfunction in Patients with Bladder Cancer. Cancer Immunol Res. 2019; 7:466–75. 10.1158/2326-6066.CIR-18-0336 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, Glass DJ, Klickstein LB. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014; 6:268ra179. 10.1126/scitranslmed.3009892 [PubMed] [CrossRef] [Google Scholar]
Mannick JB, Morris M, Hockey HP, Roma G, Beibel M, Kulmatycki K, Watkins M, Shavlakadze T, Zhou W, Quinn D, Glass DJ, Klickstein LB. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci Transl Med. 2018; 10:eaaq1564. 10.1126/scitranslmed.aaq1564 [PubMed] [CrossRef] [Google Scholar]
Demopoulos L, Polinsky M, Steele G, Mines D, Blum M, Caulfield M, Adamkovic A, Liu Q, Harler MB, Hahn C, Singh A. Reduced risk of cytomegalovirus infection in solid organ transplant recipients treated with sirolimus: a pooled analysis of clinical trials. Transplant Proc. 2008; 40:1407–10. 10.1016/j.transproceed.2008.03.084 [PubMed] [CrossRef] [Google Scholar]
Hill JA, Hummel M, Starling RC, Kobashigawa JA, Perrone SV, Arizón JM, Simonsen S, Abeywickrama KH, Bara C. A lower incidence of cytomegalovirus infection in de novo heart transplant recipients randomized to everolimus. Transplantation. 2007; 84:1436–42. 10.1097/01.tp.0000290686.68910.bd [PubMed] [CrossRef] [Google Scholar]
Pascual J, Royuela A, Fernández AM, Herrero I, Delgado JF, Solé A, Guirado L, Serrano T, de la Torre-Cisneros J, Moreno A, Cordero E, Gallego R, Lumbreras C, Aguado JM, and Spanish Society of Transplantation Virological and Immune Response Investigation Study Group. Role of mTOR inhibitors for the control of viral infection in solid organ transplant recipients. Transpl Infect Dis. 2016; 18:819–31. 10.1111/tid.12601 [PubMed] [CrossRef] [Google Scholar]
Keating R, Hertz T, Wehenkel M, Harris TL, Edwards BA, McClaren JL, Brown SA, Surman S, Wilson ZS, Bradley P, Hurwitz J, Chi H, Doherty PC, et al.. The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nat Immunol. 2013; 14:1266–76. 10.1038/ni.2741 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Diken M, Kreiter S, Vascotto F, Selmi A, Attig S, Diekmann J, Huber C, Türeci Ö, Sahin U. mTOR inhibition improves antitumor effects of vaccination with antigen-encoding RNA. Cancer Immunol Res. 2013; 1:386–92. 10.1158/2326-6066.CIR-13-0046 [PubMed] [CrossRef] [Google Scholar]
Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, Kirk AD, Larsen CP, Ford ML. Cutting edge: rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses.J Immunol. 2010; 185:2004–08. 10.4049/jimmunol.1001176 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Hurez V, Dao V, Liu A, Pandeswara S, Gelfond J, Sun L, Bergman M, Orihuela CJ, Galvan V, Padrón Á, Drerup J, Liu Y, Hasty P, et al.. Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune-deficient mice. Aging Cell. 2015; 14:945–56. 10.1111/acel.12380 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Hinojosa CA, Mgbemena V, Van Roekel S, Austad SN, Miller RA, Bose S, Orihuela CJ. Enteric-delivered rapamycin enhances resistance of aged mice to pneumococcal pneumonia through reduced cellular senescence. Exp Gerontol. 2012; 47:958–65. 10.1016/j.exger.2012.08.013 [PMC free article][PubMed] [CrossRef] [Google Scholar]
Kuss-Duerkop SK, Wang J, Mena I, White K, Metreveli G, Sakthivel R, Mata MA, Muñoz-Moreno R, Chen X, Krammer F, Diamond MS, Chen ZJ, García-Sastre A, Fontoura BM. Influenza virus differentially activates mTORC1 and mTORC2 signaling to maximize late stage replication. PLoS Pathog. 2017; 13:e1006635. 10.1371/journal.ppat.1006635 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Ranadheera C, Coombs KM, Kobasa D. Comprehending a Killer: The Akt/mTOR Signaling Pathways Are Temporally High-Jacked by the Highly Pathogenic 1918 Influenza Virus. EBioMedicine. 2018; 32:142–63. 10.1016/j.ebiom.2018.05.027 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004; 18:446–49. 10.1111/j.1399-0012.2004.00188.x [PubMed] [CrossRef] [Google Scholar]
Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies.Transplantation. 2005; 80:883–89. 10.1097/01.TP.0000184006.43152.8D [PubMed] [CrossRef] [Google Scholar]
Campistol JM, Eris J, Oberbauer R, Friend P, Hutchison B, Morales JM, Claesson K, Stallone G, Russ G, Rostaing L, Kreis H, Burke JT, Brault Y, et al.. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. J Am Soc Nephrol. 2006; 17:581–89. 10.1681/ASN.2005090993 [PubMed] [CrossRef] [Google Scholar]
Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, Broeders N, del Marmol V, Chatelet V, Dompmartin A, Kessler M, Serra AL, Hofbauer GF, et al., and TUMORAPA Study Group. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012; 367:329–39. 10.1056/NEJMoa1204166 [PubMed] [CrossRef] [Google Scholar]
Dantal J, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, Broeders N, Del Marmol V, Chatelet V, Dompmartin A, Kessler M, Serra A, Hofbauer GF, et al., and TUMORAPA Study Group. Sirolimus for Secondary Prevention of Skin Cancer in Kidney Transplant Recipients: 5-Year Results. J Clin Oncol. 2018; 36:2612–20. 10.1200/JCO.2017.76.6691 [PubMed] [CrossRef] [Google Scholar]
Patlolla JM, Kopelovich L, Qian L, Zhang Y, Kumar G, Madka V, Mohammed A, Biddick L, Sadeghi M, Lightfoot S, Rao CV. Early and delayed intervention with rapamycin prevents NNK-induced lung adenocarcinoma in A/J mice. Oncol Rep. 2015; 34:2925–34. 10.3892/or.2015.4277 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Stallone G, Infante B, Prisciandaro C, Grandaliano G. mTOR and Aging: An Old Fashioned Dress.Int J Mol Sci. 2019; 20:E2774. 10.3390/ijms20112774 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Blagosklonny MV. Prevention of cancer by inhibiting aging. Cancer Biol Ther. 2008; 7:1520–24. 10.4161/cbt.7.10.6663 [PubMed] [CrossRef] [Google Scholar]
Granville CA, Warfel N, Tsurutani J, Hollander MC, Robertson M, Fox SD, Veenstra TD, Issaq HJ, Linnoila RI, Dennis PA. Identification of a highly effective rapamycin schedule that markedly reduces the size, multiplicity, and phenotypic progression of tobacco carcinogen-induced murine lung tumors.Clin Cancer Res. 2007; 13:2281–89. 10.1158/1078-0432.CCR-06-2570 [PubMed] [CrossRef] [Google Scholar]
Kopelovich L, Fay JR, Sigman CC, Crowell JA. The mammalian target of rapamycin pathway as a potential target for cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2007; 16:1330–40. 10.1158/1055-9965.EPI-07-0045 [PubMed] [CrossRef] [Google Scholar]
Mercier I, Camacho J, Titchen K, Gonzales DM, Quann K, Bryant KG, Molchansky A, Milliman JN, Whitaker-Menezes D, Sotgia F, Jasmin JF, Schwarting R, Pestell RG, et al.. Caveolin-1 and accelerated host aging in the breast tumor microenvironment: chemoprevention with rapamycin, an mTOR inhibitor and anti-aging drug. Am J Pathol. 2012; 181:278–93. 10.1016/j.ajpath.2012.03.017 [PMC free article][PubMed] [CrossRef] [Google Scholar]
Kawabata S, Mercado-Matos JR, Hollander MC, Donahue D, Wilson W 3rd, Regales L, Butaney M, Pao W, Wong KK, Jänne PA, Dennis PA. Rapamycin prevents the development and progression of mutant epidermal growth factor receptor lung tumors with the acquired resistance mutation T790M. Cell Rep. 2014; 7:1824–32. 10.1016/j.celrep.2014.05.039 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Blagosklonny MV. Immunosuppressants in cancer prevention and therapy. OncoImmunology. 2013; 2:e26961. 10.4161/onci.26961 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Antoch MP, Blagosklonny MV. Rapamycin extends maximal lifespan in cancer-prone mice.Am J Pathol. 2010; 176:2092–97. 10.2353/ajpath.2010.091050 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Komarova EA, Antoch MP, Novototskaya LR, Chernova OB, Paszkiewicz G, Leontieva OV, Blagosklonny MV, Gudkov AV. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/- mice. Aging (Albany NY). 2012; 4:709–14. 10.18632/aging.100498 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Hasty P, Livi CB, Dodds SG, Jones D, Strong R, Javors M, Fischer KE, Sloane L, Murthy K, Hubbard G, Sun L, Hurez V, Curiel TJ, Sharp ZD. eRapa restores a normal life span in a FAP mouse model. Cancer Prev Res (Phila). 2014; 7:169–78. 10.1158/1940-6207.CAPR-13-0299 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Livi CB, Hardman RL, Christy BA, Dodds SG, Jones D, Williams C, Strong R, Bokov A, Javors MA, Ikeno Y, Hubbard G, Hasty P, Sharp ZD. Rapamycin extends life span of Rb1+/- mice by inhibiting neuroendocrine tumors. Aging (Albany NY). 2013; 5:100–10. 10.18632/aging.100533 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Ehninger D, Neff F, Xie K. Longevity, aging and rapamycin. Cell Mol Life Sci. 2014; 71:4325–46. 10.1007/s00018-014-1677-1 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Blagosklonny MV. Fasting and rapamycin: diabetes versus benevolent glucose intolerance. Cell Death Dis. 2019; 10:607. 10.1038/s41419-019-1822-8 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis.J Cell Biol. 2004; 167:399–403. 10.1083/jcb.200408161 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Um SH, D’Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006; 3:393–402. 10.1016/j.cmet.2006.05.003 [PubMed] [CrossRef] [Google Scholar]
Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006; 124:471–84. 10.1016/j.cell.2006.01.016 [PubMed] [CrossRef] [Google Scholar]
Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Rüegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, et al.. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011; 121:2181–96. 10.1172/JCI44771 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011; 12:21–35. 10.1038/nrm3025 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev. 2013; 23:53–62. 10.1016/j.gde.2012.12.005 [PubMed] [CrossRef] [Google Scholar]
Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, Roth E, Fürnsinn C, Promintzer M, Anderwald C, Bischof M, Roden M. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007; 56:1600–07. 10.2337/db06-1016 [PubMed] [CrossRef] [Google Scholar]
Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, Nowotny P, Waldhäusl W, Marette A, Roden M. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005; 54:2674–84. 10.2337/diabetes.54.9.2674 [PubMed] [CrossRef] [Google Scholar]
Zhou W, Ye S. Rapamycin improves insulin resistance and hepatic steatosis in type 2 diabetes rats through activation of autophagy. Cell Biol Int. 2018; 42:1282–91. 10.1002/cbin.11015 [PubMed] [CrossRef] [Google Scholar]
Ueno M, Carvalheira JB, Tambascia RC, Bezerra RM, Amaral ME, Carneiro EM, Folli F, Franchini KG, Saad MJ. Regulation of insulin signalling by hyperinsulinaemia: role of IRS-1/2 serine phosphorylation and the mTOR/p70 S6K pathway. Diabetologia. 2005; 48:506–18. 10.1007/s00125-004-1662-6 [PubMed] [CrossRef] [Google Scholar]
Reifsnyder PC, Flurkey K, Te A, Harrison DE. Rapamycin treatment benefits glucose metabolism in mouse models of type 2 diabetes. Aging (Albany NY). 2016; 8:3120–30. 10.18632/aging.101117 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
He S, Zhang Y, Wang D, Tao K, Zhang S, Wei L, Chen Q. Rapamycin/GABA combination treatment ameliorates diabetes in NOD mice. Mol Immunol. 2016; 73:130–37. 10.1016/j.molimm.2016.01.008 [PubMed] [CrossRef] [Google Scholar]
Leontieva OV, Demidenko ZN, Blagosklonny MV. Rapamycin reverses insulin resistance (IR) in high-glucose medium without causing IR in normoglycemic medium. Cell Death Dis. 2014; 5:e1214. 10.1038/cddis.2014.178 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Makki K, Taront S, Molendi-Coste O, Bouchaert E, Neve B, Eury E, Lobbens S, Labalette M, Duez H, Staels B, Dombrowicz D, Froguel P, Wolowczuk I. Beneficial metabolic effects of rapamycin are associated with enhanced regulatory cells in diet-induced obese mice. PLoS One. 2014; 9:e92684. 10.1371/journal.pone.0092684 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Latest Longevity Research Straight to your Inbox
Sign up for The Longevity Blueprint, a weekly newsletter from Healthspan analyzing the latest longevity research.
Sign up for The Longevity Blueprint, a weekly newsletter from Healthspan analyzing the latest longevity research.