What is Rapamycin?
Rapamycin is a nonribosomal peptide that is an antifungal, immunomodulator, anti-proliferative, and anti-aging metabolite that is produced by Streptomyces hygroscopicus. Rapamycin is a key regulator as an allosteric inhibitor that targets the protein, the mammalian target of rapamycin (mTOR), which is produced by the mTOR gene. mTOR itself is a protein kinase that regulates metabolism, cell growth, cell proliferation, cell survival, cell motility, protein synthesis, autophagy, and transcription. When the mTOR pathway suffers from deregulation, far-reaching and catastrophic problems arise with mTOR being implicated in diseases such as cancer, diabetes, obesity, neurological diseases, genetic disorders, various age-related diseases, and is a key regulatory component of the aging process. Johnson et al., 2013a, Laplante and Sabatini, 2012
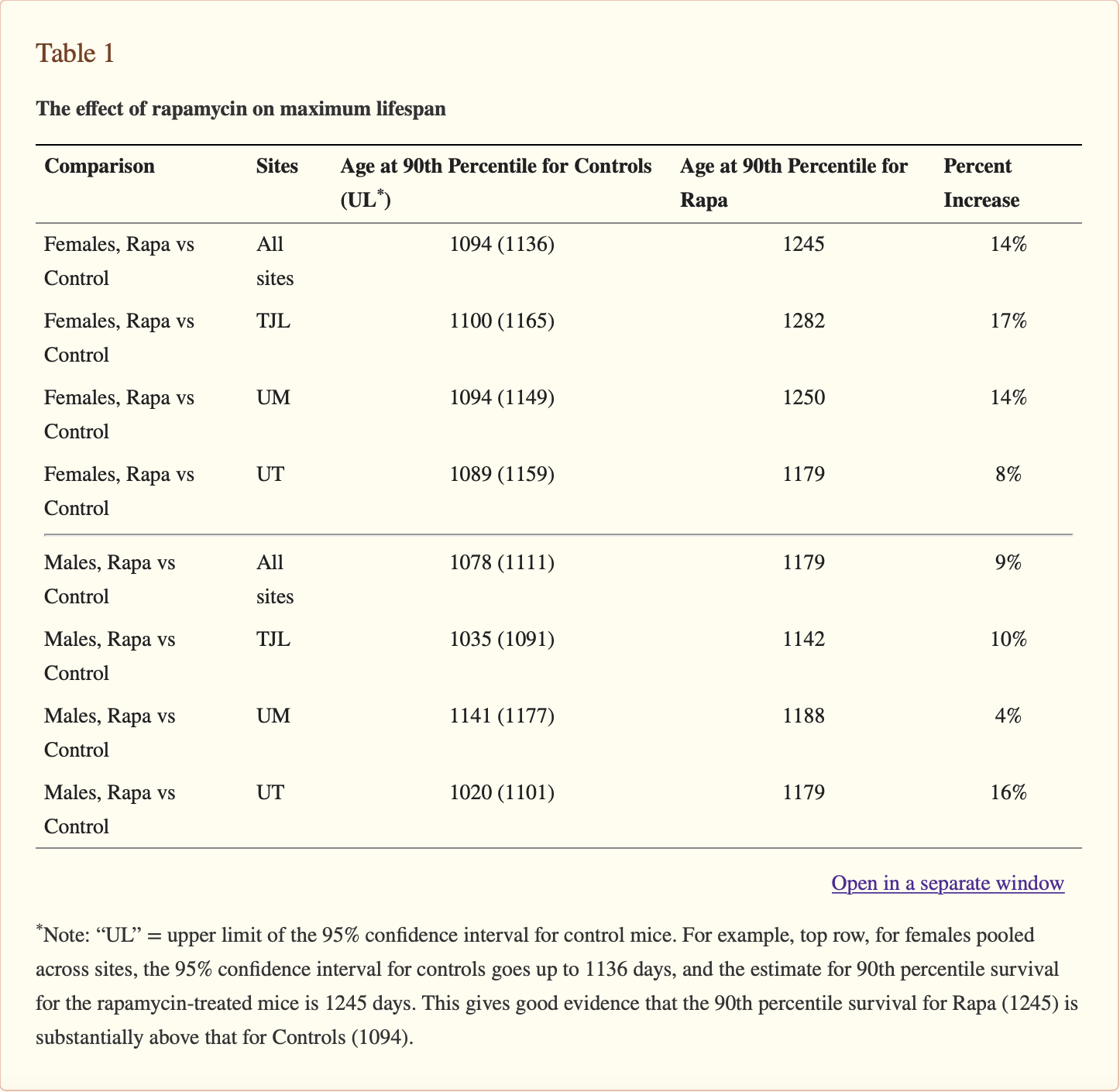
Li, Jing et al. “Rapamycin: one drug, many effects.” Cell metabolism vol. 19,3 (2014): 373-9. doi:10.1016/j.cmet.2014.01.001
A more recent discovery, rapamycin and its interactions with the human body have only begun being understood since 2011. Firstly, rapamycin joins with FKBP12, a binding protein, thus forming a complex. This rapamycin-FKBP12 complex then goes on to specifically bind to mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 along with mTORC2 are the two complexes that utilize mTOR, with mTOR forming one of the core components for the two functionally distinct complexes. Note that only mTORC1 is acutely sensitive to rapamycin inhibition and mTORC2 only shows inhibition when undergoing long-term or chronic rapamycin exposure via sequestering newly synthesized mTOR molecules that are “free floating.”
Loewith and Hall, 2011, Laplante and Sabatini, 2012, Ma and Blenis, 2009
Do you need a prescription for Rapamycin?
Yes.
What is mTOR?
mTOR is a serine/threonine protein kinase of the phosphatidylinositol-3-OH kinase (PI(3)K)-related family of enzymes, which works as a key regulator of cellular growth and metabolism with regards to nutrient and hormonal fluctuations. mTOR stems from the independent studies of rapamycin in S. cerevisiae with the TOR1 and TOR2 genes acting as genetic mediators of rapamycin’s ability to inhibit growth. Once purified and isolated from mammalian cells the mTOR protein was determined to be the specific and physical target of rapamycin. Cornu et al., 2013, Laplante and Sabatini, 2012, 2
The disruption of mTORC1 by the rapamycin-FKBP12 complex is achieved via the disruption of the interaction between mTOR and raptor. This interaction between mTOR and raptor is quintessential for mTOR to carry out its functions. Lipid synthesis regulation by mTORC1 is believed to occur primarily through sterol-regulatory-element-binding protein transcription factors (SREBP1). mTORC1 negatively regulates autophagy through various mechanisms
Components of mTORC1 and mTORC2
mTORC1 -
- mTOR - mammalian target of rapamycin
- mLST8 - mammalian lethal with sec-13 protein 8
- raptor - regulatory associated protein of TOR
- deptor - DEP-domain containing mTOR interacting protein
- PRAS40 - Proline-rich Akt substrate 40kDa
- TTI1- TEL2 interacting protein
mTORC2 -
- mTOR - mammalian target of rapamycin
- Rictor - rapamycin insensitive companion of mTOR
- mSIN1 - stress-activated protein kinase-interacting protein 1
- mLST8 - mammalian lethal with sec-13 protein 8
- deptor - DEP-domain containing mTOR interacting protein
- Protor ½ - protein observed with rictor ½
- TTI1- TEL2 interacting protein
Cornu et al., 2013, Laplante and Sabatini, 2012
mTOR and Rapamycin
Understanding the relationship between mTOR and rapamycin is key to a healthy future. mTORC1 is a signal integrator which responds to a plethora of signals from growth factors, nutrients, energy, and oxygen to regulate and control processes that are necessary for the survival, growth, and proliferation of cells such as autophagy, energy metabolism, the creation and synthesis of mRNA, protein, lipid, and nucleotides (Fig. 1). Therefore, deregulation of mTORC1 is consistently found in cancers, genetic disorders, and aging and age-related diseases. When it comes to mTORC2 we know it is a regulatory body in cell survival via the activation of Akt and SGK1 as well as being a regulator of actin cytoskeleton organization via the activation of PKCα, paxillin and the small GTPases, Rho and Rac. Johnson et al., 2013a, Laplante and Sabatini, 2012
Johnson, Simon C et al. “mTOR is a key modulator of aging and age-related disease.” Nature vol. 493,7432 (2013): 338-45. doi:10.1038/nature11861
Aging and Rapamycin
With the regulation of aging by mTOR, rapamycin has a profound opportunity to scale back the clock if you will and reverse aging. Inhibition of mTOR has been found to extend the lifespan of invertebrates such as yeast, nematodes, and fruit flies. In 2009, Harrison et al displayed the ability of rapamycin to extend median and maximal life of both male and female heterogeneous mice with treatment beginning at 9 or 20 months of age for a duration of 1.5 to 2 years. Harrison et al set a further precedent with the trials being the first conducted on mammals. Harrison et al., 2009, Lamming et al., 2013
Increases in activation of mTORC1 is consistently seen in a number of cancers due to gain-of-function mutations occurring in oncogenes, and/or loss-of-function mutations occurring in tumor suppressors, which are upstream regulators of mTORC1. Because these mutations provide cancer cells with a selective growth advantage. This “advantage” forces the cells to go through drastic changes in nutrient uptake and energy metabolism, which are directly controlled by mTORC1, to meet the high demand of cancer growth. Similarly, the ramp up in protein synthesis isn't the only problem to arise with oncogenic activation of mTORC1 causing cancerous cells to undergo metabolic reprogramming. This reprogramming entails the increase in glycolysis, which is the process utilized by our cells to break down glucose into usable energy for our metabolism. This occurs via the upregulating of Hypoxia-inducible factor alpha (HIF1α) and c-Myc. The upregulation of HIF1α and c-Myc stimulates the production of lipids and the activation of the pentose phosphate pathway via the binding protein SREBP-1; and positively controls glutamine metabolism by repressing SIRT4. Therefore, if cancer metabolism is controlled by mTORC1 then rapamycin which specifically targets mTORC1, can be expected to hinder cancer metabolism. Menon and Manning, 2008, Yecies and Manning, 2011, Csibi et al., 2013
mTOR’s ability to control and regulate the aging process is rapamycin’s opportunity to control and regulate the aging process. With genetic and pharmacological inhibition of mTOR signaling extending the lifespans of a plethora of various species. Research conducted by both Harrison et al and Lamming et al have shown that rapamycin has a positive influence on lifespan. Furthermore, Harrison et al showed that rapamycin has an indiscriminate positive effect on non-clonal mice, with several analogous studies producing evidence that confirms this theory. In other words, rapamycin is a genetically indiscriminate positive controller of the aging process. Lamming et al., 2013, Harrison et al., 2009
The ultimate explanation on how rapamycin achieves such a dramatic influence on longevity is between two possible explanations: Firstly, rapamycin increases lifespan via the slowing of aging; or secondly, these feats of longevity is due to rapamycin inhibiting the metabolic diseases or lethal neoplastic diseases, independent of aging and its detrimental effects. Wilkinson et al tested the first hypothesis that rapamycin increases lifespan via the slowing or retardation of aging.
To test the veracity of the claim that rapamycin retards aging, Wilkinson et al utilized a heterogeneous mouse model, or mice with high genetic variability from one another; ie not clones of one another. With his mice, Wilkinson et al analyzed age-related pathologies and age-dependent spontaneous activity of said mice with rapamycin treatment occurring at 9 months of age for a treatment duration of 1 year. The results suggest that age-dependent changes occur more slowly within the rapamycin-treated mice, which includes age-related alterations in the heart, liver, endometrium, adrenal gland, and tendon elasticity. Additionally, Wilkinson et al was successful in showing that rapamycin diminishes age-related decline in spontaneous activity of the mice as well. Wilkinson et al., 2012
Rapamycin and cancer:
In addition to its possible anti-aging properties, rapamycin has anti-proliferative properties in various forms of cancer as well. Now, please note that rapamycin’s effect on longevity could be due to the suppression of specific pathologies that decrease longevity, like cancer. A dysfunctional increase in mTORC1 activation is consistently observed in various cancers due to gain-of-function mutations in oncogenes and/or loss-of-function mutations in tumor suppressors which are upstream regulators of mTORC1. These mutations are key to proliferation of cancer due to the selective growth advantage they provide. These cancer cells often have drastic and fundamental alterations in metabolism and nutrient uptake to meet the demand for growth. Both these processes’ are directly controlled by the mTORC1 pathway. Additionally oncogenic activation of mTORC1 promotes gene expression that helps with the metabolic reprogramming of cancer cells. Activation of mTORC1 also promotes glycolysis via the upregulation of Hypoxia-inducible factor alpha (HIF1α) and c-Myc; stimulates the biosynthesis of lipids and the pentose phosphate pathway through sterol regulatory element binding protein 1 (SREBP-1); and positively regulates glutamine metabolism via the repression of SIRT4. Therefore, drugs that selectively target mTORC1, such as rapamycin, can be fully expected to impair cancer metabolism and are considered to be promising anti-cancer therapies. Menon and Manning, 2008, Yecies and Manning, 2011, Csibi et al., 2013
Rapamycin has poor solubility and its pharmacokinetics is also less than stellar, which have been the main proponents behind the development of various rapamycin analogs (rapalogs). Temsirolimus and everolimus are two water-soluble derivatives of rapamycin that were approved in 2007 and 2009, for the treatment of renal cancer carcinoma by the Food and Drug Administration (FDA). In 2011, the FDA also approved the use of everolimus for patients with progressives neuroendocrine tumors of pancreatic origin. In 2009, the EU, had several clinical trials with temsirolimus treatment for advanced neuroendocrine carcinoma, advanced or recurrent endometrial cancer, and relapsed or refractory mantle cell lymphoma. Everolimus trials were conducted with patients who had advanced gastric cancer, advanced non-small cell lung cancer, and advanced hepatocellular carcinoma. In 2011, Wander et al conducted trials of another rapalog, ridaforolimus, via clinical trials for advanced bone and soft-tissue sarcomas and a variety of other tumors. Wander et al., 2011
As promising as these rapalogs sound, they have only had a modest effect in major solid tumors in clinical trials. While the true reason behind such muddling success hasn't been established, it might be due to the large number of mTORC1-regulated negative feedback loops that suppress upstream signaling systems such as activation of receptor tyrosine kinases, PI3K-Akt signaling and the Ras-ERK pathway and which can also be re-activated via rapamycin. With the limitations in mind, several other options have also been explored, such as the development of ATP-competitive mTOR inhibitors, which block both mTORC1 and mTORC2 activity. Due to the high sequence homology shared between mTOR and PI3K, some compounds that were originally defined and identified as PI3K inhibitors actually were shown to be inhibitors of mTOR. While rapamycin is a specific allosteric inhibitor of mTORC1, the ATP-competitive inhibitors target the catalytic site directly and prevent feedback-mediated PI3K/Akt activation and could potentially provide a broader, more potent, and a longer sustained inhibition of mTOR. Benjamin et al., 2011
How does rapamycin impact metabolism?
Unsurprisingly mTOR is a keystone regulator of cellular metabolism and responds accordingly to nutrients and growth factors to maintain the balance between anabolic and catabolic processes. We know that when you're fasting, your muscles and liver produce glucose via the breakdown of glycogen and synthesize glucose. Additionally the generation of fatty acids via lipolysis occurs in adipose tissue when fasting. We also know that once feeding occurs glycogen synthesis is favored in your muscles and liver and lipid uptake is favored in adipose tissue. Furthermore, dysregulation of mTOR signaling has been linked to the development of several metabolic diseases, with prime examples being diabetes and obesity. Inhibition of mTOR via rapamycin has been shown to be both beneficial as well as detrimental with its effects on metabolism. For example, when acute rapamycin treatment is given it improves insulin sensitivity in both vitro and in vivo via the disruption of a S6K-mediated feedback loop. Additionally, rapamycin treatment has been shown to also inhibit human adipocyte differentiation in vitro as well as protects against high fat diet-induced obesity in C57BL/6J mice. With rapamycin appearing to be capable of extending the lifespan of mice and preventing the onset of various age-related diseases. Please note that in other studies conducted there were deleterious metabolic effects reported in subjects. For example, Fraenkel et al discovered that a 2 week treatment of rapamycin worsened hyperglycemia in a nutrition-dependent type 2 diabetes mouse model. Quite similarly, Chang et al showed exacerbation of glucose intolerance in diet-induced obesity KK/HIJ mice when they were given a 6 week rapamycin treatment. However, Houde et al’s study demonstrated that a 2 week treatment of rapamycin promoted insulin resistance and hyperlipidemia in rats. Unfortunately, so far it is still unclear how rapamycin is capable of being both a positive and negative effector. However, we do know that rapamycin exerts the different effects via separate mechanisms from Lamming et al’s study in 2012. They determined that reduced mTOR1 activity via rapamycin increases longevity and maintains glucose homeostasis while reduced mROR2 activity via rapamycin contributes to insulin resistance in vivo. Fang et al further suggests that these previous findings can be explained via the duration of rapamycin treatment. This study, conducted in 2013, compared various metabolic effects of rapamycin treatment with male mice with treatments of 2, 6, or 20 weeks. The mice displayed a smaller pancreas and an enlarged liver. However, with prolonged treatment, these changes revert back to their previous pre-rapamycin levels while adiposity, body weight, and food consumption were significantly reduced. More interestingly though, insulin sensitivity was altered with respect to the different lengths of rapamycin treatment. So we know that under normal physiological conditions, your body's insulin suppresses hepatic gluconeogenesis while increasing lipogenesis, but if you have impaired insulin sensitivity, your body produces excess insulin to compensate for the insulin insensitivity. In 2010, Houde et al increased insulin levels in mice after they had undergone a rapamycin treatment of 2 weeks. The mice became glucose intolerant and insulin resistant. Unsurprisingly, the mice exhibited an improvement in insulin sensitivity after just 6 weeks of rapamycin treatment and by the 20th week of rapamycin treatment, the mice’s insulin levels were decreased while insulin sensitivity increased. Please note, additional metabolic effects were altered dependent on treatment duration including the mice’s lipid profile, oxygen consumption and ketogenesis. These findings in mouse models are pivotal in understanding how long-term rapamycin treatment extends longevity. However promising these results are, how they translate to human aging is still yet to be defined. Laplante and Sabatini, 2012, Krebs et al., 2007, Tremblay and Marette, 2001, Bell et al., 2000, Chang et al., 2009a, Fraenkel et al., 2008, Chang et al., 2009b, Lamming et al., 2012, Fang et al., 2013, Houde et al., 2010
Despite rapamycin being such a recent discovery, it already is well storied with multiple studies and promising discoveries. Understanding rapamycin’s mechanism of action and further understanding mTOR and its respective signaling network has progressed by leaps and bounds. Uncontrolled mTORC1-mediated signaling is often seen within human diseases. Thus it was thought that pharmacological inhibition of mTOR via rapamycin would have a significant and wide range of clinical effects. Despite rapamycin-based therapies being shown to have benefits for patients with RCC, TSC, and LAM-related tumors, the application of rapamycin as a monotherapy in a wide range of metabolic diseases, especially in treating cancers, is somewhat limited due to its modest efficacy. This could be exp,ained via the inability of rapamycin to complete cblock mTORC1-mediated signaling events, the presence of several feedback loops as well the up-regulation of compensatory pathways that promote cell survival and growth. Therefore, there’s a crucial need to further define and understand these signaling processes as well as develop strategies that are able to bypass these drawbacks. With the recent emergence of combination therapies with rapamycin the ability to increase efficacy and bypass feedback activation of survival pathways. Further efforts that focus on the exploration of novel drug combinations with optimal doses will have remarkable potential to yield improvements in efficacy and safety profiles. Additionally, a significant boone to public health remains for the discovery of new pathway inhibitors, as well as the possibility that existing bioactives may directly or indirectly reduce mTORC1 and/or mTORC2 activity in monotherapy or in combination therapy.
- Alain T, Morita M, Fonseca BD, Yanagiya A, Siddiqui N, Bhat M, Zammit D, Marcus V, Metrakos P, Voyer LA, et al. eIF4E/4E-BP ratio predicts the efficacy of mTOR targeted therapies. Cancer research. 2012;72:6468–6476. [PubMed] [Google Scholar]
- Bell A, Grunder L, Sorisky A. Rapamycin inhibits human adipocyte differentiation in primary culture. Obesity research. 2000;8:249–254. [PubMed] [Google Scholar]
- Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nature reviews Drug discovery. 2011;10:868–880. [PubMed] [Google Scholar]
- Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, Sauter M, Nonomura N, Brakemeier S, de Vries PJ, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:817–824. [PubMed] [Google Scholar]
- Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151.[PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Rapamycin extends life- and health span because it slows aging. Aging. 2013;5:592–598. [PMC free article][PubMed] [Google Scholar]
- Bove J, Martinez-Vicente M, Vila M. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci. 2011;12:437–452. [PubMed] [Google Scholar]
- Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer cell. 2011;19:58–71.[PMC free article] [PubMed] [Google Scholar]
- Chang GR, Chiu YS, Wu YY, Chen WY, Liao JW, Chao TH, Mao FC. Rapamycin protects against high fat diet-induced obesity in C57BL/6J mice. Journal of pharmacological sciences. 2009a;109:496–503. [PubMed] [Google Scholar]
- Chang GR, Wu YY, Chiu YS, Chen WY, Liao JW, Hsu HM, Chao TH, Hung SW, Mao FC. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin Pharmacol Toxicol. 2009b;105:188–198.[PubMed] [Google Scholar]
- Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17414–17419. [PMC free article] [PubMed] [Google Scholar]
- Chung J, Grammer TC, Lemon KP, Kazlauskas A, Blenis J. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994;370:71–75.[PubMed] [Google Scholar]
- Chung J, Kuo CJ, Crabtree GR, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992;69:1227–1236.[PubMed] [Google Scholar]
- Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Current opinion in genetics & development. 2013;23:53–62. [PubMed] [Google Scholar]
- Csibi A, Fendt SM, Li C, Poulogiannis G, Choo AY, Chapski DJ, Jeong SM, Dempsey JM, Parkhitko A, Morrison T, et al. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell. 2013;153:840–854.[PMC free article] [PubMed] [Google Scholar]
- Fang Y, Westbrook R, Hill C, Boparai RK, Arum O, Spong A, Wang F, Javors MA, Chen J, Sun LY, et al. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 2013;17:456–462. [PMC free article] [PubMed] [Google Scholar]
- Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, Berthault MF, Magnan C, Cerasi E, Kaiser N, et al. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008;57:945–957. [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395.[PMC free article] [PubMed] [Google Scholar]
- Houde VP, Brule S, Festuccia WT, Blanchard PG, Bellmann K, Deshaies Y, Marette A. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59:1338–1348. [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013a;493:338–345. [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J, Wall VZ, Gagnidze A, et al. mTOR Inhibition Alleviates Mitochondrial Disease in a Mouse Model of Leigh Syndrome. Science 2013b [PMC free article] [PubMed] [Google Scholar]
- Kang SA, Pacold ME, Cervantes CL, Lim D, Lou HJ, Ottina K, Gray NS, Turk BE, Yaffe MB, Sabatini DM. mTORC1 phosphorylation sites encode their sensitivity to starvation and rapamycin. Science. 2013;341:1236566. [PMC free article][PubMed] [Google Scholar]
- Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, Roth E, Furnsinn C, Promintzer M, Anderwald C, et al. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007;56:1600–1607.[PubMed] [Google Scholar]
- Kuo CJ, Chung J, Fiorentino DF, Flanagan WM, Blenis J, Crabtree GR. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature. 1992;358:70–73. [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643.[PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Sabatini DM, Baur JA. Rapalogs and mTOR inhibitors as anti-aging therapeutics. The Journal of clinical investigation. 2013;123:980–989. [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. [PMC free article] [PubMed] [Google Scholar]
- Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189:1177–1201.[PMC free article] [PubMed] [Google Scholar]
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318.[PubMed] [Google Scholar]
- Marsh DJ, Trahair TN, Martin JL, Chee WY, Walker J, Kirk EP, Baxter RC, Marshall GM. Rapamycin treatment for a child with germline PTEN mutation. Nat Clin Pract Oncol. 2008;5:357–361.[PubMed] [Google Scholar]
- McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. [PMC free article] [PubMed] [Google Scholar]
- Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends in biochemical sciences. 2011;36:320–328. [PMC free article] [PubMed] [Google Scholar]
- Menon S, Manning BD. Common corruption of the mTOR signaling network in human tumors. Oncogene. 2008;27(Suppl 2):S43–51. [PMC free article] [PubMed] [Google Scholar]
- Muncy J, Butler IJ, Koenig MK. Rapamycin reduces seizure frequency in tuberous sclerosis complex. J Child Neurol. 2009;24:477. [PMC free article] [PubMed] [Google Scholar]
- Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schroder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, et al. Rapamycin extends murine lifespan but has limited effects on aging. The Journal of clinical investigation. 2013;123:3272–3291.[PMC free article] [PubMed] [Google Scholar]
- Nyfeler B, Chen Y, Li X, Pinzon-Ortiz M, Wang Z, Reddy A, Pradhan E, Das R, Lehar J, Schlegel R, et al. RAD001 enhances the potency of BEZ235 to inhibit mTOR signaling and tumor growth. PloS one. 2012;7:e48548. [PMC free article] [PubMed] [Google Scholar]
- Price DJ, Grove JR, Calvo V, Avruch J, Bierer BE. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992;257:973–977. [PubMed] [Google Scholar]
- Sato A, Kasai S, Kobayashi T, Takamatsu Y, Hino O, Ikeda K, Mizuguchi M. Rapamycin reverses impaired social interaction in mouse models of tuberous sclerosis complex. Nat Commun. 2012;3:1292. [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Sabatini DM. Rapamycin inhibits mTORC1, but not completely. Autophagy. 2009;5:725–726. [PubMed] [Google Scholar]
- Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. The Journal of biological chemistry. 2001;276:38052–38060. [PubMed] [Google Scholar]
- Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. The Journal of clinical investigation. 2011;121:1231–1241. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682.[PMC free article] [PubMed] [Google Scholar]
- Yecies JL, Manning BD. Transcriptional control of cellular metabolism by mTOR signaling. Cancer research. 2011;71:2815–2820. [PMC free article] [PubMed] [Google Scholar]
- Yu J, Henske EP. mTOR activation, lymphangiogenesis, and estrogen-mediated cell survival: the “perfect storm” of pro-metastatic factors in LAM pathogenesis. Lymphatic research and biology. 2010;8:43–49. [PMC free article] [PubMed] [Google Scholar]
- Zaytseva YY, Valentino JD, Gulhati P, Evers BM. mTOR inhibitors in cancer therapy. Cancer Lett. 2012;319:1–7. [PubMed] [Google Scholar]