Methylene Blue for the Aging Brain: Mitochondrial Mechanisms Driving Neuroprotective and Cognitive Benefits
Methylene Blue Enhances Mitochondrial Efficiency and ATP Production: One of methylene blue’s most consistently documented effects is its ability to enhance mitochondrial energy production by supporting electron flow through the electron transport chain (ETC). Studies show that in its reduced form, leucomethylene blue, it donates electrons directly to cytochrome c—bypassing damaged segments of the ETC and sustaining ATP synthesis. In cell culture and animal models, low doses (0.5–4 mg/kg) of methylene blue increased cellular oxygen consumption by up to 70% and boosted ATP production by approximately 30%. These changes indicate a robust increase in mitochondrial output and set the stage for its broad utility in energy-deficient states.
Redox Cycling Makes Methylene Blue a Reusable Antioxidant: Most antioxidants neutralize a single ROS molecule and are then consumed in the process. Methylene blue, by contrast, can cycle between oxidized and reduced states indefinitely, acting more like a rechargeable antioxidant than a one-time-use agent. This property allows it to buffer redox imbalances continuously, reducing oxidative stress at its source rather than simply cleaning up damage after the fact. Its delocalized π-electron system and structural stability also make it resilient in the face of oxidative stress, contributing to its unique therapeutic potential.
Cytochrome Oxidase Deficits Are Central to Alzheimer’s Pathophysiology: In Alzheimer’s disease (AD), the enzyme cytochrome c oxidase (CO), also known as Complex IV of the ETC, is disproportionately impaired—particularly in the posterior cingulate cortex (PCC), a region crucial for memory retrieval. Quantitative histochemistry in AD patients revealed up to a 39% reduction in CO activity in superficial cortical layers (I and II) of the PCC. This region is among the earliest to show signs of hypometabolism, and such reductions precede the appearance of amyloid plaques or tau tangles. These findings support the vascular-hypometabolism hypothesis, suggesting that impaired oxidative metabolism may be a root cause of neurodegeneration, not merely a downstream effect.
Methylene Blue Targets Energy Hotspots in the Brain: Thanks to its small size, lipophilicity, and positive charge, methylene blue crosses the blood-brain barrier and accumulates in the mitochondrial matrix—especially in metabolically active neurons. This preferential localization allows it to directly assist the energy machinery in regions of the brain with high energy turnover, such as the hippocampus and prefrontal cortex. In doing so, it supports cognitive functions like memory consolidation, spatial navigation, and emotional regulation, particularly under conditions of cellular stress or age-related mitochondrial decline.
Methylene Blue Boosts Cytochrome Oxidase Activity in a Dose-Dependent Manner: Multiple studies confirm that low doses of methylene blue enhance CO activity, a key mitochondrial enzyme. For example, a single 1 mg/kg injection in rats resulted in a 30% increase in CO activity in brain tissue after 24 hours. In vitro application of 500 nanomolar methylene blue similarly raised CO activity by 25%. However, this response followed a hormetic curve: higher concentrations (e.g., 10 μM) suppressed enzyme function. This inverted U-shaped effect reinforces the importance of precise dosing, as benefits plateau and reverse beyond the optimal window.
Methylene Blue Improves Memory Across Diverse Behavioral Paradigms Animal studies consistently show that methylene blue enhances memory in a variety of tasks, including object recognition, spatial navigation, and fear extinction. For instance, in the Morris water maze, methylene blue reversed scopolamine-induced memory deficits and improved performance in mouse models of Alzheimer’s disease. In object recognition tasks, rats receiving 1–4 mg/kg of methylene blue after exposure demonstrated significant memory retention 24 hours later. These enhancements were associated with increased metabolic activity in memory-critical brain regions like the hippocampus and prefrontal cortex.
Alzheimer’s Disease May Involve a Metabolic Bottleneck—Not Just Plaques: Dr. Francisco Gonzalez-Lima’s research proposes that Alzheimer’s disease is fundamentally a disorder of mitochondrial hypometabolism, particularly in the posterior cingulate cortex (PCC)—a brain region essential for memory and cognition. His studies show that deficits in cytochrome oxidase activity occur early, often decades before clinical symptoms, and do not correlate with traditional markers like amyloid plaques. This suggests that energy failure may precede and possibly drive the classical histopathology of Alzheimer’s, offering a compelling rationale for targeting metabolic pathways in treatment.
Methylene Blue Offers a Dual Solution for Hypoperfusion and Hypometabolism: In the context of Alzheimer’s and other neurodegenerative diseases, methylene blue may offer a uniquely comprehensive approach by addressing both mitochondrial dysfunction and impaired cerebral blood flow. Not only does it help neurons produce ATP more efficiently—even under low-oxygen conditions—but it also triggers localized nitric oxide (NO) release, enhancing cerebral blood flow through vasodilation. This dual action—improving both oxygen utilization and delivery—makes methylene blue a promising candidate for therapeutic strategies aimed at breaking the vicious cycle of energy failure and neurodegeneration.
Hormetic Curve Explains Dose-Dependent Benefits and Risks. Methylene blue’s effects follow a classic hormetic (inverted U-shaped) curve: low to moderate doses improve mitochondrial function and cognitive performance, while high doses can be harmful. In rat studies, 1–4 mg/kg improved memory and CO activity, but doses above 50 mg/kg caused motor impairments, increased oxidative stress, and disrupted mitochondrial function. This response is partly explained by the molecule’s switch from forming helpful dimers at low doses to disruptive monomers at high doses. Maintaining doses within the “hormetic zone” is thus crucial for safety and efficacy.
Background
First synthesized in the late 19th century as a textile dye, methylene blue holds the distinction of being the first fully synthetic drug used in medicine. It gained early clinical adoption as a treatment for malaria and methemoglobinemia, and became a mainstay in biomedical research due to its vivid color and versatile redox properties. For decades, it served as a reliable tool in histological staining and cellular respiration experiments—its utility seemingly confined to the laboratory bench.
Yet in recent years, methylene blue has undergone a quiet scientific revival—this time not as a dye or a diagnostic reagent, but as a mitochondrial modulator with broad implications for neurological health.
This resurgence was sparked by early investigations from Dr. Francisco Gonzalez-Lima and colleagues at the University of Texas, who demonstrated that methylene blue acts as a potent electron cycler within mitochondria. Rather than binding a specific receptor like most pharmaceuticals, methylene blue supports core bioenergetic processes: it donates and accepts electrons, reinforces weak points in the electron transport chain, suppresses the formation of damaging reactive oxygen species (ROS), and boosts the resilience of neurons—cells with among the highest energy demands in the body.
These foundational findings prompted a new wave of inquiry: How exactly does methylene blue influence mitochondrial respiration? What doses are safe and effective? Could it be harnessed to treat or prevent neurodegenerative disease, cognitive decline, or psychiatric disorders?
Unlike many drugs that act on a single molecular target, methylene blue engages the metabolic architecture of the cell itself, tuning redox balance and energizing vulnerable neurons under stress. As evidence accumulates, its potential roles have expanded—from protecting the brain during acute injuries like stroke or chemical toxicity, to slowing chronic degenerative processes in conditions like Alzheimer’s disease and frontotemporal dementia.
A critical insight that emerged alongside this research is that methylene blue follows a hormetic dose-response curve: small or moderate amounts enhance mitochondrial function and protect neurons, but higher doses can overwhelm redox balance and become harmful. This inverted U-shaped curve underscores the importance of dose precision and purity in both experimental and clinical applications.
This narrative review synthesizes key discoveries in methylene blue research, from its biochemical properties and redox cycling to its role in supporting memory, protecting neurons, and targeting energy hotspots in the brain. We examine both foundational studies and emerging clinical findings, and consider how methylene blue may serve as a platform for metabolism-targeted interventions in neurology and psychiatry.
A Primer on Methylene Blue’s Mitochondrial Mechanisms of Action
Most drugs are precision tools—they bind to a specific receptor or block a single enzyme. Methylene blue works differently. It acts as a redox-active compound, meaning it can gain and donate electrons, much like a chemical courier. This ability allows it to influence multiple cellular processes at once, especially those tied to energy production and oxidative stress. Instead of simply flipping a switch on or off in the body, methylene blue reshapes how cells manage their electrical and metabolic balance.
This is particularly important in neurons, the brain’s signaling cells, which use enormous amounts of energy to fire signals and maintain internal stability. Neurons are also especially vulnerable when that energy supply falters—a common problem in aging and disease.
To understand how methylene blue helps, it’s worth revisiting the mitochondria—the energy factories inside our cells. Inside each mitochondrion is the electron transport chain (ETC), a series of proteins that pass along electrons like runners in a relay race. These electrons, extracted from nutrients, eventually combine with oxygen to form water. Along the way, they help pump protons across the mitochondrial membrane, creating a kind of electrochemical pressure. That pressure powers an enzyme called ATP synthase, which produces ATP—the universal energy molecule that fuels nearly everything cells do.
But when mitochondria are under stress—whether from aging, inflammation, or disease—this system can break down. Electrons may get stuck or leak out prematurely, especially at vulnerable points in the chain. This disrupts ATP production and generates reactive oxygen species (ROS)—unstable molecules that can damage DNA, proteins, and cell membranes.
Methylene blue helps smooth the flow of electrons. It can step in as a kind of molecular shortcut, accepting electrons upstream and passing them directly to a later part of the chain. This detour helps bypass damaged segments, keeping the energy pipeline open and reducing the buildup of ROS in the process.
Importantly, methylene blue works upstream of many traditional antioxidants. Rather than cleaning up oxidative damage after it happens, it prevents the problem at its source—by making the mitochondrial system run more cleanly and efficiently. The result is better energy production, less oxidative stress, and a stronger foundation for cellular health. [1]
Why Methylene Blue Is Reusable When Most Antioxidants Are Not
One of the key reasons methylene blue stands out is because of its molecular structure, which makes it unusually stable and adaptable. Its chemical backbone includes a special pattern of alternating single and double bonds—called a delocalized π-electron system—that allows electrons to flow freely across the molecule. This electron-sharing design gives methylene blue the flexibility to shift between two forms: its oxidized version, which is vividly blue, and its reduced version, known as leucomethylene blue, which is colorless. [1]
This redox shapeshifting is central to how methylene blue works in the body. Because it can accept and donate electrons repeatedly, it functions as a kind of electronic middleman, helping to move electrons where they’re needed without being permanently altered or used up. A small chemical group in its structure—the imine group—adds even more stability, helping the molecule withstand stress without breaking apart. [1]
Most antioxidants are one-and-done: they neutralize a single reactive molecule and are then spent. Think of them like disposable fire extinguishers. Methylene blue, on the other hand, behaves more like a rechargeable tool. After it does its job—whether accepting an electron or donating one—it naturally resets itself by reacting with oxygen in the environment. This process, called autoxidation, restores methylene blue to its active form so it can keep going. That’s why it’s sometimes described as a redox mediator—it keeps traffic flowing in the cell’s energy network without creating a jam or exhausting itself in the process. [1]
This durability made methylene blue a favorite in early cell biology research, long before its therapeutic potential was widely explored. Scientists used it as an artificial electron donor in laboratory experiments to simulate how cells produce energy. Because methylene blue was so dependable—readily handing off electrons and bouncing back to do it again—it helped researchers map out how mitochondria function and how cells manage oxygen. In many ways, it was a workhorse of basic biochemistry: a molecule that didn’t just reveal how life runs on energy, but that could one day help sustain it.
How Methylene Blue Reaches—and Powers—the Brain
One of the features that makes methylene blue especially promising in neurology is its ability to cross the blood-brain barrier—a dense, highly selective filter that shields the brain from harmful substances in the bloodstream. Many drugs and nutrients never make it past this gate. But methylene blue’s chemical structure—its small size, positive charge, and moderate fat solubility—allows it to pass through and enter the central nervous system, where it can reach neurons directly. [1]
Once inside a neuron, methylene blue shows a strong tendency to accumulate in mitochondria, the energy-producing compartments we've already explored. This targeting is driven by the electrochemical gradient that mitochondria maintain across their inner membranes—a natural attraction for positively charged molecules. As a result, methylene blue concentrates in the mitochondrial matrix, precisely where electron transport and ATP production occur. [1]
This mitochondrial localization is key to methylene blue’s ability to support energy metabolism, especially in the brain. Rather than acting from a distance, it positions itself right in the middle of the cell’s energy machinery. As discussed earlier, it can insert itself into the electron transport chain, acting as a backup route for electrons when the system is impaired. This is especially valuable in neurons, which not only consume vast amounts of energy but also suffer disproportionately when mitochondrial function is disrupted.
Keeping the Energy Pipeline Open: How Methylene Blue Supports the Electron Transport Chain
As we’ve already seen, the electron transport chain (ETC) is the final stage of cellular energy production—a biological assembly line that passes electrons from one protein complex to the next, using the released energy to create ATP. In healthy cells, this system runs smoothly: electrons flow from entry points like Complex I or II, move through carriers such as coenzyme Q and cytochrome c, and ultimately reach Complex IV, where they help convert oxygen into water. The energy generated along the way powers the production of ATP—the fuel that drives nearly every cellular process. [1]
But in neurons, the margin for error is slim. These cells are constantly firing signals, building new synapses, and resetting ion gradients, all of which require enormous amounts of energy. When mitochondria are stressed—by disease, toxins, or the cumulative effects of aging—parts of the ETC can slow down or stall, compromising energy output at the very moment it’s needed most.
This is where methylene blue shows one of its most important advantages. In its reduced form—known as leucomethylene blue—it can donate electrons directly to cytochrome c or other points further down the ETC, effectively creating a shortcut around damaged or sluggish segments. This ability to reroute electron traffic allows the rest of the chain to keep running, even when upstream components falter. [1]
This concept isn’t just theoretical. Classic experiments in cellular biology found that methylene blue could increase oxygen consumption in cells—an indicator that energy production was ramping up. More recent neuroscience studies show that it also boosts glucose and fat metabolism, and enhances the activity of ion pumps like the Na⁺/K⁺-ATPase, which neurons rely on to reset after firing. All of these processes are ATP-intensive, and methylene blue helps make sure the supply meets the demand. [1]
By keeping electrons moving through the chain and preserving the proton gradient that drives ATP synthesis, methylene blue helps neurons maintain their high-energy lifestyle. From firing signals to recycling neurotransmitters to strengthening neural connections, these tasks all depend on a steady flow of ATP. You can think of methylene blue as a relief pitcher—stepping in when the cell’s usual energy machinery is under strain and helping carry the game through to the next inning.
Preventing Cellular “Rust”: How Methylene Blue Lowers Oxidative Stress
As we’ve discussed, the electron transport chain is essential for producing ATP, but it also comes with a risk: leaking electrons. When this happens—particularly under stress or when the chain becomes inefficient—electrons may prematurely interact with oxygen to form reactive oxygen species (ROS), such as superoxide. These molecules are highly unstable and can damage DNA, proteins, and cellular membranes. Over time, the cumulative effect of ROS is like rust spreading through machinery—slowly degrading function and structure. In the brain, excessive oxidative stress has been linked to the gradual loss of neurons seen in neurodegenerative diseases like Alzheimer’s and Parkinson’s. [1]
Methylene blue helps mitigate this oxidative damage in two key ways.
First, by keeping the electron transport chain running smoothly, it reduces the likelihood of electrons straying off course. When the chain is backed up or sluggish, electrons are more likely to escape and form ROS. But when flow is consistent—as methylene blue helps maintain—there are fewer opportunities for that kind of misfire. [1]
Second, methylene blue has a unique chemical ability: it can cycle between its oxidized and reduced forms over and over again. This lets it act like an electron sponge, temporarily picking up stray electrons before they can interact with oxygen in harmful ways. Rather than forming superoxide or other damaging radicals, those electrons are steered toward safer outcomes, like the formation of water. [1]
This makes methylene blue a kind of unconventional antioxidant. Unlike traditional antioxidants, which neutralize one ROS molecule and are then used up, methylene blue can reset itself—ready to go again. It doesn’t just clean up after oxidative stress; it helps prevent it at the source by smoothing energy flow and capturing loose electrons before they cause harm.
In neurons—where energy demands are high and tolerance for damage is low—this dual function may be especially valuable. Methylene blue doesn’t just support energy production; it helps protect the machinery that makes that energy possible.
Methylene Blue, Nitric Oxide, and the Importance of Dose
Beyond its well-known role in energy production, methylene blue also interacts with another major signaling system in the body—nitric oxide (NO). This small, gaseous molecule plays a vital role in regulating blood flow and facilitating communication between brain cells. When functioning normally, nitric oxide helps relax blood vessels to control blood pressure and supports neurotransmission by fine-tuning the behavior of neurons and glial cells.
But as with many biological messengers, too much of a good thing can become a problem. Under conditions of inflammation, infection, or oxidative stress, certain forms of the enzyme that produce nitric oxide—known collectively as nitric oxide synthases (NOS)—can go into overdrive. This leads to excess NO, which combines with other molecules to form reactive nitrogen species. These byproducts can damage DNA, proteins, and cell membranes, fueling a process called nitrosative stress, which is often implicated in both neurodegenerative disease and vascular collapse. [1]
Methylene blue can intervene in this pathway by dialing back NO production. At low to moderate doses, it inhibits overactive NOS enzymes, reducing both nitric oxide itself and its harmful derivatives. This property has even been used in emergency medicine: in cases of septic shock, where runaway nitric oxide causes blood pressure to plummet and threatens survival, methylene blue has been shown to help restore vascular tone when other treatments fail. [1]
Yet this helpful action comes with an important caveat: dose matters—a lot. Like many compounds that affect fundamental cellular processes, methylene blue follows a pattern known as hormesis. In a hormetic response, low doses are helpful, but high doses may become harmful. This principle is especially important for redox-active compounds like methylene blue, where the balance between antioxidant and pro-oxidant effects is delicate.
Used properly, methylene blue supports mitochondrial function, regulates nitric oxide signaling, and buffers oxidative stress. But in excess, it can disrupt the very systems it protects. It may interfere with normal electron flow in the mitochondria, create metabolic traffic jams, or even generate its own reactive byproducts, pushing cells toward oxidative damage instead of away from it.
This dose-dependent “flip” underscores a central theme of methylene blue’s biology: more is not necessarily better. Like a skilled technician fine-tuning a circuit, the key to harnessing its benefits lies in precision—using just enough to reinforce cellular function without overloading the system.
Why the Brain Benefits Most
As we’ve seen, methylene blue doesn’t act like a typical drug that flips a single molecular switch. Instead, it targets something more fundamental: the core metabolic machinery that keeps cells—especially neurons—alive and functioning. By supporting the electron transport chain, it helps neurons maintain a steady supply of ATP, the energy needed to perform their most essential tasks. At the same time, it reduces oxidative stress by capturing stray electrons that might otherwise form harmful radicals. And by tuning down excess nitric oxide, it protects against inflammatory and toxic insults that can derail brain function.
This is especially important in the brain, an organ that burns through more energy than any other in the body. Neurons are constantly firing, forming new connections, and remodeling themselves in response to experience—a process known as neuroplasticity. These activities demand not just energy, but energy consistency. When mitochondria start to falter—whether from aging, environmental toxins, or genetic vulnerabilities—cognitive decline often follows. [1, 2]
Methylene blue offers a way to shore up the system from within. Because it concentrates in mitochondria and keeps electron flow moving, it provides neurons with a kind of metabolic safety net. Animal studies suggest that this support can improve memory, protect neurons from toxins, and help preserve learning-related processes that are highly energy dependent.
Rather than locking onto one receptor or blocking a single enzyme, methylene blue works more like a cellular metabolic optimizer—enhancing how neurons make and manage energy, while buffering them against the side effects of oxygen metabolism. In the energy-hungry environment of the brain, this support can be the difference between adaptation and exhaustion. That’s why methylene blue continues to draw scientific interest—not only as a potential cognitive enhancer, but also as a long-term neuroprotective agent aimed at preserving brain function over time.
The Dose Makes the Difference: Methylene Blue and the Hormetic Curve
One of the most important—and often misunderstood—features of methylene blue is that its effects depend heavily on the dose. At low concentrations, it supports mitochondrial function, boosts cellular energy, and helps buffer oxidative stress. But at higher doses, those same benefits begin to unravel. This pattern is known as hormesis—a biological principle where a compound can be helpful in small amounts but harmful in excess.
Methylene blue’s response curve follows a shape known as the inverted U (or β curve). Picture a graph with dose on the x-axis and benefit on the y-axis. At first, as the dose increases, so do the advantages—enhanced ATP production, stronger antioxidant defenses, and improved cognitive performance in preclinical models. But these gains peak in the middle, within what researchers call the hormetic zone. Beyond this optimal window, the curve bends downward. Push the dose too high, and methylene blue can interfere with normal electron flow, act as a pro-oxidant, and potentially harm the very cells it was meant to help.
This U-shaped curve isn’t unique to methylene blue—it’s seen with many molecules that act on core cellular systems, especially those involved in redox balance. But it’s particularly important here because methylene blue recycles itself and interacts with energy metabolism directly, meaning a miscalibrated dose can quickly shift from helpful to harmful.
In practical terms, the sweet spot lies in very low concentrations—typically in the nanomolar to low micromolar range—which are enough to stimulate mitochondrial performance and keep reactive molecules in check, without overloading the system. Go above that range, and the compound’s effects can plateau—or even backfire.
The lesson is clear: with methylene blue, more is not better.
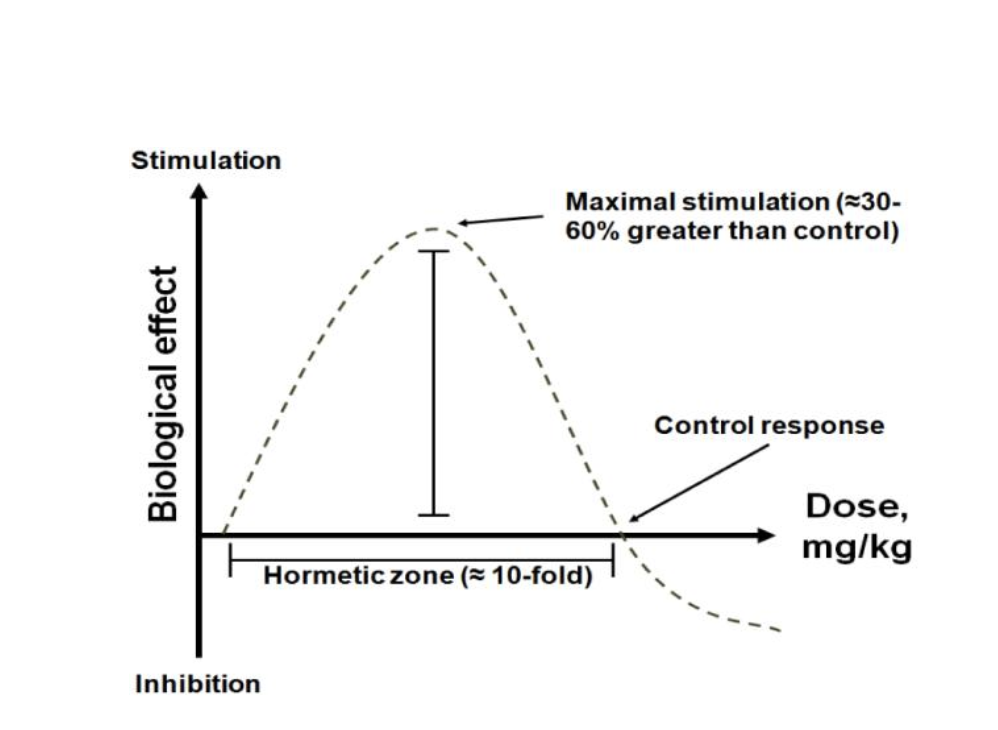
Figure A. A visual representation of the hormetic β curve for methylene blue. The upward slope at low doses reflects improvements in mitochondrial activity, antioxidant capacity, and overall cellular health. The curve then reaches an optimal plateau (the midrange or “hormetic zone”) before descending, illustrating how further increases in dose can lead to harmful effects. [1]
What Drives the Hormetic Curve? The Chemistry Behind the Dose
The reason methylene blue can help or harm depending on the dose lies in the chemistry of how it moves electrons inside mitochondria. At moderate concentrations, methylene blue tends to pair up into dimers—two molecules linked together. These dimers appear to interact safely with the cell’s energy machinery, helping to smooth the flow of electrons through Complex IV (also called cytochrome oxidase), the final stop in the electron transport chain. In doing so, they help boost energy production while reducing the risk of oxidative damage. [1]
But at higher concentrations, this balance breaks down. Instead of forming dimers, methylene blue mostly stays in its monomeric form—individual molecules that don’t play by the same cooperative rules. These monomers can begin pulling electrons from other parts of the electron transport chain, upsetting the flow and creating electron traffic jams. The result? An increase in reactive oxygen species, or free radicals, which damage the very components methylene blue is supposed to protect. [1]
This shift—from cooperative dimers to disruptive monomers—is one reason why the benefits of methylene blue peak in a narrow dose window. In neurons, where consistent energy and low oxidative stress are crucial for tasks like memory formation and synaptic plasticity, this shift can tip the balance from protection to harm.
Another key factor is methylene blue’s positive charge, which causes it to accumulate in mitochondria, the most negatively charged space in the cell. At low to moderate doses, this localization is helpful—it places methylene blue right where energy production happens, allowing it to act as a backup electron shuttle. But at high doses, the buildup becomes too much. The excess molecules can start competing with the cell’s natural electron carriers, overwhelming the system, disrupting ATP synthesis, and amplifying oxidative stress. [1]
Importance of Chemical Purity
The dose-related toxicity of methylene blue can be further complicated by contaminants. Even pharmaceutical-grade (USP) methylene blue can contain trace levels of metals such as arsenic, lead, or mercury. At low doses, these impurities typically remain too small in quantity to cause serious problems. As doses increase, however, contaminants can accumulate to levels that lead to inflammation or toxicity, confounding the results of research or treatment. Some non-USP or industrial grades of methylene blue contain 8–10% or more of various impurities, making them unsafe for therapeutic use and unreliable for scientific studies. This is why anyone using methylene blue for medical or investigative purposes must ensure the substance is of the highest possible purity. [1]
What the Studies Show: The Real-World Impact of Hormesis
Animal research has brought the concept of methylene blue’s hormetic curve to life, showing how different doses produce sharply different outcomes. In rat studies, small doses—typically between 1 and 4 milligrams per kilogram of body weight—consistently lead to improved memory performance, such as better recognition of new objects and faster adaptation to unfamiliar environments. These cognitive benefits are achieved without noticeable side effects. [1]
But when the dose climbs much higher—around 50 mg/kg or more—the effects flip. Not only do the memory gains disappear, but the animals often show signs of disrupted motor function, reduced alertness, and even general behavioral impairments. In other words, once methylene blue moves beyond its optimal zone, the very systems it was supporting begin to suffer. [1]
Similar patterns show up in studies of mitochondrial function. At moderate doses, methylene blue enhances energy production and protects cells from oxidative stress. But at high doses, it overwhelms the system, increasing oxidative damage and interfering with ATP synthesis. These findings reinforce what we’ve already seen at the molecular level: methylene blue operates within a narrow therapeutic window, and stepping outside of it—especially in the direction of excess—can reverse its benefits. [1]
This inverted U-shaped response sets methylene blue apart from many conventional drugs, whose effects typically scale more predictably with dose. With methylene blue, more is not better. Small, precise doses activate beneficial pathways like cytochrome oxidase activity and mitochondrial defense, while larger doses can push neurons into oxidative stress and metabolic imbalance.
Given these properties, researchers and clinicians must choose doses carefully and use methylene blue of high purity to ensure they harness its powerful mitochondria-protecting and antioxidant actions without tipping over into toxicity. This nuanced dose response also sheds light on why methylene blue has sometimes produced inconsistent results in older or poorly controlled studies: dosing errors or impure preparations can easily hide or negate its beneficial effects.
Behavioral Paradigms Demonstrating Methylene Blue’s Memory-Enhancing Effects
Over decades of research, scientists have found that methylene blue consistently enhances memory in animal models—whether in healthy rodents or those with cognitive impairments. What’s especially striking is that these improvements show up across a wide variety of behavioral tasks, suggesting that methylene blue is tapping into a core process underlying memory itself. [1]
In particular, it seems to strengthen the consolidation phase of memory—the critical period after learning, when short-term information is stabilized and stored for long-term use. This is a time when neurons need a surge of energy to reinforce connections and build durable memory traces. Methylene blue supports this phase by increasing the activity of mitochondrial enzymes, especially cytochrome oxidase, which plays a key role in cellular respiration. By boosting mitochondrial output, it helps ensure that neurons have the energy they need to lock in new information. [1]
Animal studies have tested this effect using a wide range of behavioral paradigms, from fear extinction and spatial navigation to object recognition tasks. A recurring pattern emerges: low doses of methylene blue—typically between 0.5 and 4 mg/kg, delivered by injection—reliably improve memory retention without causing side effects. These gains often correlate with higher metabolic activity in brain regions tied to learning, such as the hippocampus and prefrontal cortex.
Fear Extinction Training: How Methylene Blue Enhances Emotional Learning
One of the most compelling demonstrations of methylene blue’s memory-enhancing effects comes from studies on fear extinction—a process that mirrors the kind of emotional relearning that happens in therapy for anxiety or post-traumatic stress disorder (PTSD). In these experiments, animals are first trained to associate a harmless cue, like a tone or a specific environment, with something unpleasant, such as a mild foot shock. This leads them to develop a conditioned fear response—a learned association between the cue and danger.
During extinction training, the cue is presented again, but without the shock. Over time, the animal begins to unlearn the fear, recognizing that the cue no longer signals a threat. This is the behavioral foundation of exposure therapy, where patients repeatedly encounter anxiety-inducing cues in a safe setting until their fear responses fade. [1]
Here’s where methylene blue comes in: when moderate doses—around 4 mg/kg—were given to rats shortly after extinction sessions, the animals were better able to retain the new “no-fear” memory. They were less likely to fall back into old fear patterns when re-exposed to the original cue later—a phenomenon known as fear renewal. In essence, methylene blue helped the brain hold onto the emotional update: this is no longer something to fear. [1]
What was happening beneath the surface? Brain tissue analysis revealed that methylene blue increased cytochrome oxidase activity in key areas of the prefrontal cortex—a region deeply involved in emotion regulation and fear inhibition. These findings suggest that methylene blue supported the energy demands of neurons actively remodeling emotional memory networks. By boosting mitochondrial output during this window of consolidation, it helped lock in a calmer, more adaptive response. [1]
This line of research highlights a broader idea: that strengthening cellular energy metabolism at the right moment—particularly in brain regions that govern emotion—can enhance therapeutic learning. Methylene blue may not erase fear, but it appears to amplify the brain’s capacity to reframe it.
Methylene Blue and Spatial Memory
Spatial learning—the ability to navigate an environment and remember where things are—is one of the most energy-intensive cognitive tasks the brain performs. It relies heavily on the hippocampus, a region deeply involved in both spatial navigation and memory formation, and one that’s particularly sensitive to changes in cellular metabolism.
One classic test of spatial learning in rodents is the Morris water maze. In this setup, rats or mice must swim through a pool to locate a hidden platform using visual cues around the room. Over repeated trials, the animals learn to find the platform more quickly—unless something disrupts the learning process.
Researchers have found that small doses of methylene blue can significantly improve performance in this task, especially under challenging conditions. For instance, when animals are given scopolamine, a drug that impairs the brain’s acetylcholine system (which is critical for memory), their ability to learn the maze declines. But when methylene blue is added, it restores performance, suggesting that it can counteract energy deficits that would otherwise block learning. [1]
Even more strikingly, methylene blue has improved spatial memory in mouse models of Alzheimer’s disease and tauopathies, which are known for mitochondrial dysfunction and progressive memory loss. In these models, methylene blue’s support of mitochondrial function appears to partially rescue cognitive performance, reinforcing the idea that energy supply is a major bottleneck in diseased brains. [1]
These findings echo what we’ve seen across other behavioral studies: methylene blue seems to offer its biggest benefits at moments of high metabolic demand, when neurons are working hard to encode, update, or retrieve information. Spatial navigation is one of those peak-demand moments—when the brain is integrating cues, mapping space, and forming lasting memories.
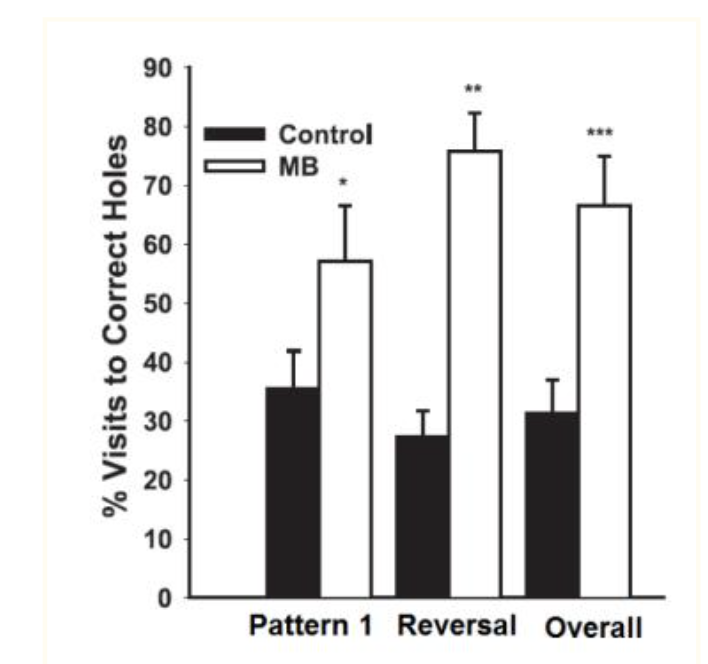
Figure B. Methylene blue (MB) improved spatial memory retention in a holeboard task where rats learned to find sweetened cereal in baited holes using visual cues. This figure shows the percentage of correct hole visits for both MB-treated and vehicle control groups. Rats given Methylene Blue made about twice as many correct choices as controls under the initial baiting pattern (Pattern 1), the reversed pattern (Reversal), and across both tests (Overall). [1]
Methylene Blue and Recognition Memory
Another line of research supporting methylene blue’s cognitive benefits comes from tests of object recognition, a simple but telling measure of memory. In these experiments, animals are first exposed to an object. Later, they’re presented with both the familiar object and a new one—and researchers measure how much time they spend exploring each. If the animal remembers the first object, it will naturally spend more time investigating the novel one.
In a study by Dr. Francisco-Lima, titled Memory facilitation by methylene blue: dose-dependent effect on behavior and brain oxygen consumption, a single low dose of methylene blue—between 1 and 4 mg/kg, given shortly after the first exposure—significantly enhanced memory for the familiar object, even when tested 24 hours later. This suggests that methylene blue strengthened the brain’s ability to consolidate the memory of the original encounter, helping it survive beyond the short-term. [3]
A similar effect was seen in the open field habituation test, where rats are placed in a new environment and gradually become less anxious and more exploratory over time. This process of habituation—recognizing that a space is safe—depends on memory, not just emotional regulation. Here too, low-dose methylene blue enhanced long-term retention of the experience: animals became more exploratory when reintroduced to the environment, indicating they remembered it from before.
Importantly, these improvements occurred without changes in overall movement levels or signs of agitation, showing that the effects weren’t due to increased arousal or hyperactivity. In other words, methylene blue didn’t just make the animals more energetic—it made them more efficient learners, strengthening memory without stimulating the nervous system in a nonspecific way.
These findings reinforce a central theme: methylene blue enhances cognitive function not by overriding brain systems, but by supporting the energetic foundation of memory.
Evidence at the Molecular Level: How Methylene Blue Boosts Brain Energy
Laboratory studies have repeatedly confirmed what behavioral research has suggested: low doses of methylene blue enhance memory by increasing the brain’s energy capacity. A key part of this effect involves the enzyme cytochrome oxidase, which plays a central role in the electron transport chain and is a major driver of oxygen-based energy production in neurons.
In one foundational study, researchers from Dr. Francisco-Lima's lab gave rats a single low dose of methylene blue (1 mg/kg) and observed a 30% increase in brain cytochrome oxidase activity—but interestingly, this boost didn’t occur immediately. The increase became apparent 24 hours later, suggesting that methylene blue initiates a sustained metabolic shift rather than just a short-term spike in activity. [4]
Follow-up studies confirmed this effect in both live animals and in vitro experiments using rat brain tissue. For instance, applying 500 nanomolar methylene blue—a concentration corresponding to the 1 mg/kg dose used in rats—led to a 25% increase in cytochrome oxidase activity in brain homogenates. But as the dose rose beyond this optimal range, the benefits disappeared: 5 micromolar concentrations had no effect, and 10 micromolar concentrations actually suppressed enzyme activity, demonstrating the same inverted U-shaped response seen in behavioral tests. [5]
Other studies reinforced the finding that repeated low-dose methylene blue enhances not only behavior but also brain metabolism. In one experiment, rats given three daily doses of 1 mg/kg showed markedly better performance in a memory task requiring them to distinguish between rewarded and unrewarded options. Their brains were then analyzed using spectrophotometry, revealing a 70% increase in cytochrome oxidase activity compared to controls. This dramatic rise points to more than a temporary enzyme boost—it likely reflects a broader upregulation of mitochondrial energy systems, preparing the brain for sustained cognitive demands. [6]
At the molecular level, these effects are thought to be driven by the activation of nuclear respiratory factors—genetic regulators that increase the expression of mitochondrial enzymes like cytochrome oxidase. These pathways kick in during periods of persistent neural activity, when the brain’s demand for oxygen and energy spikes—such as during memory consolidation, neurotransmission, and synapse formation. [3]
Additional research has shown that leucomethylene blue, the reduced (active) form of the molecule, also increases brain oxygen consumption when applied to rat brain tissue. Consistent with this, low doses of methylene blue administered in live animals have been associated with significantly higher oxygen use in the brain 24 hours later—again, at doses that also improved object recognition and habituation in behavioral tasks. [3]
Altogether, these findings strengthen the case that methylene blue enhances memory by supporting the brain’s respiratory machinery, allowing neurons to generate the ATP they need for high-demand tasks. It’s a rare example of a molecule whose chemical properties align elegantly with the biology of memory—boosting energy where and when it matters most.
Evidence of Methylene Blue as a Neuroprotective Agent
While methylene blue first captured attention for its ability to enhance memory, a growing body of research shows that it also acts as a neuroprotective agent, shielding neurons from damage and degeneration. What makes this particularly compelling is that the same mechanisms that support learning and memory—sustaining mitochondrial energy production and reducing oxidative stress—also appear to help neurons survive under duress.
Neurons are uniquely vulnerable to metabolic disruption. They burn through energy at a relentless pace to fire electrical signals, maintain ion gradients, and remodel connections during learning. These demands leave little room for error. If mitochondrial respiration falters—whether from toxic insults, oxygen deprivation, or disease-related changes—the result can be swift and irreversible damage.
Methylene blue addresses these risks at multiple levels:
- First, it can reroute electron flow around damaged sections of the electron transport chain. This prevents a full system failure and keeps ATP production alive, even when parts of the chain are compromised.
- Second, by accepting stray electrons, methylene blue helps prevent the formation of reactive oxygen species (ROS)—molecules that damage DNA, proteins, and membranes. This interceptive action reduces oxidative stress at its source.
- Third, methylene blue’s ability to cycle between oxidized and reduced states allows it to enhance the cell’s use of oxygen, especially during metabolic stress. This supports essential functions like synaptic signaling and calcium buffering, both of which are critical for keeping neurons healthy and communicative.
In effect, methylene blue strengthens the metabolic backbone of neurons. Rather than acting as a shield that deflects injury from the outside, it works from within, stabilizing the internal machinery that powers and protects the brain.
The following examples illustrate how these actions manifest in different experimental and clinical scenarios, ranging from acute toxic insults to chronic neurodegenerative conditions. [1]
A Clinical Case Study in Mitochondrial Rescue: Ifosfamide-Induced Encephalopathy
One of the most compelling real-world examples of methylene blue’s neuroprotective power comes from its use in ifosfamide-induced encephalopathy—a dangerous neurological complication that can arise during chemotherapy. Ifosfamide, a chemotherapy drug used to treat solid tumors, is metabolized into toxic byproducts that disrupt mitochondrial function. These byproducts specifically target flavoproteins, which are key components of the electron transport chain. When this happens, neurons can no longer efficiently produce ATP, leading to symptoms such as confusion, disorientation, drowsiness, and in severe cases, coma. [7]
But in these high-stakes situations, methylene blue can make a measurable difference. Clinical observations have shown that administering methylene blue—typically around 50 mg per day, taken orally or delivered intravenously—can halt or shorten the duration of these encephalopathic episodes. When given early, or even as a preventive measure, it often leads to rapid neurological improvement.
Mechanistically, methylene blue is believed to act as a partial substitute for the impaired flavoproteins, stepping in to support the electron transport chain and sustain NADH oxidation—a crucial step in cellular respiration. This helps prevent the buildup of aldehyde-based toxic intermediates, which are thought to play a major role in the onset of encephalopathy. [7]
Early case reports and clinical series have validated this approach. In two documented cases from the 1990s, methylene blue successfully reversed symptoms of ifosfamide-induced brain dysfunction. Later studies confirmed that even short courses—such as four to six days of low-dose methylene blue—can significantly reduce symptom duration, often bringing it down to less than a single day. [7]
These findings highlight an important broader point: methylene blue isn’t just a lab tool or a memory enhancer—it’s a clinically viable treatment for acute mitochondrial distress in the human brain. By restoring energy metabolism in neurons that are on the brink of failure, methylene blue can, in some cases, prevent full-blown neurological collapse.
Battling Neurodegeneration: Methylene Blue in Models of Mitochondrial Failure
Some of the strongest evidence for methylene blue’s neuroprotective power comes from its performance in animal models designed to mimic the cellular damage seen in neurodegenerative diseases. A standout example involves rotenone, a compound that blocks Complex I of the mitochondrial electron transport chain. This blockade causes a rapid drop in ATP production, a spike in oxidative stress, and ultimately, widespread neuronal damage—conditions that resemble the metabolic breakdown seen in disorders like Parkinson’s disease and Leber’s hereditary optic neuropathy. [8, 9]
Retinal Protection: A Model of Optic Neuropathy
In one set of experiments, researchers injected rotenone directly into the eyes of rodents, causing targeted damage to retinal ganglion cells—the neurons responsible for transmitting visual information to the brain. This approach simulates the kind of mitochondrial optic neuropathy that leads to vision loss in humans. However, when low doses of methylene blue (ranging from 0.7 to 70 µg/kg) were co-administered, they preserved both the structure and function of the retinal ganglion layer. [10]
The protective effects were dose-dependent: while the smallest dose (0.7 µg/kg) had little effect, higher doses significantly prevented retinal thinning and cell loss, and also maintained normal vision-related behaviors in the animals. Methylene blue appeared to act by bypassing the Complex I blockade, keeping ATP production steady and avoiding the metabolic collapse that rotenone typically triggers. It also boosted cytochrome oxidase activity in retinal tissue—an indicator of healthier mitochondrial function. [10]
Striatal Protection: Preventing Metabolic Lesions
A similar pattern emerged in studies targeting the striatum, a brain region involved in movement and reward processing. When rotenone was injected into this area, it caused lesion-like damage similar to what’s seen in metabolic strokes. But animals treated with methylene blue had smaller lesions, better motor function, and lower oxidative stress levels. Again, methylene blue’s ability to redirect electrons and preserve mitochondrial output helped protect neurons from dying under toxic pressure.
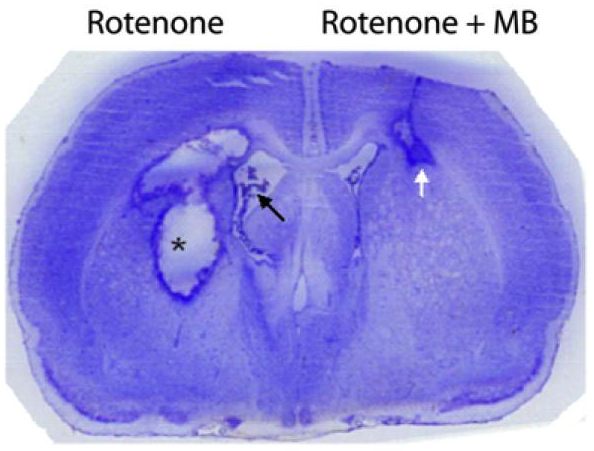
Figure C. Stained coronal section of a rat forebrain illustrating the contrasting effects of rotenone alone (left hemisphere) versus rotenone plus methylene blue (right hemisphere). Rotenone—a mitochondrial Complex I inhibitor—caused a large area of liquefactive necrosis (asterisk) and robust reactive gliosis, along with an expanded lateral ventricle (black arrow). In contrast, co-treatment with methylene blue significantly reduced tissue loss, confining damage primarily to the corpus callosum (white arrow). This protective outcome aligns with methylene blue’s known ability to support mitochondrial function and limit oxidative injury. [1]
Translational Insight
What makes these findings particularly compelling is that rotenone is not just a research tool—it’s also considered a potential environmental neurotoxin, and its mechanism of harm mirrors what’s thought to happen in several neurodegenerative diseases. These experiments demonstrate that methylene blue can rescue neurons not only by blocking damage after it begins, but by maintaining the energy infrastructure that neurons need to survive in the first place.
Protecting the Brain After Cardiac Arrest: Methylene Blue’s Role in Reperfusion Injury
In moments of cardiac arrest, the brain faces one of the most extreme metabolic crises possible. Oxygen delivery drops to zero, energy production halts, and neurons are left vulnerable. Even when the heart is restarted, the return of oxygen-rich blood—a process known as reperfusion—can paradoxically trigger a second wave of injury, as oxidative stress and inflammation surge. This kind of damage, known as ischemia-reperfusion injury, is a major contributor to long-term cognitive impairment in cardiac arrest survivors.
Researchers have explored whether methylene blue can help stabilize the brain during this critical window, using animal models of cardiac arrest, particularly in pigs, whose cardiovascular systems closely resemble our own. In these studies, animals receive intravenous methylene blue during or immediately after resuscitation. The results are striking: methylene blue reduces signs of brain injury, including swelling, blood-brain barrier disruption, and degradation of myelin, the protective insulation around neurons. [11]
One particularly telling marker is S-100β, a protein released when astrocytes—the brain’s support cells—are under stress. In methylene blue–treated animals, S-100β levels remain significantly lower than in controls, suggesting less hypoxic brain damage. Additional studies have shown that methylene blue also preserves vascular integrity, limits oxidative injury, and helps prevent leakage of proteins like albumin into the brain—hallmarks of a well-maintained blood-brain barrier. [11]
But methylene blue’s protective effects extend beyond the physical structure of the brain. On a molecular level, it activates gene networks involved in cell survival and repair. In one study, animals treated with low-dose methylene blue showed increased expression of genes that combat apoptosis (programmed cell death), reduce inflammation, and stimulate neuronal regeneration. It also helped normalize protein trafficking within neurons, reversing the stress-induced breakdown in communication between the endoplasmic reticulum and other parts of the cell. [11]
Importantly, methylene blue improved hemodynamics—blood pressure and circulation—within minutes of resuscitation and increased short-term survival after prolonged cardiac arrest. Taken together, these findings suggest that methylene blue doesn’t just help restart the heart—it may also help preserve the brain in the aftermath, by reinforcing mitochondrial function, limiting oxidative stress, and supporting the brain’s own repair systems. [11]
Rethinking Alzheimer’s: A Mitochondrial and Vascular Perspective
For decades, Alzheimer’s disease (AD) has been framed largely through the lens of amyloid plaques and tau tangles. But a growing body of research suggests that another pathology may precede these hallmarks: a failure of energy metabolism, rooted in mitochondrial dysfunction.
As we've established, neurons are especially vulnerable to even modest disruptions in energy supply. Their high metabolic demands support not just signaling, but also structural maintenance, synapse formation, and the cleanup of toxic proteins. When mitochondrial respiration falters, neurons can no longer meet these demands—leading to oxidative stress, inflammation, and, eventually, cell death. This energy crisis is increasingly seen as a driving force in neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and frontotemporal dementia.
The Vascular-Hypometabolism Hypothesis of Alzheimer’s
In the early 2000s, Dr. Francisco Gonzalez-Lima and his colleagues proposed an alternative to the amyloid-centric model of AD. Drawing on both neuroimaging and postmortem tissue analysis, they identified early and pronounced declines in cytochrome c oxidase activity in specific brain regions—particularly the posterior cingulate cortex (PCC), a hub for memory retrieval and cognitive integration.
Using quantitative histochemistry, the team measured cytochrome c oxidase (CO) activity across all six cortical layers. They found especially steep drops—up to 39% in the superficial layers (I and II)—in AD patients. These layers are rich in synapses and vital for coordinating communication between brain regions. Reduced CO activity in these areas leads to ATP shortages, weakened neurotransmission, and disrupted protein synthesis—a molecular recipe for cognitive decline.
What made this finding striking was its independence from traditional AD pathology. The metabolic deficits in CO did not track with the density of amyloid plaques or neurofibrillary tangles. This suggests that energy failure may be an initiating factor, rather than a secondary effect of protein accumulation.
Further studies using PET imaging support this view, revealing that regions like the PCC show signs of hypometabolism long before clinical symptoms emerge. In fact, energy deficits may begin 20 years before diagnosis, providing a potential window for early detection and intervention.
What makes this even more significant is that CO dysfunction correlates with disease duration, highlighting its potential role as both a driver and marker of disease progression. As CO activity declines, neurons produce less ATP, generate more oxidative stress, and experience “functional uncoupling”—a breakdown in the coordination between different cortical layers. The result is a cascading energy deficit that compromises the brain’s core operations.
This vulnerability is rooted in the brain’s extraordinary energy needs. Although it accounts for only about 2% of body weight, the brain consumes roughly 20% of the body’s daily energy budget. It also has limited capacity for anaerobic metabolism, meaning it relies almost entirely on oxygen-dependent ATP production. When blood flow or oxygen availability drops—even modestly—mitochondria begin to struggle, and neurons are among the first to falter.
These dynamics help explain certain behavioral symptoms of dementia—such as a craving for sugars—which may reflect a shift toward less efficient anaerobic pathways as neurons attempt to compensate for mitochondrial shortfalls. And they help contextualize why CO is considered the rate-limiting step in the electron transport chain: its dependence on oxygen and its sensitivity to hypoxia make it a metabolic chokepoint, especially under stress.
Indeed, studies show that partial occlusion of cerebral blood vessels—even without causing a stroke—can lead to reactive downregulation of CO activity, setting off a destructive cycle of poor mitochondrial respiration and worsening hypoperfusion.
From a clinical standpoint, this model provides a powerful lens through which to view Alzheimer’s: not simply as a disorder of misfolded proteins, but as a disease of chronic energy insufficiency, fueled by both vascular dysfunction and mitochondrial failure. The extended preclinical phase of AD—often spanning 10 to 20 years—offers a valuable window for early detection and intervention, particularly if we focus on the right targets: oxygen delivery, mitochondrial function, and oxidative metabolism.
Methylene Blue: A Mitochondrial Rescue Agent
This energy-centric model of Alzheimer’s points to a different kind of therapeutic target: stabilizing mitochondrial function rather than clearing plaques. Here, methylene blue (MB) offers a promising intervention.
MB can act as an alternative electron carrier in the electron transport chain. When CO is underperforming, MB effectively bypasses the bottleneck, helping to maintain ATP production by transferring electrons to oxygen directly. In both animal models and human cell lines, low doses of MB (0.5–4 mg/kg) have been shown to increase oxygen consumption by up to 70% and boost ATP levels by 30%. [6]
But MB does more than just power up mitochondrial engines. In low-oxygen environments—a common problem in Alzheimer’s due to poor cerebral perfusion—MB maintains its ability to support respiration. Functional MRI studies show that MB helps convert oxygen to water more efficiently, keeping neurons running even under hypoxic stress. [6]
This oxygen efficiency can also trigger a secondary benefit: the production of nitric oxide (NO), a molecule that dilates blood vessels. When MB accelerates oxygen use, it briefly lowers local oxygen levels, which prompts CO to generate NO, increasing blood flow and nutrient delivery to metabolically active brain regions. [6]
Clinical Implications, Practical Considerations, and Future Directions
Methylene blue’s neuroenhancing and neuroprotective potential has generated considerable interest in both experimental and clinical contexts. By stabilizing mitochondrial energy production and limiting oxidative stress, it has shown promise for improving memory, preventing neuronal damage, and possibly altering disease progression in a range of models. However, while these initial data are encouraging, they are not yet conclusive. More rigorous, large-scale investigations will be needed to determine precisely how and when methylene blue exerts its most meaningful effects.
Taken together, current findings hint that methylene blue may offer a targeted metabolic boost to neurons working at peak load, potentially enhancing memory consolidation and alleviating harmful metabolic stress. This idea of “targeted” support has fueled hope that methylene blue could focus on so-called “energy hotspots” in the brain, which might be especially useful for neurological disorders involving high oxidative burden or significant mitochondrial dysfunction.
Across multiple animal models of neurodegenerative disease—including Alzheimer’s, Parkinson’s, and various tauopathies—methylene blue has repeatedly shown the ability to support mitochondrial function, limit toxic protein aggregation, and mitigate oxidative stress. These actions open the door to broader clinical applications in conditions where neuronal energy production is compromised by pathological proteins or chronic inflammation. Translating these insights into real-world practice remains a challenge, but the compound’s dual effects on metabolism and protein aggregation make it a compelling candidate for further clinical development.
Central to its therapeutic promise is the need to establish safe and effective dosing protocols that account for its hormetic (inverted U-shaped) properties. Animal studies suggest that doses of roughly 1–4 mg/kg reliably enhance memory consolidation and protect neurons, with few apparent side effects. Converting these doses to a human equivalent requires careful attention to body weight, metabolic differences, and administration route, but early indications from clinical trials suggest that similar doses can be both well tolerated and beneficial in certain patient populations. By contrast, animal experiments using doses above 50 mg/kg often result in pro-oxidant activity, memory impairments, or overt toxicity—a pattern that underscores how exceeding methylene blue’s optimal range may disrupt cellular redox balance rather than support it.
Another vital practical concern is the chemical purity of methylene blue. Pharmaceutical-grade (USP) preparations contain fewer potentially harmful contaminants such as arsenic or lead, whereas industrial- or chemical-grade products often harbor higher levels of heavy metals and other impurities. At increased doses, these contaminants can compromise safety and distort experimental results, emphasizing the importance of using rigorously purified formulations for both medical and research purposes.
The potential target populations for methylene blue therapy are diverse. Investigators are exploring its cognitive-enhancing effects in older adults with age-related memory decline and in individuals with amnestic mild cognitive impairment or advanced neurodegenerative conditions like Alzheimer’s or Parkinson’s disease. Its capacity to support mitochondrial function also makes it a promising option in disorders featuring protein aggregation. In psychiatric contexts, low-dose methylene blue is under investigation as an adjunct to exposure therapy for patients with post-traumatic stress disorder (PTSD) or phobias, where preliminary data indicate it may strengthen the extinction of fear-based memories. Several of these applications are already in clinical trials, which measure not only memory and fear reduction but also potential changes in biomarkers of neurodegeneration. [1]
Despite these promising leads, many future directions remain open for exploration. Researchers continue to dissect the precise molecular pathways of methylene blue’s actions, particularly its interplay with cytochrome oxidase, nitric oxide synthase, and redox-sensitive transcription factors. Important questions remain about whether its apparent ability to reduce harmful protein aggregates—such as amyloid beta or pathological tau—is a distinct mechanism from its broader role in enhancing mitochondrial metabolism. Long-term safety is another crucial consideration since no major issues have surfaced at low to moderate doses, but larger-scale clinical trials must confirm that methylene blue remains safe and effective when administered for extended periods, especially in older individuals or those with coexisting health conditions. Its potential pro-oxidant effects at higher doses also necessitate careful monitoring.
Finally, there is growing interest in combining methylene blue with therapies that target complementary pathways. For instance, researchers have proposed pairing it with neurotrophic factors, anti-inflammatory medications, or other metabolic modulators to achieve synergistic benefits in complex disorders. Moving toward a more personalized, “precision medicine” approach—where methylene blue use is tailored to a patient’s unique metabolic needs—could further optimize outcomes. As dosing regimens are refined and mechanistic details clarified, methylene blue may offer a safe, versatile way to reinforce neuronal function across a wide array of clinical indications.
- Julio C. Rojas, Aleksandra K. Bruchey, F. Gonzalez-Lima, Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue, Progress in Neurobiology, Volume 96, Issue 1, 2012, Pages 32-45, ISSN 0301-0082,
- Gonzalez-Lima F, Auchter A. Protection against neurodegeneration with low-dose methylene blue and near-infrared light. Front Cell Neurosci. 2015 May 12;9:179. doi: 10.3389/fncel.2015.00179. PMID: 26029050; PMCID: PMC4428125.\
- Riha PD, Bruchey AK, Echevarria DJ, Gonzalez-Lima F. Memory facilitation by methylene blue: dose-dependent effect on behavior and brain oxygen consumption. Eur. J. Pharmacol. 2005;511:151–158. doi: 10.1016/j.ejphar.2005.02.001.
- Callaway NL, Riha PD, Bruchey AK, Munshi Z, Gonzalez-Lima F. Methylene blue improves brain oxidative metabolism and memory retention in rats. Pharmacol. Biochem. Behav. 2004;77:175–181. doi: 10.1016/j.pbb.2003.10.007.
- Callaway NL, Riha PD, Bruchey AK, Munshi Z, Gonzalez-Lima F. Methylene blue improves brain oxidative metabolism and memory retention in rats. Pharmacol. Biochem. Behav. 2004;77:175–181. doi: 10.1016/j.pbb.2003.10.007.
- Wong-Riley MT, Nie F, Hevner R, Liu S. Brain cytochrome oxidase: Functional significance and bigenomic regulation in the CNS. In: Gonzalez-Lima F, editor. Cytochrome oxidase in neuronal metabolism and Alzheimer’s disease. Plenum Press; New York: 1998. pp. 1–54
- Kupfer A, Aeschlimann C, Wermuth B, Cerny T. Prophylaxis and reversal of ifosfamide encephalopathy with methylene-blue. Lancet. 1994;343:763–764. doi: 10.1016/s0140-6736(94)91839-2.
- Rojas JC, Gonzalez-Lima F. Mitochondrial optic neuropathy: In vivo model of neurodegeneration and neuroprotective strategies. Eye and brain. 2010;2:21–37. doi: 10.2147/eb.s9363.
- Larsson NG, Andersen O, Holme E, Oldfors A, Wahlstrom J. Leber’s hereditary optic neuropathy and complex I deficiency in muscle. Ann. Neurol. 1991;30:701–708. doi: 10.1002/ana.410300511
- Rojas JC, John JM, Lee J, Gonzalez-Lima F. Methylene blue provides behavioral and metabolic neuroprotection against optic neuropathy. Neurotox. Res. 2009a;15:260–273. doi: 10.1007/s12640-009-9027-z.
- Miclescu A, Basu S, Wiklund L. Methylene blue added to a hypertonic-hyperoncotic solution increases short-term survival in experimental cardiac arrest. Crit. Care Med. 2006;34:2806–2813. doi: 10.1097/01.CCM.0000242517.23324.27.