Inhibiting Skin Senescence with Topical Rapamycin (Sirolimus): A Novel Approach to Anti-Aging Skincare
Senescent Accelerated Aging
One of the main hallmarks of aging is the accumulation of senescent cells. As we age, senescent cells grow at a compounding rate, accelerating the aging process. Senescent cells are often called "zombie cells," as they remain dysfunctional but cannot replicate or undergo programmed cell death.
Cell senescence induces a dysfunction characterized by excess growth, excessive production of disruptive cell output, and excess consumption. This cellular excess ultimately leads to the deterioration of tissue function, which accelerates the process of aging.
Manifestations of human senescence are very consistent: skin wrinkles and male baldness, insulin-resistance and osteoporosis, hypertension and atherosclerosis, obesity and diabetes, cancer and Alzheimer's disease.
Across all cell types, one of the defining characteristics of a senescent cell is its excessive release of inflammatory molecules. Senescent cells release a "witch's brew" of pro-inflammatory cytokines, chemokines, growth and mitogenic factors, pro-senescent proteins, and proteases that make up the Senescent Associated Secretory Phenotype (Senescent SASP).
The SASP can trigger an inflammatory response in nearby healthy cells. This is why we call senescent cells' zombie cells'—they don't merely release harmful chemicals; inflammatory signaling can cause adjacent healthy cells to become senescent, as the SASP and the resulting inflammation can activate senescent programming in these neighboring cells.
A vicious cycle develops—senescence begets more senescence. This process contributes to the spread of cellular dysfunction that drives tissue deterioration and an aging phenotype.
When we are young, we have regularly scheduled clearances of senescent cells—as new senescent cells are introduced, they get cleared out at an equal rate. This is what keeps our tissues functional and healthy. The proportion of functional cells to dysfunctional cells remains balanced in favor of the functional cells due to this clearance mechanism.
However, as we age, the rate at which we accumulate senescent cells outpaces the rate at which we eliminate them. The accumulation of these toxically hyper-functional senescent cells ultimately drives aging later in life. As we accumulate more and more senescent cells, the proportion of dysfunctional cells to functional cells begins to shift, contributing to the gradual decline in tissue function that characterizes the aging process.
Skin Senescence and the Potential of Rapamycin
In the skin, the accumulation of senescent cells manifests itself in various ways, including photodamage, dermal atrophy, and impaired barrier function. Photodamage is a result of prolonged exposure to UV radiation, which can lead to the breakdown of collagen and elastin fibers, resulting in wrinkles, dyspigmentation, and dull-appearing skin.
Dermal atrophy, or skin "thinning," is caused by a reduction in the underlying tissue and can result in a loss of firmness and elasticity. Impaired barrier function can cause dryness, flakiness, and a weakened skin barrier, making the skin more susceptible to damage from external factors.
If you were to take a skin biopsy of a young person and compare it to an older person's under a microscope, it would be fairly easy to identify which is which. The older person's sample would have much higher inflammation—healthy cells interspersed with dysfunctional cells, bathing in a witch's brew of pro-inflammatory molecules. Inflammation is one of the most discernible hallmarks of aging. Scientists have even coined a term for this chronic low-grade inflammation that develops with advanced age: 'inflamm-aging.'
As we grow older, we lose the phenotype of the young person's biopsy—smooth, less inflamed tissue—and enter an inflammatory state. This shift is accompanied by a transition from functional to dysfunctional tissue. This shift is due to the accumulation of senescent cells and their pernicious hyperinflammatory state—which is most apparent in aging skin.
One of the defining characteristics of senescent cells is elevated mTOR activation. mTOR is a complex in every cell type and all species. mTOR (mammalian target of rapamycin) is a protein kinase that plays a crucial role in regulating cell growth.
You can think of mTOR as the air-traffic controller for cellular growth. When cells receive signals indicating abundant nutrients or growth factors, mTOR becomes activated and stimulates cellular protein synthesis and cell growth. This activation of mTOR promotes cell growth and division, which is essential for tissue repair and growth in developing organisms.
In senescent cells, mTOR amplifies its hypergrowth and functionality, resulting in excessive growth and output that is toxic to the tissue—it makes the zombie cells bigger, more active, and more pernicious in recruiting healthy cells into a dysfunctional state. This hyperactivation of mTOR leads to the cellular dysfunction that characterizes cellular senescence.mTOR-driven hyperfunction causes the acceleration of aging skin appearance. Overexpression of insulin-like growth factor II gene (IGF-2) in keratinocytes, for example, causes overgrowth of the skin, which appears as wrinkling. Throughout the skin layer, we see the hyperfunction driving dysfunction of the skin.
If mTOR accelerates the aging of cells and tissues, then rapamycin is a potentially potent molecule to decelerate the aging process. Rapamycin binds and inhibits the activity of mTOR—slowing down the progression of senescence and cellular dysfunction.
In a recent study published in the Journal Geroscience, researchers set out to investigate the effects of applying topical rapamycin to the skin to see if it reduced the markers of senescence and aging in human skin.
Methods of the study:
The study was an exploratory, prospective, randomized trial that involved a total of 60 participants aged 40 and above.
Participants enrolled in the study were provided with a container of rapamycin cream and a container of placebo in identical dispensers with labels for right or left hand and were instructed to apply the creams to the dorsal side of each hand every 24 to 48 h in the evening before bed.
Before and after the treatment period, the researchers collected skin samples from the participants and analyzed them for markers of senescence and aging. They also assessed the participants' skin for signs of aging, such as wrinkles and sagging, using a standardized scoring system.
Results:
The results of the study showed that topical rapamycin significantly reduced markers of senescence in the skin samples collected from the treatment group. The researchers evaluated the impact of rapamycin by analyzing skin biopsies for the expression of proteins related to senescence and aging.
Rapamycin and p16 Inflammation
The researchers focused on a protein called p16INK4A (p16), which is involved in regulating the cell cycle and senescence. In the skin, senescence cells measured by increased expression of the p16 protein have been found to correlate with markers of aging such as elastic fiber morphology and wrinkling of the skin. In addition to its role in senescence, p16 has been shown to be involved in regulating inflammation in the skin. Inflammation is a driver of skin aging, and high levels of inflammation in the skin have been associated with the development of wrinkles and other signs of aging.
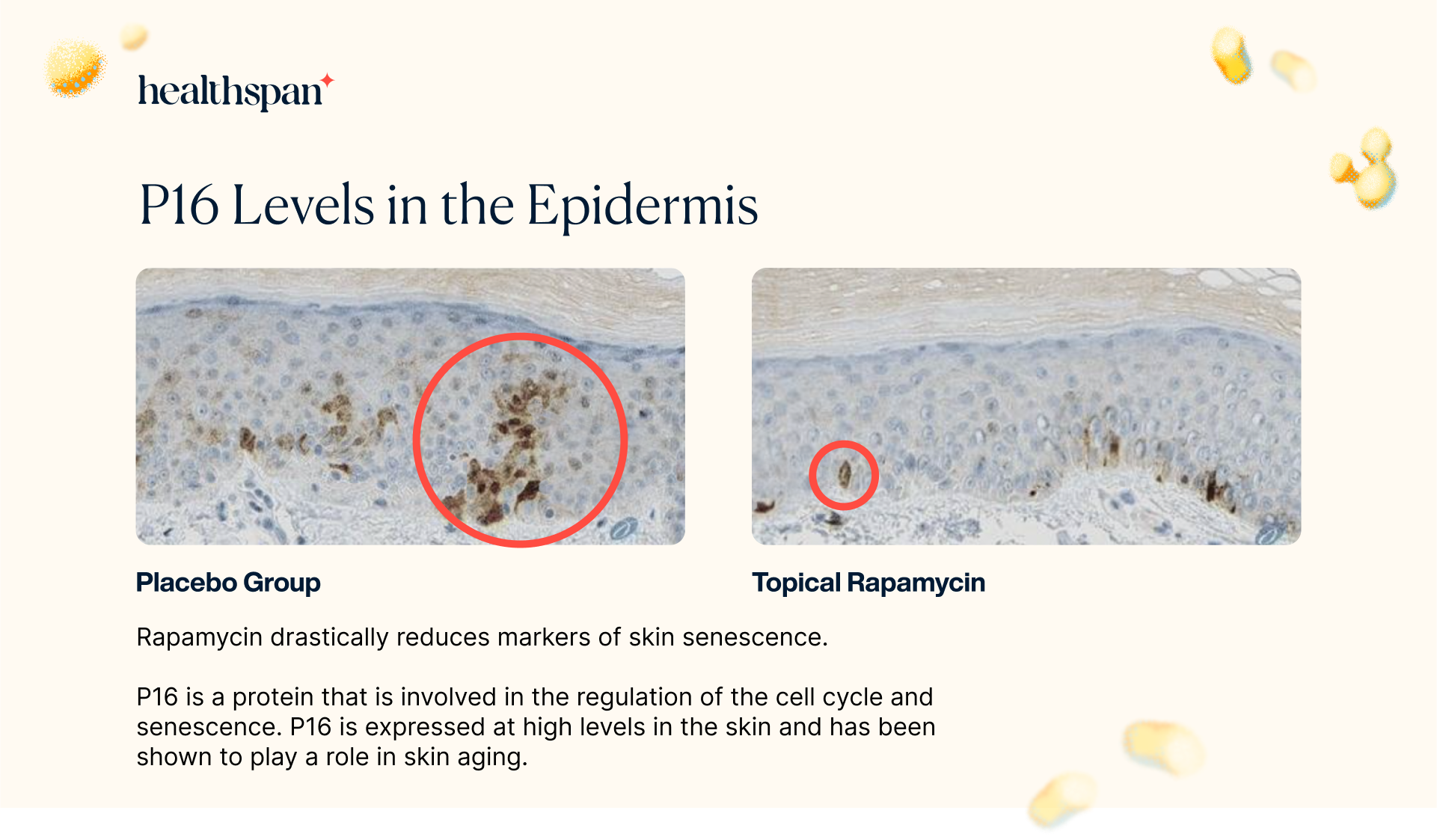
The study's findings revealed that the application of topical rapamycin led to a noteworthy 50% decrease in P16 expression in treated biopsies compared to the control group. This significant reduction in P16 expression strongly indicates that the absolute number of senescent cells in the epidermis was diminished. Unlike conventional treatments that simply modify senescent cells in the tissue, rapamycin treatment is either reducing the number of cells that enter senescence or enhancing the clearance of existing senescent cells.
Topical Rapamycin Reduces Solar Elastosis in Skin Biopsies
In the study, the researchers also assessed the levels of a skin condition known as solar elastosis. This condition is commonly seen in individuals who have experienced chronic sun exposure, particularly in the face, neck, and hands. Solar elastosis occurs when abnormal elastic fibers accumulate in the skin, resulting in a thickened and yellowed appearance.
It is a key feature of actinic keratoses, which are pre-cancerous skin lesions caused by sun damage. By evaluating the levels of solar elastosis in the skin biopsies, the researchers aimed to determine whether topical rapamycin could prevent or reduce the development of this condition.
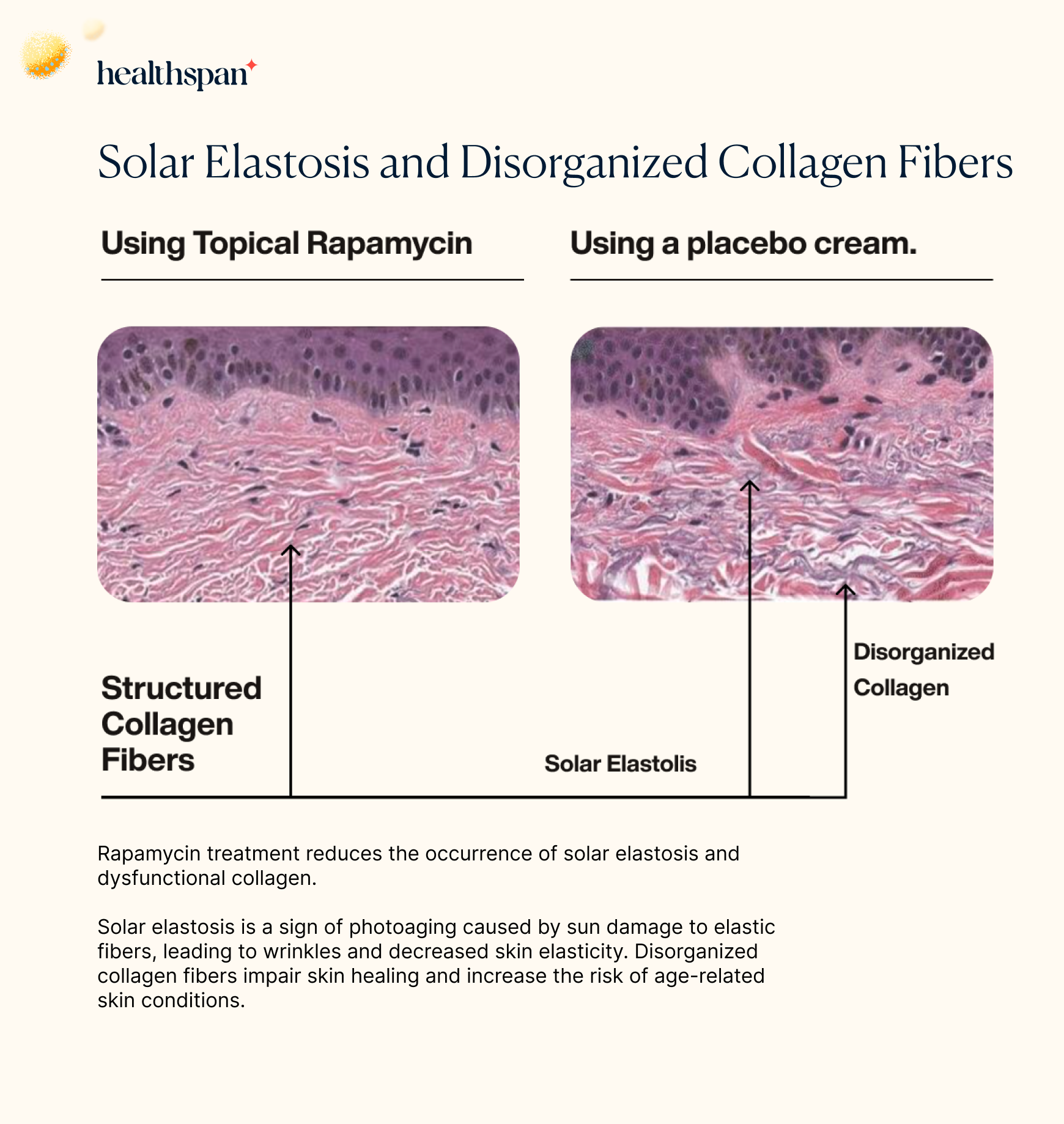
When analyzing the histological images, the researchers found that the placebo group had significantly higher levels of disorganized collagen fibers, which are associated with sun damage, as well as evidence of solar elastosis. This finding further highlights the potential of topical rapamycin as a preventative measure against the harmful effects of chronic sun exposure on the skin.
Furthermore, it appears that rapamycin treatment induces autophagy - a crucial cellular process that helps to maintain cellular health and prevent the accumulation of damaged proteins and other molecules associated with age-related diseases and tissue dysfunction. By promoting autophagy, rapamycin treatment enhances the health and function of the skin layer by increasing the clearance of damaged cellular components and byproducts.
Cytokeratin 5/6 Distribution in the Basal Layer Improves with Rapamycin Treatment
The skin biopsies also revealed that rapamycin had a positive effect on the distribution of cytokeratin 5/6 in the basal layer. Cytokeratin 5 and cytokeratin 6 are crucial components for the development and maintenance of healthy skin.
The proper distribution of cytokeratin 5 in the basal layer is vital as it provides structural support to the cells, ensuring their shape and function are maintained. Moreover, cytokeratin 5 is thought to regulate the process of mitosis, which is crucial in the replacement of the skin cells that are shed from the surface of the skin.
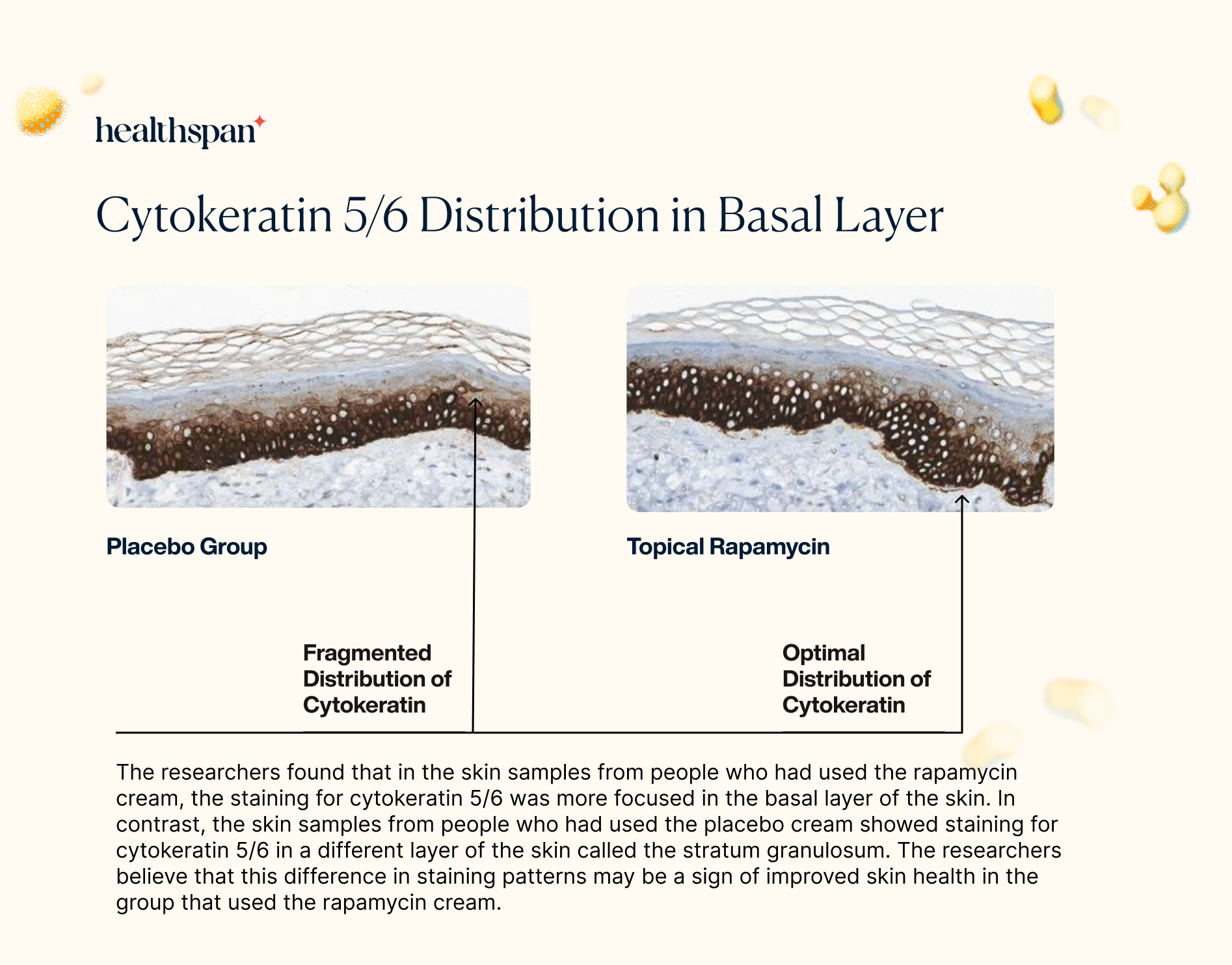
By improving the distribution of cytokeratin 5/6 in the basal layer through its stimulation of autophagy, rapamycin could have a significant impact on the health and vitality of the skin. These findings suggest that topical rapamycin may have a broader impact on the maintenance and repair of the skin, providing additional benefits beyond reducing markers of aging and preventing sun damage.
Rapamycin Increases Collagen VII Protein Levels
To explore the potential impact of rapamycin on collagen VII, the researchers utilized immunohistochemistry to assess the levels of this protein in skin samples. Collagen VII is an important structural component of the skin's extracellular matrix, which is responsible for maintaining the skin's strength and elasticity.
The findings showed that the levels of collagen VII protein were significantly increased in the skin samples treated with rapamycin. This result is particularly noteworthy since the loss of collagen VII has been associated with the development of several skin disorders, including epidermolysis bullosa and skin aging.
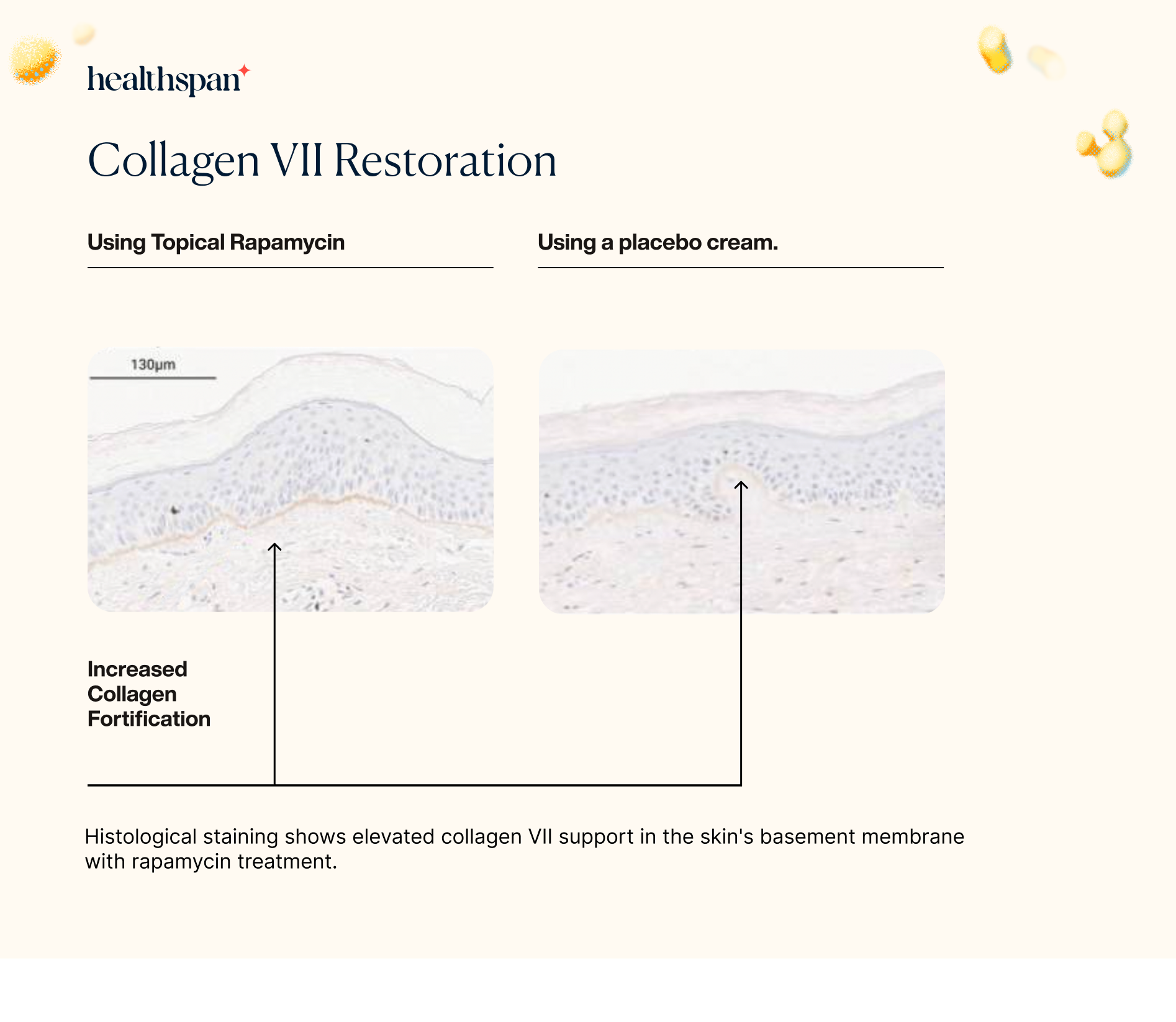
The increase in collagen VII protein levels observed in the rapamycin-treated skin samples suggests that the use of topical rapamycin could potentially help to prevent or treat these skin disorders by promoting the maintenance and repair of the skin's extracellular matrix. Overall, these findings highlight the potential of rapamycin as a promising new treatment option for various skin conditions associated with collagen loss.
The results of the study on topical rapamycin demonstrated notable improvements in several clinical signs of cutaneous aging. Observational assessments revealed a decrease in fine wrinkles, an increase in dermal volume, and a brighter, more even skin tone in the treated areas.
Additionally, sagging of the skin was reduced, indicating a tightening effect.
Of the subjects involved in the study, all but two exhibited improvements in the clinical signs of aging, including a decrease in the prominence of veins and tendons, an improvement in fine wrinkling, and an improvement in dyspigmentation and the dull appearance associated with photoaged skin. These changes were observed approximately four months after treatment began and continued to improve with subsequent visits.
Dr. Mikhail Blagosklonny's Recommendation for Topical Rapamycin
Dr. Mikhail Blagosklonny, the creator of the theory of cellular hyperfunction and renowned for his research on rapamycin, has recommended using rapamycin-containing cream as a potential cosmetic solution for skin aging. Specifically, the cream may be applied to selected areas of the skin, such as the hands and face, that are affected by age-related spots and pathologies.
However, Dr. Blagosklonny notes that the systemic use of rapamycin is likely a better option for treating the entire skin surface, as many manifestations of skin aging are related to systemic organismal aging and disease.
While topical application of any drug is generally safer than systemic administration, Dr. Blagosklonny suggests that a combined approach of systemic and topical use of rapamycin in selected areas of the skin may be the best strategy for some cases, particularly those where there are signs of aging marks.
Key Takeaways and Underlying Mechanisms
1. Rapamycin reduces the total amount of senescent cells
The data suggests that the improvement observed may be due to the reduction of the overall burden of senescent cells. This could be due to rapamycin reducing the pro-inflammatory secretions produced by senescent cells, or it could be that rapamycin is reducing the number of senescent cells.
The fact that p16 is reduced suggests that the absolute number of senescent cells in the epidermis is reduced.
Rather than simply modifying senescent cells present in the tissue, rapamycin treatment either reduces the number of cells entering senescence or increases the clearance of senescent cells.
Whether the reduction in senescent cells is due to reduced entry into senescence or increased clearance, a reduction in the burden of senescent cells would be expected to improve functionality. Beyond skin health, the implication of these results suggests that rapamycin could be a powerful tool to reduce senescence throughout the body.
2. Reduction in the SASP and inflammatory cytokines
Senescent cells produce substances called pro-inflammatory cytokines, matrix metalloproteins, and reduced levels of anti-angiogenic factors, which together create an environment that can support tumor growth and negatively affect stem cells. This group of substances is known as the Senescence-Associated Secretory Phenotype (SASP). In normal cells and tissues, the SASP is closely linked to the expression of the p16INK4A protein. The results of the study suggest that rapamycin reduces inflammatory cytokines in the skin.
3. An increase in collagen, and autophagy of misfolded collagen
Rapamycin seems to improve the incorporation of collagen VII into the basement membrane of skin, which is a measure of skin quality. Collagen VII is important for maintaining a functional skin barrier, but its levels decrease with age and in areas where wrinkles form.
The mechanism by which rapamycin increases collagen VII protein levels is not fully understood, but it is thought that rapamycin's effects on autophagy and vesicle trafficking may allow for the proper processing and localization of collagen at the cell periphery and basement membrane.
Collagen VII has also been linked to the clearance of misfolded proteins and extracellular pathogens, and may play a role in maintaining cell-cell junctional integrity.