The Metabolic Basis of Neurodegeneration: The Glucose Paradox of Fueling Cognition and Driving Decline
Background
Though relatively small, the brain consumes an outsized portion of the body’s energy—nearly 20%—to sustain its intricate and complex functions. This energy is almost entirely derived from glucose, a simple sugar essential for powering neuronal activity, enabling processes like memory formation, decision-making, and maintaining synaptic connections. However, this reliance reveals an intriguing paradox: the very fuel that drives thought and memory, when dysregulated, can also become a catalyst for cognitive decline.
A delicate balance exists within the body between glucose availability and its metabolic regulation. In the brain, this balance is particularly critical. When glucose regulation falters—whether through chronic excess or deficiency—the effects ripple through the neural network, triggering neuroinflammation, oxidative stress, and structural damage that undermine cognitive health and resilience. These processes not only impair everyday mental functions but also increase the risk of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s.
Adding to this complexity are the roles of other metabolic players, such as fructose, which can exacerbate inflammation and oxidative stress, and ketones, which serve as an alternative energy source during metabolic stress. Together, these molecules form a complex web of interactions, highlighting the brain’s remarkable adaptability while underscoring the risks of dysregulation.
In this research review, Healthspan’s clinical team delves into the intricate relationship between glucose regulation and cognitive health. Shriya Bakhshi, a longevity research specialist, explores the cellular mechanisms driving glucose’s profound impact on the brain, while Kristen Race, MS, Patient Education Specialist at Healthspan, reviews actionable strategies—spanning lifestyle modifications and pharmacological approaches—to support optimal glucose regulation. By examining these mechanisms and interventions, this article aims to provide a comprehensive understanding of how to protect cognitive function through improved metabolic health.
Glucose as the Brain's Primary Energy Source
Glucose is the brain’s primary energy source, powering nearly all its essential functions. Despite making up only about 2% of the body’s weight, the brain consumes approximately 20% of the body’s energy, underscoring its reliance on glucose. To meet this demand, glucose journeys through the bloodstream and crosses the blood-brain barrier (BBB), a selective security checkpoint designed to protect the brain from potentially harmful intruders. This passage relies on specialized gatekeeping proteins known as glucose transporters, with GLUT1 operating in the BBB's endothelial cells and GLUT3 managing glucose uptake directly into neurons. These transporters ensure that glucose—a molecule vital for cognitive performance and neuronal survival—reaches its destination efficiently and securely, sustaining the brain's energy-intensive operations.
Once inside the brain, glucose undergoes a series of chemical reactions to produce adenosine triphosphate (ATP), the molecule that stores and delivers energy for cellular activities. The production of ATP involves three key steps: glycolysis, where glucose is broken down into smaller molecules; the citric acid cycle (or Krebs cycle), which extracts more energy; and oxidative phosphorylation, where the bulk of ATP is generated. This energy is indispensable for neurons, the brain’s communicators, enabling them to transmit signals, release neurotransmitters (the chemical messengers of the brain), and regulate ionic balance to sustain the electrical activity essential for cognition and function.
Unlike many other cells in the body, neurons are entirely dependent on a continuous supply of glucose from the bloodstream—they lack storage "reservoirs" for energy. To meet this demand, astrocytes, specialized glial cells often referred to as the brain’s "caretakers," step in to assist. Astrocytes absorb glucose and convert it into lactate, a flexible energy source that neurons can tap into during moments of high demand, such as intense learning or problem-solving. This cooperative dynamic between neurons and astrocytes ensures the brain’s energy requirements are met, particularly in regions like the hippocampus, which is vital for forming memories, and the prefrontal cortex, critical for decision-making and executive control.
Beyond fueling brain activity, glucose also underpins the synthesis of the brain’s two key neurotransmitters: glutamate, which excites neurons and drives processes like learning and memory, and GABA, which counterbalances excitation by dampening excessive neuronal activity. This careful equilibrium between excitation and inhibition is like the brain’s "volume control," maintaining the harmony essential for cognitive health and function.
Glucose plays a critical role in the brain’s defense systems, acting as a key contributor to its "antioxidant arsenal." It fuels the pentose phosphate pathway, a biochemical route that produces NADPH, a molecule indispensable for regenerating glutathione—the brain’s primary antioxidant and a frontline defender against cellular damage. Glutathione neutralizes reactive oxygen species (ROS), unstable byproducts of normal metabolism that, if left unchecked, act like sparks capable of igniting widespread damage to neurons.
When glucose levels are insufficient to sustain this pathway, ROS can accumulate, tipping the balance toward oxidative stress—a state where the brain’s defenses are overwhelmed. Over time, this oxidative stress can injure neurons and contribute to neurodegenerative processes, underscoring the importance of glucose not just as an energy source but as a vital protector of brain health.
If glucose levels are unstable—whether too high or too low—the brain’s ability to perform these vital processes is compromised. Chronic high glucose levels (hyperglycemia) are commonly associated with conditions like diabetes and can lead to symptoms such as fatigue, excessive thirst, frequent urination, and slow wound healing. Low glucose levels (hypoglycemia) can cause dizziness, confusion, and even loss of consciousness.
What is less commonly understood is the cumulative impact of glucose dysregulation on long-term brain health. Chronic hyperglycemia has been implicated in the development of insulin resistance within the brain, a key feature of Alzheimer’s disease often referred to as "type 3 diabetes." The combination of inflammation, oxidative damage, and disrupted neuronal signaling significantly increases the risk of neurodegenerative diseases like Alzheimer’s and Parkinson’s, as well as general cognitive decline. Hypoglycemia, although typically acute, can also have lasting effects if episodes are frequent, exacerbating neuronal stress and vulnerability over time. Maintaining stable glucose levels is therefore not only critical for overall health but also essential for protecting the brain from the insidious effects of dysregulation, safeguarding cognitive function and resilience against age-related changes.
Mechanisms Linking Glucose Dysregulation to Cognitive Decline
The brain's reliance on glucose makes it exceptionally vulnerable to disruptions in glucose regulation. When blood sugar levels are too high (hyperglycemia) or too low (hypoglycemia), the brain experiences metabolic imbalances that impair its energy supply, cellular function, and structure. These imbalances initiate a cascade of harmful processes, including neuroinflammation, oxidative stress, blood-brain barrier breakdown, vascular damage, and synaptic dysfunction. Together, these mechanisms can lead to cognitive decline and increase the risk of neurodegenerative diseases.
Neuroinflammation and Oxidative Stress
Glucose dysregulation triggers several interconnected processes that harm the brain, starting with neuroinflammation and oxidative stress:
Hypoglycemia (Low Blood Sugar)
When blood sugar drops below optimal levels, neurons—the brain’s communication cells—are deprived of the glucose they need to produce energy in the form of ATP. ATP is crucial for maintaining the electrical signals that neurons use to communicate and for basic cellular maintenance. Without sufficient energy, neurons cannot function properly, leading to confusion, difficulty concentrating, and irritability. If hypoglycemia persists, it can cause more severe effects, including neuron damage or death, especially in highly active brain areas like the hippocampus, which is responsible for memory formation. [1]
Hyperglycemia (High Blood Sugar)
Chronic high glucose levels overwhelm the brain’s ability to manage glucose metabolism efficiently. Over time, this causes insulin resistance, a condition in which neurons become less sensitive to insulin, the hormone that allows cells to absorb glucose from the bloodstream. Insulin resistance deprives neurons of glucose despite its abundance in the blood, creating an energy shortage. This paradoxical state of “starvation amid plenty” disrupts neuronal function and promotes neuroinflammation, a process in which immune cells in the brain release inflammatory chemicals that damage neurons and interfere with their ability to communicate. [1]
Oxidative Stress and Advanced Glycation End-Products (AGEs)
Hyperglycemia contributes to the formation of advanced glycation end-products (AGEs), harmful compounds generated when excess glucose binds to proteins or lipids. These modified molecules accumulate in tissues, including the brain, where they disrupt normal cellular functions. AGEs interact with receptors on cell surfaces known as RAGEs (Receptors for Advanced Glycation End-products), activating inflammatory pathways and amplifying the production of reactive oxygen species (ROS).
ROS are highly reactive, unstable molecules that can act like biochemical "wrecking balls," damaging essential cellular components such as DNA, proteins, and membranes. This damage, collectively known as oxidative stress, undermines neuronal integrity and accelerates aging processes. The brain is particularly susceptible to oxidative stress due to its high metabolic demands and relatively limited antioxidant defenses compared to other organs.
Over time, the cumulative effects of oxidative stress weaken synaptic function, the "communication bridges" between neurons. This diminishes the brain’s ability to form and maintain neural networks, a process crucial for learning, memory, and adaptation. Such disruptions not only impair cognitive function but also contribute to the progression of neurodegenerative diseases, including Alzheimer’s and Parkinson’s. By fueling inflammation and oxidative damage, hyperglycemia sets the stage for a cascade of harmful processes that jeopardize brain health and resilience.
In Alzheimer’s disease, advanced glycation end-products (AGEs) and oxidative stress are deeply intertwined with the formation of amyloid-beta plaques and tau tangles—hallmark toxic protein aggregates. Amyloid-beta plaques form when fragments of the amyloid precursor protein (APP) accumulate and clump together outside neurons, while tau tangles develop inside neurons when tau proteins, crucial for stabilizing microtubules, become abnormally phosphorylated and misfolded.
Both AGEs and oxidative stress exacerbate these pathological processes. AGEs modify proteins, altering their structure and function, which can accelerate the aggregation of amyloid-beta and tau. Meanwhile, oxidative stress further destabilizes cellular environments, impairing the brain’s ability to clear these toxic aggregates. Together, these factors disrupt synaptic communication, severing the intricate signaling networks that enable memory and cognition. As these aggregates spread, they lead to widespread neuronal dysfunction and ultimately, cell death, driving the progressive cognitive decline characteristic of Alzheimer’s disease.
The role of advanced glycation end-products (AGEs) in Alzheimer’s disease (AD) pathology was explored in the seminal paper "Advanced Glycation End Products in Alzheimer's Disease and Other Neurodegenerative Diseases," published in 1998 in The American Journal of Pathology. This research provided early evidence linking AGEs to the molecular mechanisms underlying Alzheimer’s and other neurodegenerative diseases, offering foundational insights that continue to shape our understanding today.
Study Design
To investigate the role of advanced glycation end-products (AGEs) in Alzheimer’s disease (AD) pathology, the researchers employed a comprehensive, multi-method approach:
- Tissue Analysis: Postmortem brain tissue samples were obtained from individuals diagnosed with Alzheimer’s disease and compared with age-matched, non-demented controls. This approach enabled the researchers to identify patterns of AGE deposition specific to AD pathology, minimizing confounding effects related to normal aging.
- Immunohistochemistry: Using AGE-specific antibodies, the study localized AGE accumulation within key brain regions. Co-localization studies provided critical insights into the spatial overlap between AGEs and hallmark AD lesions, such as amyloid-beta plaques and neurofibrillary tangles (NFTs), revealing potential interactions between AGEs and these pathological structures.
- Quantitative and Comparative Analysis: The researchers employed quantitative measures to assess the intensity and distribution of AGE deposition across Alzheimer’s and control tissues. This comparative analysis offered a detailed perspective on the degree of AGE involvement in AD relative to normal aging, highlighting their pathological significance.
Results
Their findings revealed significant AGE accumulation in Alzheimer’s brains, particularly within amyloid-beta plaques and neurofibrillary tangles (NFTs), two hallmark pathological structures of AD. The study revealed significantly elevated levels of AGE-modified proteins in the brains of individuals with Alzheimer’s disease compared to non-demented controls. AGE deposition was particularly prominent in amyloid-beta plaques and neurofibrillary tangles (NFTs), two hallmark pathological structures of AD. These findings reinforce the association between AGE accumulation and the fundamental molecular features of Alzheimer’s disease. [7]
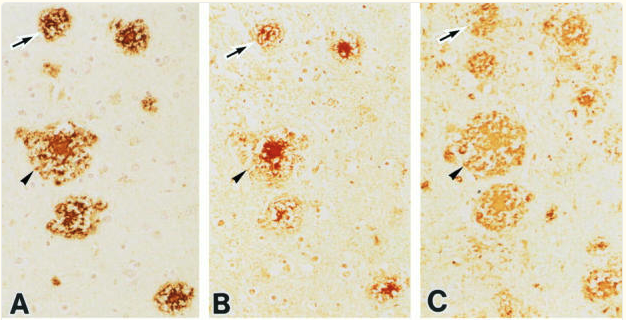
Immunohistochemical analysis demonstrated a robust co-localization of AGEs with amyloid-beta plaques and tau protein aggregates in NFTs. This suggests that AGEs may play an active role in facilitating the formation, stability, or persistence of these toxic protein aggregates, thereby contributing to the structural and functional disruptions observed in AD. [7]
The study identified a strong correlation between AGE accumulation and markers of oxidative stress and inflammation. By interacting with cellular receptors and metabolic pathways, AGEs appear to amplify oxidative damage and inflammatory responses, further exacerbating neuronal dysfunction and accelerating disease progression.
This study was among the first to establish advanced glycation end-products (AGEs) as both critical markers and active mediators of Alzheimer’s disease progression. It provided early evidence of how metabolic dysfunction, particularly hyperglycemia and associated oxidative stress, contributes to neurodegeneration. By demonstrating AGE involvement in amyloid-beta plaque and NFT formation, as well as in promoting oxidative damage and inflammation, the research highlighted AGEs as central players in the molecular cascade driving AD pathology. [7]
From a therapeutic perspective, these findings suggest that strategies targeting AGE formation, enhancing their clearance, or disrupting their interactions with neuronal structures may hold potential in mitigating the progression of Alzheimer’s disease. By breaking the cycle of protein modification, oxidative stress, and inflammation, such approaches could protect neuronal integrity and slow cognitive decline.
This pioneering work laid the foundation for subsequent research exploring the broader implications of AGEs in neurodegeneration, reinforcing the need for innovative interventions aimed at reducing their impact on brain health and resilience.
Blood-Brain Barrier (BBB) Compromise
The blood-brain barrier (BBB) is a highly selective structure that serves as a gatekeeper, regulating the exchange of substances between the bloodstream and the brain. Comprised of tightly packed endothelial cells, it allows the passage of essential nutrients such as glucose and oxygen while preventing harmful substances, including toxins, pathogens, and certain immune cells, from entering the brain’s delicate environment. However, chronic hyperglycemia—commonly observed in metabolic disorders such as diabetes—can damage the endothelial cells that form the BBB, leading to increased permeability or a "leaky" barrier.
When the BBB is compromised, harmful substances, such as inflammatory molecules, circulating toxins, and immune cells, can infiltrate the brain. Once inside, these invaders exacerbate neuroinflammation and directly contribute to neuronal damage. Inflammatory cytokines released in response to BBB breakdown interfere with synaptic plasticity—the brain’s ability to adapt and form new neural connections—undermining processes critical for learning and memory.
A weakened BBB also disrupts the brain’s waste management systems. Under normal conditions, the BBB supports the clearance of toxic proteins, including amyloid-beta, a key pathological marker of Alzheimer’s disease. When the barrier’s integrity is compromised, this clearance is impaired, allowing amyloid-beta to accumulate and contribute to the development of neurodegenerative conditions. The combined effects of neuroinflammation, synaptic disruption, and impaired clearance of toxic proteins accelerate cognitive decline and heighten the brain’s vulnerability to Alzheimer’s disease and other neurodegenerative disorders.
By compromising the BBB, chronic hyperglycemia sets off a cascade of detrimental effects that undermine the brain’s ability to maintain its internal environment, emphasizing the critical role of metabolic health in preserving cognitive function and resilience against neurodegenerative conditions.[1]
A pivotal 2020 study published in the prestigious journal Nature, titled "Hyperglycemia and Advanced Glycation End Products Disrupt Blood-Brain Barrier and Reduce Cerebral Blood Flow in Mice", provides critical insights into the mechanisms linking elevated blood glucose levels and advanced glycation end products (AGEs) to neurovascular dysfunction. Using a well-controlled murine model, the researchers examined how chronic hyperglycemia and AGE exposure compromise the integrity of the blood-brain barrier (BBB) and cerebral blood flow (CBF), offering a robust framework for understanding the pathophysiological consequences of metabolic dysregulation on brain health. [8]
The study utilized a multifaceted approach to dissect the pathological effects of hyperglycemia and AGEs. Mice served as the experimental model, allowing the researchers to replicate key features of metabolic dysregulation and its impact on neurovascular integrity. Mice are commonly used in biomedical research due to their genetic and physiological parallels with humans, particularly for studying metabolic disorders like diabetes.
The researchers induced hyperglycemia in the mice through established methods, such as dietary interventions or chemical treatments like streptozotocin. These approaches mimic the chronic high blood glucose levels observed in diabetic patients, enabling the researchers to study long-term effects on the BBB and CBF. [8]
To evaluate the specific role of AGEs, the researchers administered exogenous AGEs to the mice. This allowed them to isolate and analyze the direct impact of these modified molecules on neurovascular function, independent of hyperglycemia.
The study revealed that both hyperglycemia and AGE exposure significantly compromised BBB integrity, leading to increased permeability, or a “leaky” barrier. This disruption allowed harmful molecules, including inflammatory mediators and toxins, to penetrate the brain, exacerbating neuroinflammation and oxidative stress. Additionally, the researchers observed a notable reduction in CBF, indicating impaired cerebral perfusion and increased vulnerability of brain tissues. [8]
These findings underscore the critical role of metabolic health in preserving neurovascular integrity. Chronic hyperglycemia, as commonly seen in diabetes, may predispose individuals to neurovascular damage and increase their risk of developing neurodegenerative conditions, such as Alzheimer’s disease. The study highlights the potential therapeutic value of targeting AGEs or mitigating hyperglycemia to protect the BBB and maintain cerebral blood flow, offering promising avenues for future research and clinical intervention. [8]
By providing a detailed examination of the mechanisms linking metabolic dysfunction to brain health, this study has established a foundation for understanding how hyperglycemia and AGEs contribute to neurovascular impairment. These insights reinforce the importance of glycemic control in safeguarding cognitive resilience and preventing neurodegenerative disease progression.
Vascular Damage and Cognitive Decline
The vascular system plays an essential role in maintaining brain function by delivering oxygen and nutrients, particularly glucose, to meet its high metabolic demands. However, chronic glucose dysregulation, as seen in conditions like diabetes, severely impacts vascular health, leading to a cascade of problems that compromise cognitive function:
1. Endothelial Dysfunction
Chronic hyperglycemia acts like a corrosive agent on the endothelial cells lining blood vessels, gradually wearing down their ability to function as the body’s vascular "gatekeepers." Endothelial cells are essential for orchestrating blood flow, preventing clots, and managing inflammation. However, persistently elevated glucose levels trigger oxidative stress and glycation—akin to rust forming on metal—compromising the flexibility and resilience of these cells.
This endothelial dysfunction sets the stage for atherosclerosis, a process where plaques—composed of lipids, cholesterol, and cellular debris—accumulate like debris clogging a stream. Over time, these plaques calcify and stiffen, narrowing the blood vessels and reducing their ability to carry blood efficiently. In cerebral vessels, this narrowing restricts the delivery of oxygen and glucose, the brain’s primary energy source, much like a kinked garden hose limits water flow to plants.
When blood flow becomes insufficient, ischemia can develop—localized areas of brain tissue starved of oxygen and nutrients. This energy deficit disrupts the intricate network of synapses, the "communication hubs" of neurons, impairing their ability to send signals and maintain function. If prolonged, ischemia leads to neuronal damage and cell death, contributing to cognitive decline and increasing the risk of ischemic strokes. These strokes can further accelerate a vicious cycle of reduced cerebral perfusion and neuronal dysfunction, similar to a failing power grid struggling to sustain demand.
By undermining endothelial health and impairing cerebral blood flow, chronic hyperglycemia creates cascading effects that jeopardize brain function. Maintaining stable glucose levels is therefore not just critical for vascular health but also vital for preserving the brain’s "ecosystem," ensuring it has the resources to support cognition and resilience against neurodegenerative diseases.
2. Microvascular Damage
Chronic hyperglycemia exerts a profound impact on the brain's microvasculature, the intricate network of tiny blood vessels responsible for delivering oxygen and nutrients to neurons with pinpoint precision. This system functions like a city’s network of side streets, ensuring even the most remote neighborhoods—the brain’s cellular structures—receive their essential supplies. However, elevated blood glucose levels compromise the integrity of these microvessels, causing structural changes such as thickened basement membranes, endothelial cell damage, and loss of capillary density. These alterations, collectively referred to as microvascular remodeling, restrict blood flow at a microscopic level, akin to debris clogging narrow alleys and obstructing traffic.
Over time, these disruptions increase the risk of "silent strokes," small ischemic events that often go unnoticed due to their subtle or asymptomatic nature. Although individually minor, these strokes accumulate, leaving behind pockets of damaged brain tissue, much like small, untreated potholes gradually degrading the overall integrity of a road system. This accumulated damage undermines the brain's structural and functional resilience, impairing neuronal communication and connectivity.
The consequences of these microvascular changes extend beyond the immediate effects of ischemia. Silent strokes diminish the brain’s cognitive reserve—the capacity to adapt and compensate for injury—which is critical for maintaining cognitive health as we age. Over time, the cumulative impact of silent strokes can contribute to the progression of vascular dementia and exacerbate the effects of neurodegenerative diseases like Alzheimer’s.
This underscores the critical role of microvascular health in preserving the brain’s "supply chain." Protecting this fragile network through glycemic control is essential for maintaining neuronal vitality, reducing the risk of cognitive decline, and safeguarding against dementia.
3. Impaired Neurovascular Coupling
The brain possesses a remarkable ability to regulate blood flow dynamically, ensuring that regions engaged in high activity receive the energy they need. This process, known as neurovascular coupling, acts like a finely tuned traffic system, directing resources—oxygen and glucose—to "busy intersections" where cognitive tasks such as problem-solving or learning are taking place. When functioning optimally, neurovascular coupling ensures that neuronal demands are met in real-time, supporting activities like synaptic transmission, memory formation, and executive decision-making.
However, chronic glucose dysregulation disrupts this balance. Hyperglycemia damages endothelial cells and impairs the signaling pathways between neurons and blood vessels, causing the "traffic lights" of neurovascular coupling to malfunction. As a result, blood flow is poorly matched to areas of heightened neural activity, leading to localized energy shortages akin to a bustling city gridlock where critical supplies fail to reach their destination.
This impairment has far-reaching consequences. Without adequate delivery of glucose and oxygen, neurons struggle to sustain synaptic activity, affecting cognition, memory, and problem-solving abilities. Over time, the inability to efficiently allocate resources during cognitive tasks contributes to neuronal stress, diminished cognitive performance, and increased vulnerability to neurodegenerative diseases.
Synaptic Dysfunction and Neurotransmitter Imbalances
Glucose dysregulation also disrupts the production of neurotransmitters, the chemical messengers that neurons use to communicate. For example:
- Glutamate, the brain’s primary excitatory neurotransmitter, requires glucose for its synthesis. Glutamate is critical in learning, memory, and other cognitive functions.
- GABA, the brain’s primary inhibitory neurotransmitter, is also derived from glucose. It helps maintain balance in neuronal activity and prevents overstimulation.
When glucose levels are unstable, the production of these neurotransmitters is impaired, leading to an imbalance between excitation and inhibition in the brain. This imbalance can cause cognitive symptoms such as memory loss, difficulty concentrating, and slowed decision-making.
The combined effects of glucose dysregulation—energy shortages, oxidative stress, neuroinflammation, BBB compromise, vascular damage, and synaptic dysfunction—create a self-perpetuating cycle of harm. For example, oxidative stress exacerbates neuroinflammation, further damaging the BBB and allowing more harmful substances into the brain. Similarly, vascular damage reduces blood flow, depriving neurons of glucose and oxygen, which worsens energy deficits and neuronal dysfunction.
This complex interplay of mechanisms highlights why maintaining stable glucose levels is critical for brain health. Consistent glucose regulation supports neuronal energy supply, protects against oxidative and inflammatory damage, and preserves the brain's structural and functional integrity.
Gut-Brain Axis and Glucose Regulation
The gut, often referred to as the "second brain," plays a vital role in glucose regulation and brain health. Acting as both a digestive organ and a key player in the endocrine and immune systems, the gut influences metabolism and cognitive function through a complex bidirectional communication network called the gut-brain axis. This communication is mediated by the nervous system, immune signals, and microbial metabolites, making gut health a cornerstone of both metabolic and neurological well-being. Disruptions in the gut, such as dysbiosis or gut barrier dysfunction, can impair glucose regulation, trigger systemic inflammation, and exacerbate cognitive decline.
Role of Gut Microbiota
The gut microbiota—a vast and dynamic ecosystem of trillions of microorganisms residing in the intestines—plays a pivotal role in regulating glucose metabolism and modulating inflammation. These microbes act as the body’s "metabolic symbionts," performing essential functions that influence systemic health and brain function. Among their many roles, one of the most critical is breaking down dietary fibers into short-chain fatty acids (SCFAs), including butyrate, acetate, and propionate, which serve as powerful metabolic regulators. SCFAs regulate glucose and insulin levels in several ways:
1. Enhancing Insulin Sensitivity
SCFAs influence the release of incretin hormones, such as GLP-1 (glucagon-like peptide-1) and PYY (peptide YY), which act like molecular "traffic controllers" for glucose metabolism. GLP-1 enhances insulin secretion, reduces postprandial glucose spikes, and slows gastric emptying, creating a smoother flow of glucose into the bloodstream. This stabilization of blood sugar levels helps optimize energy availability for tissues, including the brain, while reducing metabolic strain.
2. Regulating Inflammation
SCFAs act as "anti-inflammatory mediators," reducing the production of pro-inflammatory cytokines like TNF-alpha and IL-6 while promoting anti-inflammatory cytokines such as IL-10. By calming systemic inflammation, SCFAs help prevent insulin resistance, a condition that impairs glucose uptake and can disrupt brain function. This anti-inflammatory effect creates a protective "buffer" against metabolic and cognitive decline, emphasizing the gut-brain connection.
3. Strengthening the Gut Lining
Butyrate, in particular, supports gut barrier health by nourishing intestinal epithelial cells. A robust gut barrier prevents harmful substances, such as endotoxins, from entering the bloodstream, thereby reducing systemic inflammation. This protective barrier also limits the downstream effects of inflammation on the brain, preserving cognitive health and preventing neuronal stress.
Short-Chain Fatty Acids and Neuroprotection
Butyrate, a key short-chain fatty acid produced by gut microbes during the fermentation of dietary fibers, plays a critical role in maintaining both gut and brain health. Often referred to as the "multi-tasker" of SCFAs, butyrate not only strengthens the gut lining and reduces systemic inflammation but also exerts significant neuroprotective effects through its interactions with the brain.
1. Crossing the Blood-Brain Barrier: A Direct Path to Neuroprotection
Unlike many other molecules, butyrate has the unique ability to cross the blood-brain barrier (BBB), allowing it to directly influence brain cells. Once in the brain, butyrate acts as a "fertilizer for neural growth," enhancing the production of brain-derived neurotrophic factor (BDNF), a protein critical for neuronal survival, plasticity, and repair. Elevated levels of BDNF have been linked to improved learning, memory, and resilience against neurodegenerative diseases such as Alzheimer’s and Parkinson’s, underscoring butyrate’s role in sustaining cognitive health.
2. Regulating Neuroinflammation: Calming the Brain’s Immune Response
Butyrate plays a key role in modulating the brain’s immune system by inhibiting the overactivation of microglia, the brain’s resident immune cells. In states of dysregulation, microglia can become overactive, releasing pro-inflammatory molecules that damage neurons. Butyrate acts as a "dimmer switch," suppressing the release of these inflammatory mediators and protecting neurons from inflammation-induced damage. This anti-inflammatory effect helps maintain a healthy environment for synaptic function and neural communication.
3. Improving Mitochondrial Function: Energizing the Brain
Neurons require large amounts of energy to sustain high-demand processes such as synaptic transmission. A 2017 study published in Translational Psychiatry titled "Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with Autism" provides compelling evidence supporting the role of butyrate in enhancing mitochondrial function.
The researchers investigated the effects of butyrate on mitochondrial function under conditions of oxidative stress in cell lines derived from boys diagnosed with autism spectrum disorder (ASD). Given that mitochondrial dysfunction and oxidative stress are often associated with ASD, the study aimed to determine whether butyrate could ameliorate these cellular deficits.
Butyrate treatment significantly increased mitochondrial respiration rates, akin to fine-tuning an engine to run more efficiently. This improvement indicates that butyrate optimizes the electron transport chain's function, enhancing the mitochondria's ability to generate energy. By increasing the efficiency of this biochemical "power plant," cells are better equipped to meet their energy demands, particularly during periods of stress. [9]
The study revealed elevated levels of ATP in butyrate-treated cells. This suggests that butyrate boosts the cell’s energy economy, ensuring that ATP-dependent processes, such as synaptic transmission and intracellular signaling, can operate without interruption. For neurons, this steady energy supply is essential to maintain their high metabolic activity and complex communication networks. [9]
By decreasing markers of oxidative stress, butyrate exhibits antioxidant-like properties that protect mitochondria from damage caused by reactive oxygen species. This effect is comparable to shielding a delicate mechanism from wear and tear, preserving mitochondrial integrity and ensuring long-term functionality. Reducing oxidative stress not only safeguards cellular components but also promotes overall energy efficiency. [9]
Butyrate positively influenced mitochondrial dynamics, striking a balance between mitochondrial fission (division) and fusion (joining). These processes are akin to traffic management in a city—fission allows the removal of damaged segments, while fusion facilitates the sharing of resources and repair. By optimizing this balance, butyrate ensures that mitochondria maintain their structural integrity and adaptability, critical for cellular health and resilience. [9]
In the context of neurological health, these effects are especially critical. Neurons rely heavily on mitochondrial efficiency to sustain synaptic activity, plasticity, and repair mechanisms. Mitochondrial dysfunction is a common feature of many neurodegenerative diseases, including Alzheimer’s and Parkinson’s, making butyrate’s neuroprotective properties particularly promising.
Dysbiosis and Cognitive Health
While a healthy gut microbiota plays a critical role in maintaining metabolic balance and brain health, its delicate ecosystem can be easily disrupted. When the balance of gut microbes is disrupted—a condition known as dysbiosis—the regulatory benefits of a healthy microbiome are lost. Dysbiosis often results in the overgrowth of harmful bacteria, which produce inflammatory molecules such as lipopolysaccharides (LPS). LPS, structural components of the outer membranes of certain gram-negative bacteria, act as molecular "alarm signals," triggering potent immune responses. However, when the gut barrier is weakened, these inflammatory molecules can escape into the bloodstream, leading to a condition called metabolic endotoxemia, characterized by chronic low-grade inflammation.
They can cross a weakened gut barrier and enter the bloodstream, causing metabolic endotoxemia—a state of chronic low-grade inflammation. Metabolic endotoxemia has several effects on glucose regulation and cognitive health:
Impaired Insulin Signaling: The chronic inflammation driven by LPS disrupts insulin signaling pathways in both peripheral tissues and the brain. This disruption creates a metabolic "disconnect," where cells become resistant to insulin's actions, leading to systemic insulin resistance. For neurons, this resistance deprives them of the glucose needed to fuel critical functions like synaptic transmission and plasticity. As a result, glucose dysregulation worsens, further impairing energy availability for optimal brain function.
Neuroinflammation: LPS that enters the brain via the bloodstream can breach its defenses and activate microglia, the brain's resident immune cells. Microglia, normally the "guardians" of the brain, shift into an overactive state when confronted with LPS. In this heightened state, they release pro-inflammatory cytokines and reactive oxygen species, which act like "friendly fire," damaging nearby neurons and impairing synaptic communication. This neuroinflammatory cascade not only affects cognitive function but also contributes to the progression of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, by accelerating neuronal loss and disrupting neural networks.
Dysbiosis and its downstream effects highlight the intricate connection between gut health, glucose metabolism, and cognitive function. The migration of LPS from the gut to the bloodstream represents a critical breach in the gut-brain axis, transforming what should be a symbiotic relationship into a source of chronic metabolic and neuroinflammatory stress.
Leaky Gut and Leaky Brain Phenomena
A healthy gut lining acts as a selective barrier, allowing nutrients and beneficial molecules to pass through while keeping toxins and pathogens out. However, dysbiosis or chronic inflammation can weaken this barrier, resulting in “leaky gut.”
In a leaky gut, gaps form between the epithelial cells lining the intestines, allowing harmful substances such as LPS, undigested food particles, and microbial byproducts to enter the bloodstream. These substances can trigger systemic inflammation, exacerbating insulin resistance and disrupting glucose regulation.
The systemic inflammation caused by leaky gut also affects the blood-brain barrier (BBB), leading to a phenomenon often called “leaky brain.” Like the gut lining, the BBB is a protective barrier that prevents harmful substances from entering the brain. Chronic inflammation weakens this barrier, allowing toxins, immune cells, and inflammatory molecules to infiltrate the brain. This results in neuroinflammation, oxidative stress, and neuronal damage, all of which accelerate cognitive decline.
The interconnectedness of the gut and brain extends beyond the physical barriers of the gut lining and blood-brain barrier to include intricate communication networks. Among these, the vagus nerve emerges as a key player, acting as a bidirectional "superhighway" for signals traveling between the gut and the brain. While the integrity of the gut lining and the BBB safeguard against harmful substances, the vagus nerve facilitates real-time information exchange, directly linking the health of the gut microbiota to the brain’s metabolic and cognitive processes.
Vagus Nerve Communication
The vagus nerve is a key component of the autonomic nervous system, which controls many of the body’s involuntary processes, such as heart rate, digestion, and respiratory rate. It is one of the longest nerves in the body, running from the brainstem down to the abdomen. Its branches connect to major organs, including the heart, lungs, and gastrointestinal tract.
In the context of the gut-brain axis, the vagus nerve serves as a vital communication highway between the gut and the brain, transmitting signals in both directions. About 80-90% of the vagus nerve’s fibers are sensory, which carries information from the gut to the brain. For example, the vagus nerve detects the state of the gut microbiota, the presence of nutrients, and signals of inflammation or damage. These signals are processed in the brainstem and can influence mood, cognition, and the regulation of glucose metabolism in the brain.
The vagus nerve also sends signals from the brain to the gut to regulate functions like the release of digestive enzymes, gut motility (food movement through the digestive system), and immune responses. For instance, the brain can use the vagus nerve to modulate gut inflammation by calming immune activity during stress or illness.
Beneficial gut microbes produce metabolites such as short-chain fatty acids that can activate the vagus nerve. This activation promotes an anti-inflammatory state, which helps protect the brain from inflammation-related damage. However, when the gut microbiota is disrupted (dysbiosis), harmful signals can travel along the vagus nerve to the brain, triggering neuroinflammation and impairing brain function. For example, inflammatory molecules from the gut can activate vagal pathways that stimulate the brain’s immune cells (microglia), releasing damaging pro-inflammatory chemicals. The vagus nerve is a critical link between the gut and the brain. It plays a significant role in maintaining metabolic balance, regulating inflammation, and preserving cognitive health. This involvement underscores the importance of a healthy gut for optimal brain function.
Beyond Glucose: The Role of Fructose in Cognitive Health
While glucose is the brain’s primary energy source, critical for sustaining cognitive function, not all sugars are metabolized or utilized in the same way. Fructose, a simple sugar commonly found in fruits, sugary beverages, and processed foods, follows a distinct metabolic pathway with unique implications for both systemic health and brain function. Unlike glucose, which is tightly regulated and utilized by nearly every cell in the body to meet energy demands, fructose is primarily metabolized in the liver, bypassing the intricate control mechanisms of glucose metabolism.
Fructose’s journey through the body resembles a "fast lane" that lacks regulatory stop signs, as it is rapidly processed by the enzyme fructokinase. This unrestrained metabolism overwhelms the liver, producing harmful byproducts and initiating a cascade of metabolic disturbances:
De Novo Lipogenesis: Turning Sugar into Fat Fructose metabolism accelerates de novo lipogenesis, a biochemical process where excess sugars are transformed into fatty acids. These fatty acids are either stored in the liver or released into the bloodstream as triglycerides, much like surplus cargo being offloaded into storage or sent downstream. This "overflow" of triglycerides contributes to the development of non-alcoholic fatty liver disease (NAFLD), a condition where excess fat accumulates in the liver, impairing its function.
Beyond its effects on the liver, elevated triglycerides circulate throughout the body, fueling systemic inflammation and fostering insulin resistance in peripheral tissues. This metabolic imbalance acts like a chain reaction, where the inflammatory signals and impaired glucose regulation compromise overall vascular health. The blood vessels supplying the brain are particularly vulnerable, as chronic inflammation and insulin resistance contribute to cerebral vascular damage, diminishing the delivery of oxygen and nutrients essential for cognitive function.
Over time, these disruptions promote a state of chronic low-grade inflammation in the brain, amplifying the risk of neurodegenerative conditions such as Alzheimer’s and Parkinson’s. This metabolic cascade underscores how seemingly peripheral processes like fatty acid synthesis can reverberate throughout the body, linking liver dysfunction to long-term cognitive decline.
Uric Acid Production
Fructose metabolism triggers the breakdown of nucleotides, leading to the production of uric acid, a molecule with significant systemic effects. While uric acid is a natural byproduct of cellular processes, elevated levels resulting from excessive fructose consumption can act as a "metabolic bottleneck," disrupting critical physiological pathways.
One of the most notable impacts of high uric acid levels is the inhibition of nitric oxide (NO) production. Nitric oxide, often referred to as the "vascular gatekeeper," plays an essential role in maintaining vascular health by promoting vasodilation and ensuring smooth blood flow. In the brain, NO is particularly crucial for maintaining cerebral perfusion, the process of delivering oxygen and nutrients to energy-hungry neurons.
When NO production is suppressed by elevated uric acid, the brain’s blood supply faces a "traffic jam," restricting the flow of critical resources. Over time, this reduced blood flow impairs the brain’s ability to meet its metabolic demands, leading to neuronal dysfunction and a gradual decline in cognitive performance. The combination of vascular stress and nutrient deprivation creates an environment ripe for neurodegenerative processes, increasing the risk of conditions such as Alzheimer’s disease.
Reactive Oxygen Species (ROS)
Fructose metabolism generates an excess of ROS, which damages cellular structures like proteins, lipids, and DNA. This oxidative stress affects both liver and brain cells, impairing their ability to function and triggering a feedback loop of damage and inflammation. With its high energy demands and limited antioxidant defenses, the brain is particularly vulnerable to the oxidative stress induced by chronic fructose exposure.
Fructose’s Impact on Brain Function
Fructose has the ability to cross the blood-brain barrier (BBB) and disrupt essential neuronal processes. Unlike glucose, which fuels energy production in the brain, fructose impairs cellular systems necessary for cognitive function:
- Mitochondrial Dysfunction: Mitochondria play a central role in meeting the brain’s substantial energy requirements. Fructose-induced oxidative stress damages mitochondrial membranes and enzymes, reducing their ability to efficiently produce ATP. Neurons deprived of sufficient ATP experience synaptic dysfunction, loss of plasticity, and, ultimately cell death. This energy deficit is particularly detrimental in high-demand regions like the hippocampus, which is essential for memory and learning. [6]
- Neuroinflammation: Chronic exposure to fructose activates microglia, the brain’s immune cells. Overactive microglia release inflammatory mediators, such as TNF-alpha and IL-6, which damage neuronal connections and promote a toxic environment in the brain. Over time, this inflammation disrupts synaptic signaling and contributes to neurodegenerative diseases such as Alzheimer’s. [6]
- Insulin Resistance in the Brain: Insulin is a critical modulator of brain activity, influencing synaptic plasticity, neurotransmitter release, and memory formation. Excessive fructose intake disrupts insulin signaling, leading to insulin resistance in the brain. This resistance reduces the brain's ability to metabolize glucose efficiently, creating energy deficits that impair cognitive function. Additionally, insulin resistance is associated with the accumulation of amyloid-beta plaques and neurofibrillary tangles, hallmark features of Alzheimer’s pathology. [6]
Fructose’s Contribution to Neurodegenerative Diseases
Fructose metabolism exacerbates processes underlying neurodegenerative diseases, particularly Alzheimer’s:
- Amyloid-Beta Plaques: Fructose promotes the aggregation of misfolded amyloid-beta proteins in the brain. These plaques interfere with neuronal communication, trigger immune responses, and worsen oxidative damage.
- Tau Protein Phosphorylation: Fructose metabolism increases tau phosphorylation, leading to the formation of neurofibrillary tangles. These tangles destabilize the microtubules that support intracellular transport in neurons, causing structural and functional impairments.
A paper titled "Cerebral Fructose Metabolism as a Potential Mechanism Driving Alzheimer’s Disease" by Johnson et al. (2020) highlights the pivotal role of fructose metabolism in processes contributing to Alzheimer’s disease (AD). The researchers identified a state of "fructose-driven energy depletion" in neurons, where fructose impairs mitochondrial function, reducing the production of ATP. This energy shortfall is akin to a power outage, where essential systems like synaptic plasticity and neuronal communication falter without sufficient energy to operate. Over time, this chronic energy deficit weakens neuronal networks, accelerating degeneration and cognitive decline. [5]
The study further revealed that fructose metabolism is intricately linked to hallmark features of AD. Fructose promotes the aggregation of amyloid-beta proteins into plaques, the "clogged intersections" of neuronal communication that obstruct signaling and provoke immune responses. At the same time, fructose increases the phosphorylation of tau proteins, leading to the formation of neurofibrillary tangles—"collapsed scaffolds" within neurons that destabilize microtubules, disrupting intracellular transport and compromising structural integrity. These dual effects contribute to the progressive breakdown of the brain’s communication and support systems.
A key insight from the study is that fructose’s role in AD pathology arises from both endogenous production and excessive dietary intake. Through the polyol pathway, the brain converts glucose into fructose during periods of stress or heightened energy demand. While this mechanism may have served as an evolutionary advantage for energy conservation, its chronic activation—exacerbated by modern diets high in added sugars—becomes maladaptive. This sustained fructose metabolism drives mitochondrial dysfunction, oxidative stress, and inflammation, creating a "vicious cycle" of neuronal damage and metabolic dysfunction. [5]
These findings underscore the need for further exploration of the mechanisms linking fructose metabolism to AD and other neurodegenerative diseases. They also highlight the importance of dietary interventions aimed at reducing fructose consumption to alleviate its systemic and neurological effects. Targeting fructose metabolism, whether through pharmacological approaches or lifestyle changes, offers a promising strategy for mitigating the progression of Alzheimer’s disease, protecting cognitive function, and preserving the brain’s intricate "energy economy."
How Fructose Impacts the Gut-Brain Connection
Fructose also indirectly affects cognitive health by disrupting the gut-brain axis, a communication network between the gastrointestinal and brain systems. Excessive fructose intake alters gut microbiota composition, leading to dysbiosis, a state where harmful bacteria outcompete beneficial microbes. This imbalance has cascading effects on systemic and brain health:
- Systemic Inflammation: Dysbiosis increases the release of lipopolysaccharides (LPS), inflammatory molecules derived from the cell walls of certain gut bacteria. LPS can cross both the gut and blood-brain barriers, triggering neuroinflammation and impairing neuronal health.
- Decreased Short-Chain Fatty Acids (SCFAs): Beneficial gut bacteria produce SCFAs like butyrate, which strengthen the gut lining, reduce inflammation, and support brain health. Fructose-induced dysbiosis reduces SCFA production, weakening these protective mechanisms.
Leaky Gut and Leaky Brain: Fructose contributes to leaky gut, a condition where the intestinal lining becomes more permeable, allowing toxins and pathogens to enter the bloodstream. Systemic inflammation caused by leaky gut can compromise the integrity of the blood-brain barrier, leading to a “leaky brain” that is more susceptible to inflammatory and neurodegenerative processes.
Implications of Chronic Fructose Exposure on Cognitive Health
The cumulative effects of chronic fructose exposure—mitochondrial dysfunction, oxidative stress, insulin resistance, neuroinflammation, and gut dysbiosis—underscore its role in accelerating cognitive decline. The brain’s high energy demands and reliance on efficient metabolic processes make it especially vulnerable to the disruptive effects of excessive fructose consumption.
Interventions to mitigate fructose-related damage, such as reducing intake of processed sugars, enhancing mitochondrial function, and promoting gut health, could be critical strategies for protecting cognitive function and reducing the risk of neurodegenerative diseases like Alzheimer’s. These findings emphasize the importance of reducing fructose intake for neurological well-being. [6]
The Role of Ketones in Brain Health
Ketones serve as an essential alternative energy source for the brain, particularly during periods of low glucose availability, such as fasting, prolonged exercise, or carbohydrate restriction. When the body experiences a drop in glucose levels, the liver converts fatty acids into ketone bodies—beta-hydroxybutyrate (BHB), acetoacetate, and acetone. These ketones are released into the bloodstream and readily cross the blood-brain barrier (BBB) to supply energy to neurons. Unlike glucose, which relies on insulin and tightly regulated transport systems for utilization, ketones bypass many of these requirements, making them a reliable energy source during metabolic stress.
Ketones are metabolically efficient and produce more energy (ATP) per molecule than glucose. This increased efficiency is due to their ability to directly enter the mitochondrial Krebs cycle, where they are oxidized to generate ATP. Importantly, ketones produce fewer reactive oxygen species during this process, reducing oxidative stress, a key factor in neuronal damage and aging.
Neurons, which have high energy demands, benefit significantly from this clean energy source, particularly when glucose metabolism is impaired, as seen in conditions like type 2 diabetes, insulin resistance, and neurodegenerative diseases like Alzheimer’s. In these states, ketones provide an alternative pathway to fuel neuronal activity, preserving cognitive functions like memory and decision-making.
Neuroprotective Properties of Ketones
Ketones not only supply energy but also exert direct neuroprotective effects through several mechanisms:
- Reduction of Oxidative Stress: Oxidative stress occurs when an imbalance between free radicals (such as ROS) and the body’s antioxidant defenses leads to cellular damage. Neurons are particularly susceptible to oxidative damage due to their high metabolic activity and limited capacity for repair. Ketones reduce oxidative stress by enhancing mitochondrial efficiency, limiting ROS production, and boosting antioxidant systems like glutathione activity. This protection helps preserve neuronal function and integrity, especially in aging populations and individuals with neurodegenerative diseases.
- Suppression of Neuroinflammation: Chronic inflammation in the brain, driven by overactive microglia (the brain’s immune cells), contributes to synaptic damage and cognitive decline. Ketones suppress microglial activation, reducing the release of pro-inflammatory cytokines such as TNF-alpha and IL-6. This anti-inflammatory effect is critical for protecting neuronal connections and maintaining healthy synaptic signaling.
- Enhancement of Synaptic Plasticity: Synaptic plasticity—the ability of synapses to strengthen or weaken over time in response to activity—is the foundation of learning and memory. Ketones promote synaptic plasticity by increasing brain-derived neurotrophic factor (BDNF) levels, a protein essential for neuronal survival, growth, and repair. Elevated BDNF levels improve cognitive resilience, particularly in aging individuals or those experiencing mild cognitive impairment.
Ketogenic Diet and Cognitive Function
The ketogenic diet, a high-fat, low-carbohydrate approach, induces a state of ketosis in which the body primarily produces ketones as its main energy source. This dietary intervention has been shown to address both metabolic and cognitive challenges, particularly by stabilizing blood sugar levels, reducing inflammation, and enhancing neuronal resilience through distinct mechanisms.
By minimizing carbohydrate intake, the ketogenic diet prevents glycemic spikes and reduces glycemic variability, two factors closely linked to oxidative stress and inflammation. Stable blood sugar levels help mitigate the production of reactive oxygen species (ROS), protect neurons from oxidative damage, and improve insulin sensitivity. Enhanced insulin sensitivity is particularly important for individuals with conditions like type 2 diabetes, where insulin resistance in the brain impairs glucose metabolism and accelerates cognitive decline.
A study led by Professor Dominic D'Agostino titled ‘Ketone Supplementation: Meeting the Needs of the Brain in an Energy Crisis’ demonstrated that participants with type 2 diabetes who adhered to a ketogenic diet for 12 weeks showed significant improvements in glycemic control (measured by HbA1c) and executive function, including problem-solving and decision-making skills. These findings are particularly important given the link between type 2 diabetes and impaired brain glucose metabolism, which accelerates cognitive decline. The ketogenic diet mitigates these negative effects by stabilizing blood sugar and reducing glycemic variability while enhancing cognitive performance. [4]
The ketogenic diet also exerts profound effects on brain inflammation, largely mediated by the anti-inflammatory properties of ketones. Beta-hydroxybutyrate (BHB), the most abundant ketone body, inhibits the activation of microglia, the brain’s immune cells, and suppresses the release of pro-inflammatory cytokines like TNF-alpha and IL-6. This reduction in neuroinflammation is critical for preserving synaptic integrity and preventing neuronal damage. In addition, ketones activate pathways such as Nrf2 (nuclear factor erythroid 2-related factor 2), which regulate antioxidant defenses and promote cellular survival. [3]
Another major benefit of the ketogenic diet is its role in enhancing synaptic plasticity, a process essential for learning and memory. Ketones stimulate the production of brain-derived neurotrophic factor (BDNF), a protein that supports neurons' growth, repair, and resilience. Elevated BDNF levels improve synaptic remodeling and neurogenesis, which are crucial for maintaining cognitive function in aging populations or individuals with mild cognitive impairment. Furthermore, the diet enhances mitochondrial efficiency, ensuring that neurons can generate the energy needed for synaptic communication and adaptation. [3]
The ketogenic diet has also shown potential in mitigating the pathology of neurodegenerative diseases like Alzheimer’s. By promoting the clearance of amyloid-beta plaques and reducing the hyperphosphorylation of tau proteins, ketosis addresses two hallmark features of Alzheimer’s pathology. These protective effects are complemented by improvements in cerebral blood flow, as ketones enhance vascular health by increasing nitric oxide (NO) production. Better circulation ensures that neurons receive adequate oxygen and nutrients, supporting their long-term function and survival.
Exogenous Ketones and Cognitive Benefits
Exogenous ketones, available as dietary supplements, provide an alternative method for increasing ketone levels without dietary restriction. These supplements are rapidly absorbed and metabolized, delivering ketones directly to the brain.
A study by Myette-Cote et al. (2021) found that older adults with mild cognitive impairment (MCI) who were given exogenous ketones showed significant improvements in memory performance and cerebral blood flow. These findings highlight the ability of ketones to address energy deficits in neurons and enhance brain function. Improved cerebral blood flow ensures that neurons receive adequate oxygen and nutrients, further supporting cognitive resilience. [2]
Strategies for Maintaining Stable Blood Sugar Levels
Maintaining stable blood sugar levels is essential for both metabolic and cognitive health. Effective strategies combine dietary approaches, gut health optimization, nutrient intake, and lifestyle modifications. These methods target insulin sensitivity, glycemic control, and the prevention of metabolic stressors that contribute to cognitive decline and neurodegenerative diseases.
Dietary Approaches
Dietary interventions are the cornerstone of blood sugar management. Research underscores the effectiveness of low-carbohydrate and ketogenic diets in promoting glycemic control. By significantly reducing carbohydrate intake, these diets shift the body into a state of ketosis, where it primarily burns fat for energy and generates ketones as an alternative to glucose. This metabolic state minimizes blood sugar fluctuations, enhances insulin sensitivity, and reduces inflammation, which is critical for individuals with metabolic syndrome or type 2 diabetes.
Studies have shown that ketogenic diets can lower HbA1c—a key marker of long-term blood sugar control—and reduce fasting glucose levels. For example, a study published in Diabetes Therapy found that a ketogenic diet improved HbA1c levels by up to 1.5% in individuals with type 2 diabetes, often enabling a reduction in diabetes medication.
In addition to carbohydrate restriction, balanced macronutrient intake is crucial for stabilizing blood sugar. Meals rich in protein, healthy fats, and low-glycemic-index carbohydrates slow glucose absorption, preventing sharp postprandial spikes. Fiber-rich foods like non-starchy vegetables, legumes, and whole grains play a dual role by blunting glucose absorption and promoting gut health. Their ability to feed beneficial gut bacteria and produce short-chain fatty acids (SCFAs) further supports insulin sensitivity.
Practical dietary tips include:
- Replacing refined carbohydrates with nutrient-dense alternatives like leafy greens and cruciferous vegetables.
- Incorporating protein sources such as lean meats, fish, and plant-based options like lentils to slow digestion and prolong satiety.
Including healthy fats, such as those from avocados, nuts, and olive oil, to enhance metabolic flexibility and stabilize blood sugar.
Optimizing Gut Health
The gut microbiome is pivotal in regulating glucose metabolism and insulin sensitivity. Imbalances in the gut microbiota—referred to as dysbiosis—are linked to increased systemic inflammation, which exacerbates insulin resistance. Supporting gut health through probiotics, prebiotics, and polyphenol-rich foods can significantly enhance glycemic control.
Key interventions include:
- Probiotics: Strains such as Akkermansia muciniphila and Lactobacillus rhamnosus improve glucose regulation by reducing gut permeability and increasing SCFA production. SCFAs, including acetate and butyrate, are key metabolites that enhance insulin sensitivity and reduce inflammatory markers.
- Prebiotics: Fibers like inulin and resistant starch nourish beneficial gut bacteria, improving gut integrity and reducing endotoxin-induced inflammation. Foods like garlic, onions, and unripe bananas are rich sources of prebiotics.
- Polyphenols: Found in berries, green tea, and dark chocolate, polyphenols exert antioxidant effects that protect against oxidative stress and promote a healthy gut environment. These compounds also directly enhance glucose uptake in muscle cells and improve insulin signaling.
- Acarbose: Acarbose is an alpha-glucosidase inhibitor that slows the breakdown of complex carbohydrates into simple sugars in the small intestine. This action reduces postprandial glucose spikes and provides more time for gut microbiota to metabolize undigested carbohydrates into beneficial metabolites like SCFAs, improving glycemic control and reducing inflammation.
The Role of Targeted Nutrients in Glucose Metabolism
Certain vitamins and minerals can support blood sugar regulation, acting as cofactors in metabolic processes that influence insulin function and glucose uptake. [3]
- Magnesium: This mineral is involved in over 300 enzymatic reactions, including those necessary for insulin signaling and glucose transport into cells. Low magnesium levels are associated with insulin resistance and poor glycemic control. Dietary sources include leafy greens, nuts, and seeds.
- Chromium: Chromium enhances the activity of insulin receptors, improving glucose tolerance. Supplementation has been shown to lower fasting glucose and HbA1c levels in individuals with diabetes.
- Vitamin D: Adequate vitamin D levels are essential for reducing inflammation and enhancing insulin sensitivity. Low vitamin D status is a risk factor for developing type 2 diabetes.
- Omega-3 Fatty Acids: Known for their anti-inflammatory properties, omega-3s improve insulin sensitivity and reduce triglyceride levels, further supporting metabolic health.
Incorporating these nutrients through a combination of whole foods and supplements ensures that the body has the resources necessary to maintain stable blood sugar levels.
Addressing Fructose’s Impact
Excessive fructose consumption, particularly from processed foods and sugary beverages, disrupts blood sugar regulation by promoting insulin resistance, fat accumulation, and systemic inflammation. Fructose is metabolized in the liver, where excessive intake generates harmful byproducts such as uric acid and ROS. Elevated uric acid levels, in turn, exacerbate oxidative stress and neuroinflammation, contributing to cognitive decline.
Dietary strategies should prioritize whole fruits over fructose-rich processed foods to mitigate these effects. Whole fruits contain fiber and polyphenols that slow fructose absorption, protecting against spikes in blood sugar and insulin. Additionally, reducing processed fructose intake can help normalize uric acid levels, lowering the risk of metabolic complications.
Additional Lifestyle Interventions
Beyond diet and gut health, physical activity, stress management, and sleep hygiene play integral roles in stabilizing blood sugar.
- Physical Activity: Regular exercise is one of the most effective strategies for improving glucose regulation. Aerobic exercise enhances glucose uptake in muscles independently of insulin, reducing fasting glucose and HbA1c levels. Resistance training complements these benefits by increasing muscle glycogen storage and improving insulin sensitivity, stabilizing glucose levels, and promoting metabolic flexibility. A combination of aerobic and resistance training provides comprehensive benefits for blood sugar control and overall metabolic health.
- Stress Management and Sleep Hygiene: Chronic stress elevates cortisol levels, which impairs insulin sensitivity and raises blood sugar levels. Stress management practices like mindfulness, meditation, and yoga lower cortisol levels and improve metabolic health. Sleep is equally critical; poor sleep increases insulin resistance and disrupts glucose metabolism. Prioritizing 7–9 hours of quality sleep each night supports glycemic control and overall health.
Pharmacological Support:
While lifestyle changes remain the cornerstone of blood sugar regulation, pharmacological interventions can provide critical support when these efforts prove insufficient. Medications such as acarbose, SGLT2 inhibitors, and metformin target different aspects of glucose metabolism and offer synergistic benefits when combined with dietary and lifestyle strategies. Each of these pharmacological agents has a unique mechanism of action, making them valuable tools for managing blood sugar in specific clinical contexts.
- Acarbose is an alpha-glucosidase inhibitor that slows the breakdown of complex carbohydrates into glucose within the small intestine, effectively reducing the speed at which glucose enters the bloodstream. This mechanism helps prevent postprandial glucose spikes, a significant glycemic variability, and oxidative stress driver. Acarbose is particularly beneficial for individuals with prediabetes or early-stage type 2 diabetes, where post-meal glucose control is crucial. Additionally, acarbose indirectly supports gut health by increasing undigested carbohydrates in the colon, fueling beneficial gut bacteria that produce short-chain fatty acids (SCFAs), and enhancing insulin sensitivity.
- Sodium-glucose co-transporter 2 (SGLT2) inhibitors, such as canagliflozin and bexagliflozin, regulate blood sugar levels by blocking the reabsorption of glucose in the kidneys, promoting its excretion through urine. This insulin-independent mechanism lowers fasting and postprandial glucose levels while also providing additional metabolic benefits. These include mild weight loss due to caloric loss from excreted glucose and a reduction in blood pressure thanks to the diuretic effect. Furthermore, SGLT2 inhibitors offer significant cardiovascular and renal protection, with studies demonstrating their ability to reduce heart failure risk and slow chronic kidney disease progression.
- Metformin is a widely used medication for type 2 diabetes that improves insulin sensitivity and reduces hepatic glucose production by activating AMP-activated protein kinase (AMPK). It effectively lowers fasting glucose and HbA1c levels, making it a valuable treatment for individuals with already elevated A1C or glucose levels. However, we recommend metformin primarily for cases where blood sugar dysregulation is present rather than as a preventative measure. This is due to its potential impact on mitochondrial function, as it can slightly impair oxidative phosphorylation, which may not be ideal for individuals with otherwise healthy glucose metabolism.
Closing Thoughts
The intricate relationship between glucose regulation and brain health reveals a vital connection between metabolic and cognitive processes. The brain’s extraordinary reliance on glucose as its primary energy source underscores the importance of maintaining stable blood sugar levels to preserve cognitive function and protect against neurodegenerative diseases. At the same time, alternative energy pathways, such as those provided by ketones during metabolic stress, demonstrate the brain’s adaptability and offer promising therapeutic avenues for addressing age-related cognitive decline and neurological disorders.
Future directions in research should focus on exploring the bidirectional relationship between cognitive function and glucose metabolism. While it is clear that impaired glucose regulation can contribute to cognitive decline, emerging evidence suggests that cognitive dysfunction may also influence glucose metabolism, creating a feedback loop that exacerbates both conditions. This bidirectional relationship could offer new insights into the underlying mechanisms linking glucose regulation and brain health, opening the door to novel therapeutic strategies. Understanding how cognitive decline impacts glucose homeostasis could help identify new targets for intervention while also enhancing our ability to predict and prevent cognitive impairment in individuals with metabolic disorders. Further research in this area could provide the foundation for personalized treatment plans that simultaneously address cognitive health and metabolic function, ultimately improving outcomes for individuals at risk for both conditions.
From glucose dysregulation to the harmful effects of excessive fructose and the neuroprotective potential of ketones, the evidence highlights the dynamic interplay between dietary choices, metabolic health, and brain function. Effective interventions—from nutritional strategies and exercise to gut microbiota optimization and pharmacological therapies—can significantly influence these pathways, improving metabolic stability and cognitive resilience.
As our understanding of these mechanisms deepens, it becomes increasingly clear that brain health is inseparable from the metabolic environment in which it functions. Addressing blood sugar regulation is not merely a strategy to manage chronic conditions like diabetes but a proactive measure to safeguard cognitive vitality throughout life.
As our last research review shared, Healthspan is heading into the new year with a renewed focus on metabolic health, recognizing that metabolic dysfunction lies at the root of many chronic and age-related diseases. We are committed to a multidimensional approach that emphasizes the profound interconnectedness of our body’s systems, the importance of lifestyle interventions, and the value of pharmacological tools and protocols. Stay tuned as we continue to expand our efforts, sharing actionable insights and evidence-based strategies to support better metabolic health and sustained cognitive well-being.
- Chi H, Song M, Zhang J, Zhou J, Liu D. Relationship between acute glucose variability and cognitive decline in type 2 diabetes: A systematic review and meta-analysis. PLoS One. 2023 Sep 1;18(9):e0289782. doi: 10.1371/journal.pone.0289782. PMID: 37656693; PMCID: PMC10473499.
- Myette-Côté É, Soto-Mota A, Cunnane SC. Ketones: potential to achieve brain energy rescue and sustain cognitive health during ageing. Br J Nutr. 2022 Aug 14;128(3):407-423. doi: 10.1017/S0007114521003883. Epub 2021 Sep 28. PMID: 34581265.
- Altayyar M, Nasser JA, Thomopoulos D, Bruneau M Jr. The Implication of Physiological Ketosis on The Cognitive Brain: A Narrative Review. Nutrients. 2022 Jan 25;14(3):513. doi: 10.3390/nu14030513. PMID: 35276871; PMCID: PMC8840718.
- Poff AM, Moss S, Soliven M, D'Agostino DP. Ketone Supplementation: Meeting the Needs of the Brain in an Energy Crisis. Front Nutr. 2021 Dec 23;8:783659. doi: 10.3389/fnut.2021.783659. PMID: 35004814; PMCID: PMC8734638.
- Johnson RJ, Gomez-Pinilla F, Nagel M, Nakagawa T, Rodriguez-Iturbe B, Sanchez-Lozada LG, Tolan DR, Lanaspa MA. Cerebral Fructose Metabolism as a Potential Mechanism Driving Alzheimer's Disease. Front Aging Neurosci. 2020 Sep 11;12:560865. doi: 10.3389/fnagi.2020.560865. PMID: 33024433; PMCID: PMC7516162.
- Yan J, Zheng K, Zhang X, Jiang Y. Fructose Consumption is Associated with a Higher Risk of Dementia and Alzheimer's Disease: A Prospective Cohort Study. J Prev Alzheimers Dis. 2023;10(2):186-192. doi: 10.14283/jpad.2023.7. PMID: 36946445.
- Sasaki, Nobuyuki et al. Advanced Glycation End Products in Alzheimer's Disease and Other Neurodegenerative Diseases The American Journal of Pathology, Volume 153, Issue 4, 1149 - 1155
- Rom, S., Heldt, N.A., Gajghate, S. et al. Hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin-5 protein secretion on extracellular microvesicles. Sci Rep 10, 7274 (2020).
- Rose S, Bennuri SC, Davis JE, Wynne R, Slattery JC, Tippett M, Delhey L, Melnyk S, Kahler SG, MacFabe DF, Frye RE. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl Psychiatry. 2018 Feb 2;8(1):42. doi: 10.1038/s41398-017-0089-z. PMID: 29391397; PMCID: PMC5804031.