Brown and Beige Fat's Role in Optimizing Metabolic Function
Adipose Tissue as an Endocrine Organ: Adipose tissue plays a dynamic role in regulating energy balance, insulin sensitivity, and inflammation through the release of adipokines, such as leptin and adiponectin, which influence appetite control, immune function, and lipid metabolism.
Types of Adipocytes: The three types of adipocytes—white, beige, and brown—have distinct functions in the body. While white adipose tissue (WAT) stores energy and is linked to negative health outcomes, brown and beige fat cells are metabolically active, contributing to energy expenditure and improved metabolic health.
Cold Exposure and Adipose Tissue Browning: Cold exposure promotes the browning of white adipose tissue, leading to the development of beige and brown fat, which enhances mitochondrial density, thermogenesis, and metabolic function. This is a key intervention for improving metabolic health.
Hormonal Regulation of Adipose Tissue: Hormones such as insulin, leptin, and adiponectin regulate the function of adipose tissue and are crucial in maintaining metabolic balance. Disruptions in these hormonal interactions can lead to metabolic disorders like obesity and type 2 diabetes.
Pharmacological Interventions: Agents like rapamycin and metformin show potential in mimicking the effects of caloric restriction, supporting mitochondrial function, and improving metabolic outcomes. These pharmacological strategies, combined with lifestyle modifications, offer new avenues for managing body weight and enhancing healthspan.
Therapeutic Potential of Adipose Tissue: Understanding the different roles of adipose tissue types provides valuable insights into targeting them as therapeutic approaches for combating metabolic disorders, improving insulin sensitivity, and promoting long-term health and longevity.
Adipose tissue, commonly known as body fat, is more than just a storage depot for excess calories. It is a dynamic endocrine organ that plays a pivotal role in regulating energy balance, insulin sensitivity, and inflammation. Adipose tissue releases a variety of hormones and signaling molecules known as adipokines, which influence numerous physiological processes, including appetite control, immune function, and lipid metabolism. Key adipokines like leptin help regulate hunger and energy expenditure, while adiponectin enhances insulin sensitivity and exhibits anti-inflammatory effects. In addition to these regulatory roles, adipose tissue also protects vital organs and insulates the body to help maintain core temperature.
However, excessive fat accumulation—particularly in visceral areas around internal organs—can contribute to serious metabolic disorders such as obesity, type 2 diabetes, and cardiovascular disease. This duality highlights the importance of understanding the different types of adipose tissue and how they impact overall health.
In this article, Brandon Fell, MS, Healthspan's leader of Metabolic Health programs, will provide a detailed examination of the various types of adipocytes—white, beige, and brown fat cells—and their distinct roles in metabolic health. We'll explore how these different fat cells function within the body and why certain types of fat are more beneficial than others. Specifically, the article will discuss the ways in which lifestyle interventions can promote the transformation of white fat into more metabolically active brown-like fat, which enhances mitochondrial density, improves insulin sensitivity, and reduces inflammation.
Additionally, our research review will analyze the impact of cold exposure on brown adipose tissue (BAT), highlighting how regular cold exposure can stimulate the conversion of white fat into thermogenic brown fat, enhancing metabolic function. Dr. Ryan Marshall, PhD, from the Lamming Laboratory for the Molecular Physiology of Aging at the University of Wisconsin, will contribute to this analysis, providing expert insight into the physiological mechanisms by which cold exposure influences metabolism. This section will include a detailed breakdown of the research findings, offering a nuanced understanding of how cold exposure can be leveraged to optimize metabolic health and adipose tissue composition.
We will also explore the hormonal regulation of adipose tissue, focusing on insulin, leptin, and adiponectin, and their roles in maintaining metabolic balance. Understanding these interactions is crucial for appreciating how disruptions in fat function can lead to metabolic disorders.
Finally, the article will discuss the potential of pharmacological agents such as rapamycin and metformin, which are being investigated for their ability to mimic the effects of caloric restriction, support mitochondrial function, and improve metabolic health. Through this comprehensive analysis, we aim to provide deeper insights into the role of adipose tissue in health and how this knowledge can be applied to optimize metabolic outcomes.
The Metabolic Spectrum: White, Beige, and Brown Adipose Tissue Explained
There are three types of adipocytes, each with distinct functions and characteristics: white adipose cells, beige adipose cells, and brown adipose cells. The color of these cells, observed under a microscope, reflects the amount of lipids stored and the number of mitochondria present within each cell. This color difference corresponds to the metabolic function of the adipocytes, with lighter cells storing more energy as fat and darker cells being more metabolically active due to higher mitochondrial content.
The image below illustrates this spectrum: the lighter the adipocyte’s color (such as in white adipose tissue), the larger the lipid droplet and the fewer the mitochondria. In contrast, darker adipocytes (like those in brown adipose tissue) contain more mitochondria, which are responsible for energy production. Because of this, a lifestyle that promotes the development of beige and brown adipocytes is desirable for maximizing mitochondrial density and metabolic health. [1]
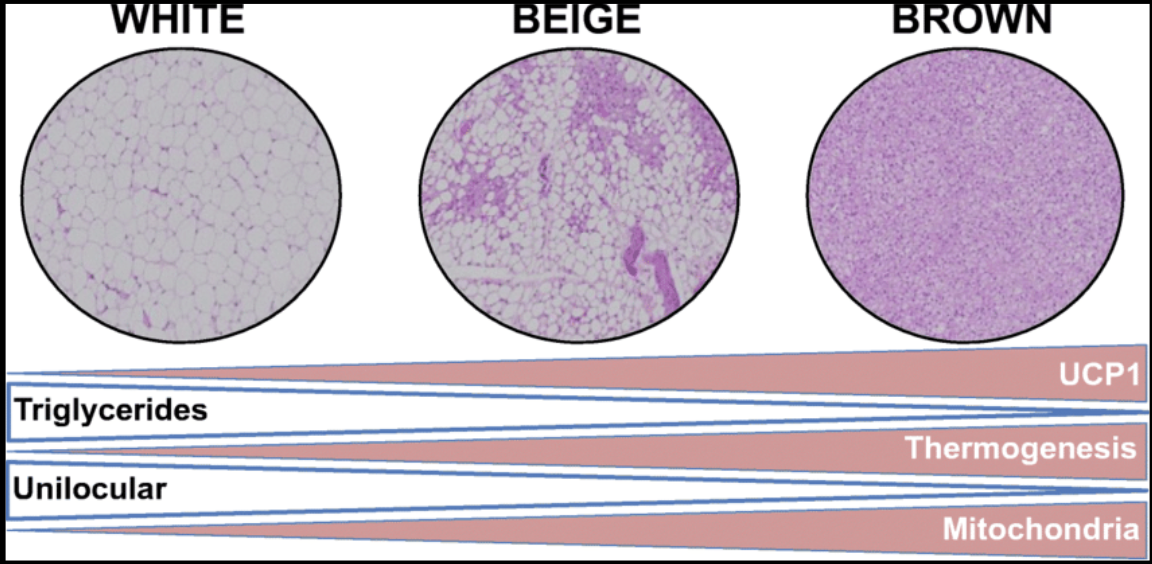
White Adipose Tissue
White adipose tissue (WAT) appears white because it contains a single large lipid droplet that occupies most of the cell’s volume, pushing the nucleus to the periphery. WAT stores energy in the form of triglycerides, releases energy during periods of fasting or energy demand, and produces hormones and cytokines that regulate metabolism, such as leptin, adiponectin, and resistin. WAT is predominantly found in subcutaneous (beneath the skin) and visceral (around internal organs) fat tissues. This type of adipocyte tends to have a lower number of mitochondria, which are the organelles responsible for producing ATP, the energy currency of the cell. The lower mitochondrial content makes WAT less metabolically active, which is why an excess of WAT is associated with poor health outcomes, including obesity-related conditions and insulin resistance (IR). Insulin resistance occurs when tissues such as skeletal muscle, adipose tissue, and the liver no longer respond effectively to insulin, impairing the body’s ability to effectively manage blood sugar levels.
Beige Adipose Tissue
Beige adipose tissue is a type of fat cell that is intermediate between white and brown adipocytes. Beige adipocytes have multiple small lipid droplets and a moderate number of mitochondria. Under certain conditions, such as cold exposure, periods of fasting, or specific hormonal signals, beige adipocytes can convert into a more brown-like state, where they become involved in thermogenesis—the process of generating heat by burning calories. Beige adipocytes are typically found scattered within white adipose tissue and represent a flexible form of fat that can enhance metabolic activity under the right conditions.
Brown Adipose Tissue
Brown adipose tissue (BAT) is a specialized form of fat tissue that differs significantly from the more common white adipose tissue. Unlike white fat, which stores energy in large lipid droplets, BAT contains multiple smaller lipid droplets and a rich supply of mitochondria, the cellular structures responsible for energy production. These abundant mitochondria not only give BAT its characteristic brown color but are also specialized for generating heat through a process known as non-shivering thermogenesis. This thermogenic process is facilitated by uncoupling protein 1 (UCP1), a protein found in the mitochondrial membrane that allows for the rapid production of heat without the need for muscle contractions, such as shivering.
The primary role of BAT is to maintain body temperature in cold environments, making it essential for thermoregulation. Brown adipocytes are less abundant in the body than white adipocytes and are typically concentrated in specific regions, such as around the neck, shoulders, and along the spine. BAT is particularly vital during infancy, as it helps newborns maintain their body temperature, but its presence and activity diminish with age.
Studies have shown that BAT activity decreases with age in both humans and rodents, which may be due to factors such as mitochondrial dysfunction associated with aging and a reduction in UCP1 activity, a protein critical for the thermogenic function of brown fat [2]. Mitochondrial dysfunction not only impacts thermogenesis but also contributes to the overall aging process and metabolic decline.
What Type of Adipose Tissue Do We Want to Favor?
When it comes to promoting health and longevity, not all adipose tissue is created equal. Among the three types of adipocytes—white, brown, and beige—brown adipose tissue (BAT) stands out as the most beneficial. As mentioned, BAT is particularly desirable due to its high density of mitochondria, the cellular powerhouses responsible for energy production. This abundance of mitochondria gives BAT its characteristic brown color and makes it a crucial player in metabolic health.
BAT’s primary function is thermogenesis, the process of generating heat by burning calories. Unlike white adipose tissue, which stores energy as fat, BAT helps to expend energy, thereby increasing the basal metabolic rate (BMR), which is the amount of energy the body uses at rest to maintain vital functions. This process not only contributes to higher energy expenditure but also supports the regulation of blood sugar and insulin levels, making BAT a valuable asset in the fight against obesity and related metabolic disorders.
Research suggests that individuals with a higher proportion of BAT tend to be leaner and have a lower risk of obesity-related diseases, such as diabetes and heart disease [3]. This is because BAT effectively burns excess calories, reducing the likelihood of fat accumulation and promoting a healthier metabolic profile. In other words, BAT acts as a metabolic ally, helping to maintain a lower body mass index (BMI) and protecting against the harmful effects of excess white fat.
Moreover, the health benefits of BAT are closely linked to its mitochondrial density. Healthy, well-functioning mitochondria are essential for optimal energy production and overall cellular health. The mitochondria in BAT are particularly efficient at converting calories into heat, a process that not only helps to regulate body temperature but also plays a role in maintaining metabolic flexibility—the ability to switch between burning carbohydrates and fats for energy. This flexibility is a key factor in metabolic resilience and the prevention of metabolic diseases.
Given the crucial role of BAT in promoting metabolic health, favoring its development and maintenance is a strategic approach to improving overall health and longevity.
How Can We Promote and Increase Beige or Brown Adipose Tissue Naturally?
The approach to promoting healthy adipose tissue is two-fold: reducing white adipose tissue (WAT) while promoting brown adipose tissue (BAT) and beige fat. While all adipocytes play crucial roles in maintaining energy balance and metabolic health, an imbalance in their function or number can lead to metabolic disorders, including obesity, type 2 diabetes, cardiovascular disease, and inflammation-related conditions.
Excessive accumulation of white adipocytes, especially in visceral fat, is associated with an increased risk of these diseases. This accumulation often results from factors like a caloric surplus, where more calories are consumed than the body expends. The excess energy is stored as fat in adipose tissue. This process is exacerbated by diets high in sugars, refined carbohydrates, and unhealthy fats, which promote fat accumulation and contribute to metabolic syndrome. The combination of poor diet and lack of physical activity further compounds the issue, leading to hormonal imbalances involving insulin, cortisol (stress hormone), leptin (which regulates hunger), and ghrelin (the hunger hormone). These hormonal imbalances can exacerbate fat storage, particularly in WAT.
To counteract this, a holistic approach is needed. This includes a balanced diet rich in whole foods, regular physical activity, effective stress management, and adequate sleep. These strategies not only help prevent the excessive accumulation of white adipose tissue but also reduce the risk of developing age-related metabolic diseases.
Conversely, enhancing the activity of brown and beige adipocytes presents a promising therapeutic approach for managing obesity and metabolic disorders, thanks to their role in energy expenditure and thermogenesis. "Beiging" or "browning" of white adipose tissue refers to the process by which white fat cells acquire characteristics similar to brown fat cells, becoming more metabolically active and aiding in energy burning. This process can significantly contribute to weight management and improved metabolic health.
Several strategies can promote the browning of white fat and the activation of brown adipose tissue:
Cold Exposure
Regular exposure to cold temperatures is one of the most effective ways to stimulate the conversion of white fat to thermogenic beige or brown fat. Cold exposure activates existing brown fat and promotes the development of new beige fat cells, enhancing the body's metabolic activity. For instance, a study found that participants exposed to mild cold for a month experienced a 42% increase in brown fat volume and a 10% increase in fat metabolic activity. These changes were independent of seasonal variations and reverted to baseline after a month of neutral temperatures, underscoring the need for consistent cold exposure to maintain these benefits [4].
Additionally, a study published in the prestigious journal Nature titled, "Brown-fat-mediated tumour suppression by cold-altered global metabolism", revealed that mild cold exposure activated significant amounts of brown adipose tissue (BAT) in cancer patients. This finding suggests that cold exposure could potentially serve as an adjuvant therapy by leveraging the body's metabolic systems to support tumor suppression and overall metabolic health [5].
We will explore the full benefits of cold exposure in stimulating brown adipose tissue in greater detail later in this article.
Physical Activity
Exercise is another powerful stimulus for fat browning. Physical activity increases the production of irisin, a hormone released from muscles during exercise that promotes the browning of white fat. This has been observed across various forms of exercise, including aerobic and high-intensity interval training (HIIT) [6, 7]. The increase in metabolically active beige fat through regular exercise can contribute to better weight management and overall metabolic health.
Dietary Interventions
Nutrition plays a critical role in promoting brown and beige fat. Certain foods and compounds have been shown to support fat browning and enhance BAT activity:
- Capsaicin: The compound responsible for the heat in chili peppers, capsaicin, can increase metabolism and promote the browning of white fat.
- Resveratrol: Found in grapes, berries, and red wine, resveratrol has been shown to activate brown fat [8].
- Catechins: These antioxidants, found in green tea, help increase energy expenditure and support the browning of white fat.
- Omega-3 Fatty Acids: Healthy fats found in fish oil, omega-3s can enhance the activity of brown fat [9].
Obesity: An Accumulation of White Adipose Tissue
When we talk about health, the focus is often on maintaining a healthy body weight, but it's important to also consider body composition—how much of your body is made up of fat, muscle, and bone. In clinical settings, health is often measured using Body Mass Index (BMI), which categorizes people based on their height and weight. However, BMI is a simple measure and doesn't give the full picture of a person's health. It doesn't tell us about body fat percentage, muscle mass, or bone density, which are more accurate indicators of health.
When an individual is overweight or obese, this often correlates with conditions like Metabolic Syndrome or Type 2 diabetes and likely indicates an undesirable body composition, favoring an abundance of white adipocytes (fat cells). This is where lifestyle factors begin to directly impact metabolism and health.
Under normal conditions, our metabolism can switch between burning carbohydrates (glucose) and fat for energy. However, the storage capacity for these two energy sources is vastly different. Stored glucose, or glycogen, is limited and can provide roughly 1,500-3,200 calories in the average adult [10]. In contrast, fat stored in white adipocytes has a much larger storage capacity. White adipocytes can continue to grow in size, allowing for seemingly endless fat storage as long as energy intake exceeds energy expenditure.
This imbalance, where energy intake outpaces energy expenditure, leads to the accumulation of white adipose tissue, contributing to obesity and its associated health risks. Recognizing this connection highlights how crucial it is to manage lifestyle factors like diet and physical activity to maintain a healthy body composition and prevent obesity-related conditions.
Bridging Adiposity Physiology and Human Metabolism
Bridging the gap between adiposity (fat accumulation) and human metabolism for weight loss involves understanding how body fat and metabolic processes are interconnected. Adiposity plays a vital role in regulating energy balance, hormone levels, and overall metabolic function. For effective weight loss strategies, it's essential to address how fat storage and release are influenced by key metabolic factors such as insulin sensitivity, calorie expenditure, and nutrient utilization. By targeting these metabolic processes, interventions can more effectively reduce fat stores, enhance energy expenditure, and promote sustainable weight loss, ultimately improving overall health. Hormones, which act as the quarterbacks of metabolism, govern much of this process.
In this section, we will delve into the specific roles of key hormones and enzymes involved in fat metabolism, such as insulin, leptin, adiponectin, and hormone-sensitive lipase (HSL). These molecules orchestrate the complex processes that regulate fat storage, energy utilization, and overall metabolic health. We will explore how insulin sensitivity affects fat storage, how leptin and adiponectin influence appetite and fat burning, and how HSL facilitates the mobilization of stored fats for energy. By understanding the interplay between these factors, we can better grasp how to design effective weight loss strategies that target the root causes of fat accumulation and metabolic imbalance.
Insulin
Insulin plays a complex and multifaceted role in regulating adipose tissue (fat tissue) function and overall metabolism. It is a hormone produced by the pancreas in response to elevated blood glucose levels, typically after eating, particularly following the intake of carbohydrates. Insulin’s primary functions include promoting glucose uptake by cells, facilitating fat storage, and supporting protein synthesis, while simultaneously inhibiting lipolysis (the breakdown of fats) and gluconeogenesis (the production of glucose from non-carbohydrate sources). These actions of insulin are crucial for maintaining energy balance and ensuring that the nutrients we consume are efficiently stored and utilized.
Insulin facilitates glucose uptake by cells throughout the body, particularly in muscle and adipose tissues. It does this by increasing the number of GLUT4 transporters on the cell membrane, which allows glucose to enter the cells from the bloodstream. Once inside the cells, glucose can be used immediately for energy or stored for later use. In muscle cells, glucose is stored as glycogen. In adipose tissue, insulin promotes the conversion of glucose into fatty acids, which are then combined with glycerol to form triglycerides, the primary form of stored fat. This storage of excess energy as fat is crucial for surviving periods of fasting or famine when food intake is low.
In addition to regulating glucose and fat metabolism, insulin also plays a significant role in protein metabolism. It enhances the uptake of amino acids (the building blocks of proteins) into cells, particularly in muscle tissue, where these amino acids are used for protein synthesis. This process contributes to muscle growth and repair, making insulin an anabolic hormone (one that promotes tissue building). Insulin also helps to reduce proteolysis, the breakdown of proteins, which further supports muscle maintenance and growth. By preserving muscle mass and promoting protein synthesis, insulin plays a key role in overall metabolic health and physical strength.
Insulin also inhibits lipolysis, the process of breaking down stored fats into free fatty acids for energy. This inhibition is essential during periods when the body has sufficient glucose available, as it encourages the body to use glucose as the primary energy source instead of breaking down fat stores. Similarly, insulin inhibits gluconeogenesis in the liver, which is the production of glucose from non-carbohydrate sources such as amino acids and glycerol. By inhibiting these processes, insulin ensures that the body prioritizes using and storing glucose when it is plentiful, rather than producing new glucose or breaking down fats.
However, when insulin resistance develops—a condition where the body's cells become less responsive to insulin—these carefully regulated processes become disrupted. With insulin resistance, the pancreas compensates by producing more insulin, leading to hyperinsulinemia (excess levels of insulin in the blood). Despite the higher insulin levels, glucose uptake by cells is impaired, leading to elevated blood sugar levels. This disruption can cause a cascade of metabolic issues, including increased fat storage (especially in the abdomen), difficulty in muscle growth, and an increased risk of metabolic disorders such as obesity, type 2 diabetes, and cardiovascular disease.
Hormone-Sensitive Lipase (HSL)
Hormone-sensitive lipase (HSL) is an enzyme that plays a crucial role in regulating lipid (fat) metabolism, specifically in mobilizing stored fats from adipocytes (fat cells). HSL is primarily responsible for driving lipolysis, the process of breaking down triglycerides (the main form of stored fat) into free fatty acids and glycerol. These triglycerides are stored in lipid droplets within adipocytes, essentially acting as the body's energy reserves.
When the body needs energy—such as during fasting, exercise, or stress—HSL is activated to break down these triglycerides, releasing energy-rich free fatty acids into the bloodstream, which can then be used by various tissues for energy.
HSL activity is tightly regulated by hormones. During periods of increased energy demand, hormones like epinephrine and norepinephrine (which are types of stress hormones called catecholamines) bind to specific receptors on the surface of adipocytes known as beta-adrenergic receptors. This binding triggers a cascade of reactions inside the cell, starting with the activation of an enzyme called adenylate cyclase. Adenylate cyclase then converts ATP (the cell’s energy currency) into cyclic AMP (cAMP), a molecule that acts as a secondary messenger. The rise in cAMP activates protein kinase A (PKA), an enzyme that phosphorylates (adds a phosphate group to) and activates HSL.
Once activated, HSL moves from the cytoplasm (the fluid inside the cell) to the surface of the lipid droplets within the adipocyte. Here, HSL begins to break down triglycerides into smaller components: first into diglycerides, then into monoglycerides, and finally into free fatty acids and glycerol. These free fatty acids are released into the bloodstream and transported to tissues like muscles and the liver, where they are oxidized (burned) to generate ATP, particularly in states of low carbohydrate intake.
In contrast, insulin, a hormone released after eating—especially after consuming carbohydrates—has the opposite effect on HSL. High insulin levels signal the body that energy (glucose) is readily available, so it’s time to store excess energy rather than break it down. Insulin accomplishes this by dephosphorylating (removing a phosphate group from) HSL, making it inactive. This inhibition of HSL prevents the breakdown of triglycerides, promoting fat storage instead. This regulatory mechanism allows the body to adapt to different nutritional states: during fasting or low carbohydrate intake, HSL becomes more active to release fatty acids for energy; during feeding, insulin suppresses HSL activity to store energy for future use.
Leptin
Leptin is a hormone primarily produced by adipose tissue (fat cells) that plays a key role in regulating energy balance, appetite, and metabolism. It acts as a communication signal between the body's fat stores and the brain. Leptin operates by binding to specific receptors in the hypothalamus, a region of the brain that controls hunger and energy expenditure. When leptin levels are high, it signals to the hypothalamus that the body has sufficient energy stored, leading to a reduction in appetite and food intake. This helps prevent overeating and excessive weight gain.
When an individual feels satiety (fullness) after eating, leptin helps promote the use of stored energy by increasing the metabolic rate—the rate at which the body burns calories. Additionally, leptin enhances thermogenesis, which is the production of heat in the body, further contributing to calorie burning. Leptin also encourages physical activity, which helps expend energy and maintain a healthy weight.
Leptin plays a critical role in metabolism by increasing the metabolic rate and promoting the burning of calories, which helps prevent excessive fat storage. It enhances the oxidation of fatty acids in muscle tissue, meaning it promotes the use of fat as a fuel source rather than storing it. By modulating appetite and energy expenditure, leptin helps regulate both the size and number of adipocytes (fat cells). Additionally, leptin promotes lipolysis, the process of breaking down stored triglycerides (the main form of stored fat) into free fatty acids and glycerol, which can then be used for energy. Through these mechanisms, leptin targets weight loss by facilitating the reduction of fat stores and enhancing energy utilization.
However, leptin resistance can occur, a condition in which the brain becomes less sensitive to leptin's signals. In leptin resistance, despite high circulating levels of leptin—often due to excessive fat accumulation—the hypothalamus does not respond appropriately to leptin's signals. This disrupts appetite regulation and energy balance, leading to continued hunger and increased food intake. Chronic exposure to elevated leptin levels, commonly seen in obesity, can desensitize the brain's response to leptin, resulting in leptin resistance. This condition is often exacerbated by chronic inflammation, which can impair leptin signaling pathways in the brain. As a result, individuals with leptin resistance may experience increased hunger and food intake, creating a cycle of overeating and weight gain that further exacerbates obesity and related metabolic disorders, such as type 2 diabetes and cardiovascular disease.
Adiponectin
Adiponectin is another important hormone predominantly produced by adipose tissue, and it plays a critical role in regulating various metabolic processes, including glucose regulation, fatty acid oxidation, and overall energy balance. It is one of the key adipokines—proteins secreted by fat tissue—that significantly influences the body's metabolic functions and plays several vital roles in metabolism, appetite regulation, and weight management.
In the regulation of adipose tissue, adiponectin enhances insulin sensitivity in key tissues such as skeletal muscle and the liver. By increasing insulin sensitivity, adiponectin promotes the uptake and utilization of glucose, which helps lower blood sugar levels. This action is crucial for preventing insulin resistance, a common feature of obesity and type 2 diabetes, where the body's cells become less responsive to insulin. Additionally, adiponectin possesses strong anti-inflammatory properties. It counteracts the effects of pro-inflammatory cytokines—proteins that promote inflammation—produced by adipose tissue, particularly in the context of obesity. By reducing chronic low-grade inflammation associated with excess fat accumulation, adiponectin helps mitigate the risk of developing metabolic disorders. Studies have shown that adiponectin levels are inversely related to C-reactive protein (CRP), a marker of inflammation that also stimulates the production of reactive oxygen species (ROS), harmful byproducts of metabolism that can damage cells and tissues.
In terms of metabolic regulation, adiponectin plays a crucial role in stimulating the oxidation of fatty acids in muscle and liver tissues. By promoting the breakdown of fats for energy, adiponectin helps reduce fat accumulation in these tissues, supporting overall metabolic health and preventing lipid buildup that can lead to metabolic complications, such as fatty liver disease. Furthermore, by improving insulin sensitivity and promoting glucose uptake, adiponectin contributes to the maintenance of stable blood glucose levels, which is vital for overall metabolic balance.
Interestingly, adiponectin levels are inversely related to body fat; higher levels of adiposity (body fat) are associated with lower adiponectin levels. This reduction in adiponectin is problematic because it contributes to the metabolic dysregulation commonly seen in obesity, including increased insulin resistance and greater fat storage. Due to its beneficial effects on metabolism and insulin sensitivity, increasing adiponectin levels is considered a potential therapeutic strategy for weight regulation and the treatment of obesity-related metabolic disorders. Interventions that boost adiponectin levels could help improve insulin sensitivity, reduce inflammation, and support healthier metabolic functioning, making it a key target in managing and preventing metabolic diseases.
Lifestyle Interventions for Optimizing Hormonal Balance and Adipose Tissue Function
Achieving optimal health through lifestyle interventions requires a holistic approach that targets the complex interplay between different types of adipose tissue and the hormones that regulate them. Here's how lifestyle modifications can enhance the function of these fat types and promote metabolic health:
Supporting Hormonal Balance
- Insulin Sensitivity: To maintain proper insulin function and prevent insulin resistance, focus on a diet that stabilizes blood sugar levels. This includes reducing refined carbohydrates and sugars, increasing fiber intake, and incorporating regular physical activity, which enhances insulin sensitivity.
- Leptin Sensitivity: Improving leptin sensitivity can be achieved by reducing chronic inflammation through a diet rich in anti-inflammatory foods, such as omega-3 fatty acids, and engaging in regular exercise. Adequate sleep and stress management are also crucial, as poor sleep and chronic stress can exacerbate leptin resistance.
- Boosting Adiponectin: To increase adiponectin levels, which improve insulin sensitivity and reduce inflammation, prioritize a diet high in healthy fats, such as those found in nuts, seeds, and fatty fish. Regular exercise also plays a key role in boosting adiponectin levels.
Promoting Beneficial Adipose Tissue
- Encouraging Brown and Beige Fat Activity: Cold exposure and physical activity are two effective strategies to activate brown adipose tissue (BAT) and promote the browning of white adipose tissue. These practices enhance mitochondrial function and increase thermogenesis, leading to improved metabolic health and weight management.
- Reducing Excess White Adipose Tissue (WAT): Managing caloric intake, particularly through balanced macronutrient distribution, along with regular exercise, can help reduce excess WAT. This reduction lowers the risk of metabolic disorders associated with obesity and insulin resistance.
A comprehensive approach that includes balanced nutrition, regular physical activity, stress management, and sufficient sleep is essential for optimizing the interplay between adipose tissue and hormonal health. By adopting these lifestyle interventions, individuals can support the healthy function of adipocytes, improve hormonal balance, and enhance overall metabolic health.
Cold Exposure and Metabolic Health: In-Depth Insights into Brown Adipose Tissue Research
Cold exposure has become ever-present in the health, fitness, and longevity community in recent years, with several high-profile celebrities, such as Chris Hemsworth, Kevin Hart, and Joe Rogan, endorsing the benefits of brief exposure (2-3 minutes) of cold water from a shower, plunge pool or a jump in the sea.
Interestingly, the practice of cold exposure is nothing new and has been dated back to 3500 BC, with the ancient Greeks using this method to treat various ailments for medicinal purposes. Since then, several research groups worldwide have produced ground-breaking research deciphering the effects of short-term cold exposure on human health, metabolic function, and as a recovery tool following exercise training.
Furthermore, many podcasters, bloggers and 'fitness experts' emphasize and potentially exaggerate the effects of cold water exposure benefits. Here we will provide a deep-dive on the advantages and disadvantages of cold water immersion on human physiology.
Background on Cold Exposure
Cold water immersion has been popularised in the last decade by the likes of Dutch extreme athlete Wim Hof and, more recently, a Ph.D. graduate from the University of Copenhagen, Dr. Susanna Soberg, Ph.D. The latter, Dr. Susanna Soberg, completed her Ph.D. in the world-leading lab of Professor Camilla Scheele, within the Center of Inflammation and Metabolism and the Center for Physical Activity Research, researching human metabolism and the effects of stressors on brown and white fat.
Since completing her Ph.D., Dr. Soberg has published several high-impact publications and a book titled "Winter Swimming: The Nordic Way Towards a Healthier and Happier Life." Her research has led to the development of the 'Soberg Principle', which is described as the minimal amount of cold exposure needed per week to see health and metabolic benefits.
In her research, she proposes that a minimum of only 11 minutes of cold exposure per week is enough to reap the health benefits, which can be enhanced by increased exposure and the addition of heat (we’ve discussed the benefits of heat in length in a previous research review article: The Longevity Benefits of Heat: Dissecting the Science Behind Sauna Therapy for Optimal Healthspan)
As with anything, this 'trend' of exposing yourself to cold water for a specified duration has been overly stated on social media platforms and podcasts to improve immunity, fat loss, inflammation, anxiety, depression, and metabolism. In a world where the general public derive a lot of their science information from podcasts, Instagram, and ticktock, how true are some of these statements? Here we will separate fact from fiction in an attempt to give you the best understanding of the benefits or disadvantages of cold water exposure.
Molecular Response to Cold Exposure
As humans, we are consistently battling external stressors in an attempt to maintain homeostasis. Following a stimulus, our body responds via hundreds, if not thousands, of signals between cells and tissues to adapt to the stressor to handle the stimulus again. As humans in the 21st century, we are typically accustomed to wearing multiple layers of clothes, enjoying the comfort of our warm homes, and having heated seats while driving.
We have become so habituated to the warmth that exposure to the cold is incredibly stressful and can even lead to cold shock, and death in certain extreme circumstances when not done correctly [17]. Nevertheless, the basic biology behind the brief exposure is fascinating, and even a short [18-19] minutes out of your comfort zone and under a cold shower or in an ice bath can have on your metabolism [18–21] & mental health [22, 23]. Here we will discuss the main molecular responses to cold exposure and how this translates into improved health.
Thermogenesis & Brown Tissue Activation
Immediately upon exposure to a cold environment, our bodies enter their 'fight or flight' response, commonly termed increased sympathetic nervous system activity. This response stimulus activates brown adipose tissue (BAT) [24], which is responsible for thermogenesis and producing heat in response to a cold environment and increasing energy expenditure [21]. Much research has been published on brown adipose tissue in mice, with little research in humans. This is primarily due to the technical difficulty in measuring its activity but also due to the limited depots of BAT in humans, which are only located in small amounts around the clavicles, upper back, and spine [25] (see PET CT scan below).
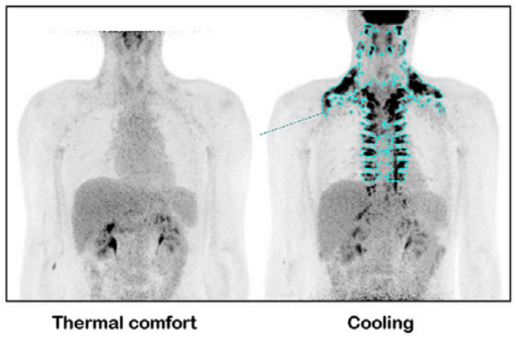
In Dr. Soberg's landmark 2021 publication from her Ph.D. thesis [21], she measured the effects of cooling via an unconventional method of cooling blankets. These are effectively water-cooled blankets (found here) that are capable of temperatures as low as 10°C.
In this study, she compared two groups of Danish men; one group were open water swimmers, that were accustomed to cold exposure, and a control group of men with no prior experience of cold exposure [21]. Following a rather long ~3hrs of cold cooling, the control group observed a ~90% increase in BAT activation.
Remarkably, the open-water swimmers observed a ~3000% increase in BAT activation [21]. The increased activation of BAT subsequently led to an extra ~425 calories and ~930 calories in the control and open water swimmers, respectively.
One of the primary mechanisms of the increased energy expenditure may be due to the increased shivering during and after cold exposure as a mile ‘exercise mimetic’ due to muscle activity & contraction [26].
"The involuntary contraction of skeletal muscle during shivering leads to heat production, energy utilization, and secretion of small molecule growth factors FGF21 & Irisin [27], thereby increasing energy expenditure."
In her 2021 publication, Dr. Solberg indirectly measured 'muscle activity' with EMG electrodes placed on the pectoralis and quadriceps in an attempt to gain insights into cold exposure's effect on muscle activity [20].
As a result, cold exposure had a significant increase in muscle activity. Similar studies using the cold water cooling blankets by the Diabetes, Endocrinology, and Obesity Branch at the National Institute of Diabetes and Digestive and Kidney Diseases, have shown a 96% increase in muscle activity, and a ~50% increase in energy expenditure over a 24 hours [20].
Overall, the increased energy expenditure shows incredibly promising data that cold exposure could potentially help with weight loss when practiced over weeks to months. However, a large limitation of the above studies is the use of cold water cooling blankets, which people do not use, nor have access to.
Instead, the use of cold plunge pools, ice baths, and cold showers is preferred. Although they likely have similar responses, further research is needed on more widely available cold exposure methods to see if there are similarities between methods.
Insulin Sensitivity & Glycaemic Control
The increase in BAT activation as a result of cold exposure increases the amount of glucose utilized by BAT in an attempt to increase energy expenditure and heat production to maintain homeostasis [24]. As a result, whole-body insulin sensitivity is improved [18]. Researchers from German Center for Diabetes Research in Neuherberg, Germany, showed a 20% increase in insulin sensitivity following a single session of cold exposure, with a concurrent rise in circulating fatty acids [19]. Similar to the above research, the authors used a wearable water-perfused suit for 100 minutes at a temperature of ~18°C [19].
Another method of cold exposure researchers use is living in a cold environment. Notably, Professor Patrick Schrauwen from Maastricht University has been at the forefront of this research. With access to a free-living research facility at Maastricht Medical Centre, Patrick and his research team can set the room's temperature to a moderate 14°C to determine how exposure to a mild cold temperature for 10 days alters human metabolism.
In a landmark study in Nature Medicine, Professor Schrauwen and his team showed that as little as 10 days of living in the cold room wearing only shorts and a t-shirt (to maximize cold exposure) resulted in a 43% increase in insulin sensitivity and ~57% increase in brown adipose tissue activation in adults with type II diabetes [2].
The paper's authors also showed that the improvements in insulin sensitivity may be driven by an increase in skeletal muscle GLUT4 expression [18].
"GLUT4 is a protein that helps cells take up glucose from the blood. It does this in response to a hormone called insulin. When insulin levels rise, GLUT4 moves from inside the cell to its surface, creating channels that allow glucose to enter. This process helps regulate blood glucose levels and provides cells with the energy they need to function properly."
Blunted Muscle Hypertrophy - Will Ice Baths Kill Your Gains?
Contrary to the aforementioned benefits, cold exposure may negatively affect muscle mass gains. This has been supported by several high-impact and well-controlled clinical trials worldwide. Most notably, Dr. Cas Fuchs from Maastricht University in the Netherlands showed a blunting of ~20% in muscle protein synthesis rates (i.e., the building of new muscle) following resistance training.
In this study, Cas had groups of volunteers place their legs in either a 30°C or 8°C for 20 minutes following exercise after a single session, then as part of a 2-week training intervention. This is important because each volunteer was subjected to neutral and cold water. The thermoneutral leg served as the control, making this an incredibly powered research investigation, as this limits participant-to-participant variation.
As part of the study, participants were required to maintain cold-water immersion following a further six training sessions. At the end of the 2-weeks, long-term muscle protein synthesis rates were ~26% lower in the leg that underwent cold water immersion.
So, what happens if you continue cold water exposure as part of a longer-duration exercise training program?
One of the first long-term studies of exercise training and cold water immersion was published in 2015 by Dr. Llion Roberts, Ph.D, observing the effects of 10 minutes of cold exposure at 10°C during a 12-week training intervention [28]. Overall, both the thermoneutral and cold water groups gained muscle mass and strength. However, the gains in both measures were severely blunted in the cold water group [28]. This was followed up by a research group at Victoria University, Melbourne, led by Professor David Bishop, a world leader in exercise physiology research.
The project was run by a PhD student at the time, Dr Jackson Fyfe, PhD, where he determined the effects of 15 minutes of 10°C cold water on muscle hypertrophy & strength [29]. Similar to the previous studies, Jackson's observed a blunting in muscle hypertrophy, but interestingly no differences in muscle strength improvement [29].
Despite concerns about potential impacts on muscle hypertrophy, the metabolic advantages, such as enhanced fat metabolism and increased glucose uptake, remain valuable for overall health and longevity. Continued research will further refine the optimal use of cold exposure to harness these benefits while balancing its effects on different aspects of health.
Pharmaceutical Protocols
Oral rapamycin and metformin offer promising therapeutic options to complement lifestyle modifications aimed at promoting healthspan. Both rapamycin and metformin interact with the mechanistic target of rapamycin (mTOR) pathway, which plays a critical role in regulating cell growth, proliferation, and metabolism. Rapamycin directly inhibits mTOR by binding to the mTORC1 complex, whereas metformin indirectly downregulates mTOR activity by activating AMP-activated protein kinase (AMPK).
Both drugs influence glucose metabolism, though through distinct mechanisms. Rapamycin, by inhibiting mTOR, affects glucose uptake and utilization in cells, potentially altering energy balance. Metformin, primarily through AMPK activation, enhances insulin sensitivity and increases glucose uptake by peripheral tissues, particularly muscle. Additionally, both agents exhibit anti-proliferative effects through different pathways: rapamycin’s inhibition of mTORC1 promotes autophagy and reduces cellular senescence, while metformin’s activation of AMPK supports catabolic processes and inhibits anabolic pathways, indirectly reducing cell proliferation.
These mechanisms make both drugs relevant for metabolic regulation and weight management through their effects on mitochondrial health. By modulating the mTOR pathway, rapamycin can influence adipocyte function and size, potentially reducing lipogenesis (fat creation) and promoting lipolysis (fat breakdown). Animal studies suggest that rapamycin can decrease fat mass and adiposity by altering fat storage and utilization [11]. Concurrently, metformin has been shown in human trials to exhibit anti-obesity properties, reducing body weight and modulating glucose metabolism. Part of metformin's effectiveness may be due to its impact on brown adipose tissue (BAT), where it enhances mitochondrial function and promotes energy expenditure in preclinical obesity models [12].
Given that insulin is a primary activator of mTORC1, rapamycin’s inhibitory effects on this pathway may improve insulin sensitivity in certain contexts, potentially aiding in the regulation of blood glucose levels and contributing to weight management. When combined with metformin, which reduces blood glucose levels and insulin response, the potential for weight management is further strengthened. An animal study demonstrated that a combination of rapamycin and metformin led to a significant reduction in weight gain compared to treatment with either drug alone or with water [13]. Other studies have shown that rapamycin can reduce food intake and induce weight loss, while metformin has been associated with weight loss in individuals with or without diabetes. Metformin's ability to increase fat oxidation in the liver may contribute to these effects [14,15].
Both rapamycin and caloric restriction (CR) are known to mimic certain physiological effects, particularly in relation to weight loss and improved metabolic health. Both interventions target nutrient-sensing pathways centered on mTORC1, with rapamycin acting as an mTORC1 inhibitor. Both rapamycin and CR have been shown to activate autophagy, a process that enhances cellular function and recycles cellular components. Additionally, studies have demonstrated that both CR and rapamycin can increase lifespan across a wide range of evolutionarily diverse species, including yeast, Caenorhabditis elegans, Drosophila, and mice, as well as reduce age-related pathology and improve physiological functions in animal models [16].
Understanding the implications of rapamycin and metformin on the mTOR pathway, glucose metabolism, and mitochondrial function provides a foundation for exploring their potential in enhancing health span and managing weight. Both drugs offer distinct yet complementary mechanisms that target key aspects of metabolic health, making them valuable candidates for further investigation in the context of aging and metabolic disorders. As research continues, these therapies may provide new insights into the management of age-related diseases and the promotion of long-term health.
Closing Thoughts
The relationship between adipose tissue and overall health is both complex and essential, with adipose tissue serving as a dynamic endocrine organ that significantly influences energy balance, metabolism, and insulin sensitivity. Each type of adipocyte—white, beige, and brown—plays a distinct role in these processes. White adipose tissue (WAT) primarily functions in energy storage, while beige and brown adipose tissues (BAT) are key players in energy expenditure and thermogenesis.
Excessive accumulation of WAT, particularly in visceral regions, is linked to adverse health outcomes, including obesity, insulin resistance, type 2 diabetes, and cardiovascular disease. In contrast, increasing the activity and quantity of beige and brown adipocytes can enhance metabolic health by boosting mitochondrial density, improving insulin sensitivity, and reducing fat mass. Lifestyle interventions, such as regular physical activity, cold exposure, and dietary modifications, can promote the conversion of WAT to more metabolically active beige and brown adipose tissues.
The regulation of adipose tissue by key hormones like insulin, leptin, and adiponectin highlights the crucial role of hormonal balance in maintaining metabolic health and preventing obesity-related conditions. Additionally, pharmacological agents like rapamycin and metformin offer promising strategies to modulate these metabolic pathways, potentially mimicking the effects of caloric restriction and enhancing mitochondrial function.
Understanding the roles and regulation of adipose tissue is vital for developing effective strategies to combat obesity, improve metabolic health, and extend healthspan. Future research should continue to explore the therapeutic potential of targeting adipose tissue and its associated pathways in the context of mitochondrial health, chronic diseases, and aging. By closely monitoring biomarkers and combining pharmacological protocols with lifestyle modifications, we can work towards helping you achieve the most optimal version of yourself.
- Cypess, A. M., Lehman, S., Williams, G., Tal, I., Rodman, D., Goldfine, A. B., & Kahn, C. R. (2009). Identification and importance of brown adipose tissue in adult humans. New England Journal of Medicine, 360(15), 1509-1517. DOI:10.1056/NEJMoa0810780
- Darcy J, Tseng YH. ComBATing aging-does increased brown adipose tissue activity confer longevity? Geroscience. 2019 Jun;41(3):285-296. doi: 10.1007/s11357-019-00076-0. Epub 2019 Jun 22. PMID: 31230192; PMCID: PMC6702504
- Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, Beguinot F, Miele C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int J Mol Sci. 2019 May 13;20(9):2358. doi: 10.3390/ijms20092358. PMID: 31085992; PMCID: PMC6539070.
- Paul Lee, Sheila Smith, Joyce Linderman, Amber B. Courville, Robert J. Brychta, William Dieckmann, Charlotte D. Werner, Kong Y. Chen, Francesco S. Celi; Temperature-Acclimated Brown Adipose Tissue Modulates Insulin Sensitivity in Humans. Diabetes 1 November 2014; 63 (11): 3686–3698.
- Seki, T., Yang, Y., Sun, X. et al. Brown-fat-mediated tumour suppression by cold-altered global metabolism. Nature 608, 421–428 (2022). https://doi.org/10.1038/s41586-022-05030-3
- Fu, P., Zhu, R., Jia, J. et al. Aerobic exercise promotes the functions of brown adipose tissue in obese mice via a mechanism involving COX2 in the VEGF signaling pathway. Nutr Metab (Lond) 18, 56 (2021). https://doi.org/10.1186/s12986-021-00581-0
- Tanimura R, Kobayashi L, Shirai T, Takemasa T. Effects of exercise intensity on white adipose tissue browning and its regulatory signals in mice. Physiol Rep. 2022 Mar;10(5):e15205. doi: 10.14814/phy2.15205. PMID: 35286020; PMCID: PMC8919700.
- Wang, S., Liang, X., Yang, Q., Fu, X., Zhu, M., Rodgers, B. D., et al. (2017). Resveratrol enhances brown adipocyte formation and function by activating AMP-activated protein kinase (AMPK) α1 in mice fed high-fat diet. Mol. Nutr. Food. Res. 61:1600746. doi: 10.1002/mnfr.201600746
- Noriega, L., Yang, C., & Wang, C. (2023). Brown Fat and Nutrition: Implications for Nutritional Interventions. Nutrients, 15(18), 4072.
- Bergström, J., Hermansen, L., Hultman, E., & Saltin, B. (1967). Diet, Muscle Glycogen and Physical Performance. Acta Physiologica Scandinavica, 71(2-3), 140-150. https://doi.org/10.1111/j.1748-1716.1967.tb03720.x
- den Hartigh LJ, Goodspeed L, Wang SA, Kenerson HL, Omer M, O'Brien KD, Ladiges W, Yeung R, Subramanian S. Chronic oral rapamycin decreases adiposity, hepatic triglycerides and insulin resistance in male mice fed a diet high in sucrose and saturated fat. Exp Physiol. 2018 Nov;103(11):1469-1480. doi: 10.1113/EP087207. Epub 2018 Sep 13. PMID: 30117227; PMCID: PMC6446929
- Ziqubu K, Mazibuko-Mbeje SE, Mthembu SXH, Mabhida SE, Jack BU, Nyambuya TM, Nkambule BB, Basson AK, Tiano L, Dludla PV. Anti-Obesity Effects of Metformin: A Scoping Review Evaluating the Feasibility of Brown Adipose Tissue as a Therapeutic Target. Int J Mol Sci. 2023 Jan 23;24(3):2227. doi: 10.3390/ijms24032227. PMID: 36768561; PMCID: PMC9917329
- Albawardi, A., Saraswathiamma, D., Sharma, C., Elomami, A., Souid, A., & Almarzooqi, S. (2023). Effect of Sirolimus/Metformin Co-Treatment on Hyperglycemia and Cellular Respiration in BALB/c Mice. International Journal of Molecular Sciences, 24(2), 1223. https://doi.org/10.3390/ijms24021223
- Kim, E. K., Min, H. K., Lee, Y., Kim, S., Ryu, G., Na, H. S., Jung, K. A., Choi, J. W., Park, H., & Cho, L. (2020). Metformin rescues rapamycin-induced mitochondrial dysfunction and attenuates rheumatoid arthritis with metabolic syndrome. Arthritis Research & Therapy, 22. https://doi.org/10.1186/s13075-020-02174-3
- Albawardi, A., Saraswathiamma, D., Sharma, C., Elomami, A., Souid, K., & Almarzooqi, S. (2023). Effect of Sirolimus/Metformin Co-Treatment on Hyperglycemia and Cellular Respiration in BALB/c Mice. International Journal of Molecular Sciences, 24(2). https://doi.org/10.3390/ijms24021223
- Unnikrishnan, A., Kurup, K., Salmon, A. B., & Richardson, A. (2020). Is Rapamycin a Dietary Restriction Mimetic? The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 75(1), 4-13.
- Tipton MJ, Collier N, Massey H, Corbett J, Harper M. Cold water immersion: kill or cure? Exp Physiol 2017; 102: 1335–1355.
- Hanssen MJW, Hoeks J, Brans B, Van Der Lans AAJJ, Schaart G, Van Den Driessche JJ et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nature Medicine 2015 21:8 2015; 21: 863–865.
- Alexander Iwen K, Backhaus J, Cassens M, Waltl M, Hedesan OC, Merkel M et al. Cold-Induced Brown Adipose Tissue Activity Alters Plasma Fatty Acids and Improves Glucose Metabolism in Men. J Clin Endocrinol Metab 2017; 102: 4226–4234.
- Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C et al. Cell Metabolism Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. doi:10.1016/j.cmet.2013.12.017.
- Søberg S, Lö J, Philipsen FE, Pedersen BK, Karstoft K, Jensen M et al. Altered brown fat thermoregulation and enhanced cold-induced thermogenesis in young, healthy, winter-swimming men. Cell Rep Med 2021; 2. doi:10.1016/j.xcrm.2021.100408.
- Kelly JS, Bird E. Improved mood following a single immersion in cold water. Lifestyle Medicine 2022; 3: e53.
- Néma J, Zdara J, Lašák P, Bavlovič J, Bureš M, Pejchal J et al. Impact of cold exposure on life satisfaction and physical composition of soldiers. BMJ Mil Health 2023; : e002237.
- Greenhill C. Cold exposure increases brown adipose tissue in humans. Nature Reviews Endocrinology 2013 9:10 2013; 9: 566–566.
- Lee P, Zhao JT, Swarbrick MM, Gracie G, Bova R, Greenfield JR et al. High Prevalence of Brown Adipose Tissue in Adult Humans. J Clin Endocrinol Metab 2011; 96: 2450–2455.
- Virtanen KA. BAT Thermogenesis: Linking Shivering to Exercise. Cell Metab 2014; 19: 352–354.
- Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C et al. Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. Cell Metab 2014; 19: 302–309.
- Roberts LA, Raastad T, Markworth JF, Figueiredo VC, Egner IM, Shield A et al. Post-exercise cold water immersion attenuates acute anabolic signalling and long-term adaptations in muscle to strength training. Journal of Physiology 2015; 593: 4285–4301.
- Fyfe JJ, Broatch JR, Trewin AJ, Hanson ED, Argus CK, Garnham AP et al. Cold water immersion attenuates anabolic signaling and skeletal muscle fiber hypertrophy, but not strength gain, following whole-body resistance training. J Appl Physiol 2019; 127: 1403–1418.