Beyond Plaques: How Methylene Blue and Ketones Address Vascular-Hypometabolism in Alzheimer’s Disease
Amyloid Versus Hypometabolism. Alzheimer’s disease (AD) has historically been linked to amyloid plaques and neurofibrillary tangles. Yet, up to 30% of cognitively healthy individuals have been found to harbor these same plaques, challenging the notion that amyloid alone drives AD. This discrepancy has spurred growing interest in vascular and metabolic contributors to the disease.
Vascular-Hypometabolism Hypothesis. Research by Dr. Francisco Gonzalez-Lima demonstrates that reduced cerebral perfusion and cytochrome c oxidase (CO) dysfunction often appear before amyloid accumulation in late-onset AD (LOAD). In one study, superficial layers of the posterior cingulate cortex (PCC) showed 39% and 32% decrements in CO activity compared to controls—a stark indication of early metabolic failure in AD.
Early Cortical Vulnerability. Even the primary motor cortex (PMC), vital for motor function, was relatively spared in comparison to the PCC, which displayed an overall 28% reduction in CO activity. This selective vulnerability correlates with the PCC’s critical roles in memory retrieval and integrative cognition—functions that can fail early in AD.
EOAD Versus LOAD Divergence. Although early-onset AD (EOAD) comprises only 4–6% of all AD diagnoses, it has historically dominated research. EOAD correlates strongly with familial mutations (e.g., PSEN1, PSEN2, APP), while late-onset AD affects 90–95% of AD cases and is typically linked to APOE ε4, vascular comorbidities (e.g., hypertension), and pronounced hypometabolism.
Overlap with Metabolic Disorders. Some literature labels AD “type 3 diabetes,” noting that 8 out of 10 AD patients also have type 2 diabetes (T2D) or abnormal glucose levels. A meta-analysis of 144 prospective studies found a 1.25- to 1.91-fold increased risk of dementia in diabetes. These figures further connect metabolic dysfunction and vascular insufficiency to AD’s pathology.
Cytochrome c Oxidase (CO) as the Mitochondrial Choke Point. Emerging evidence from Dr. Francisco Gonzalez-Lima’s research points to cytochrome c oxidase (CO)—the terminal enzyme in the mitochondrial electron transport chain—as a critical choke point in Alzheimer’s disease (AD). Studies show up to a 39% decline in CO activity in the posterior cingulate cortex, correlating with disease duration. Because CO reduces oxygen to water to power ATP production, even moderate deficits can severely limit energy output and heighten oxidative stress, especially in high-demand regions like the PCC that govern memory. This highlights AD’s vascular-hypometabolic framework, where poor perfusion exacerbates mitochondrial dysfunction.
Methylene Blue for Mitochondrial Support. Studies show methylene blue (MB) in doses of 0.5–4 mg/kg boosts oxygen consumption by 37–70% and raises ATP production by up to 30% in human cell lines and animal models. This electron-donating function helps compensate for CO shortfalls and may enhance cerebral blood flow via nitric oxide–mediated vasodilation
Ketones as Alternative Fuel. Researchers like Dr. Stephen Cunnane report that ketones can supply up to 60% of the brain’s energy needs under certain conditions, bypassing glycolytic impairments. By converting to acetyl-CoA within mitochondria, ketones reduce reactive oxygen species (ROS) and help sustain ATP production when glucose metabolism is compromised .
Multimodal Future Therapies. Combining MB (which improves electron transport), ketones (which bypass glycolysis), and near-infrared (NIR) light therapy (which photostimulates CO) could address multiple choke points in AD pathophysiology. These synergistic treatments—alongside lifestyle modifications aimed at vascular health—offer a comprehensive strategy that may surpass purely amyloid-focused interventions and open new avenues for prevention, management, and possible delay of Alzheimer’s disease progression.
Background
Alzheimer’s disease (AD) is classified as a leading cause of dementia in individuals with advancing age, accounting for up to 80% of all dementia cases [1]. AD is often accurately referred to as Alzheimer’s dementia, and it is the leading neurodegenerative disorder affecting the elderly population [2]. Globally, AD affects millions of individuals by impairing cognitive function—manifested through language difficulties (aphasia), failure to recognize sensory inputs (agnosia), memory loss, and behavioral changes—thereby placing significant strain on patients, families, and communities [3].
It is estimated that by 2050 the global population of AD patients will surpass 150 million [3]. Within the United States alone, projections from the 2024 Alzheimer’s Disease Facts and Figures suggest the AD population will nearly double to 14 million by that same year [4]. Unfortunately, there are few medications in existence that have been found to treat or reverse clinical AD, as the current options only impede progression [3]. AD poses a formidable research challenge, marked by subtle early symptoms, prolonged disease course, and lingering debates about its precise cause. Yet, promising new hypotheses and findings—some contending that vascular and metabolic imbalances are central to pathogenesis—have reinvigorated the field. Indeed, some experts are optimistic that effective AD prevention strategies could materialize as early as 2025 [5]!
In this review, we will analyze how these evolving perspectives—particularly those championed by Dr. Francisco Gonzalez-Lima, through his investigation of mitochondrial function in AD autopsy brains and his exploration of molecules that target electron transport function—recast AD as a hypometabolic disorder rooted in vascular insufficiency and mitochondrial dysfunction. By highlighting these integrative viewpoints, the review underscores promising opportunities for therapeutic intervention and sheds new light on the direction of AD research.
Alzheimer’s Disease Etiology - Theoretical Landscape
Efforts to understand and treat Alzheimer’s disease (AD) have long been guided by two principal theories of its etiology. The first—commonly referred to as the amyloid hypothesis or amyloid cascade hypothesis—emerged from Alois Alzheimer’s early-1900s observations of amyloid plaques and neurofibrillary tangles in the brains of individuals with dementia. According to this hypothesis, the abnormal accumulation of amyloid beta (Aβ) initiates a toxic cascade that leads to neuroinflammation, neuronal injury, and ultimately the cognitive and behavioral manifestations of AD [6]. Over the past few decades, this amyloid-centric view has been the driving force behind the development of many pharmacological approaches, including recent monoclonal antibodies designed to reduce amyloid burdens in the brain.
Despite considerable investment in amyloid-targeting drugs, the field has encountered ongoing debates. For instance, clinical trials have often yielded mixed or limited efficacy, prompting some to question whether amyloid buildup is a cause or a symptom of the disease process. The presence of amyloid pathology in cognitively normal individuals has further fueled these uncertainties, suggesting that additional factors likely contribute to AD pathogenesis.
In contrast, the vascular hypometabolism hypothesis argues that chronic hypoperfusion and mitochondrial respiratory failure precede and drive the disease, with amyloid pathology emerging as a later or secondary effect [7].
According to this model, reduced blood flow and impaired cellular respiration result in an energy deficit within neurons—particularly in high-demand brain regions. This deficit triggers downstream consequences that include cognitive impairment, memory loss, and, eventually, amyloid plaque formation. From this perspective, vascular insufficiency and metabolic dysfunction constitute the initial steps in AD pathology, setting in motion the neurodegenerative cascade.
The key distinction between these two perspectives is that the vascular hypometabolism hypothesis prioritizes energy failure as the fundamental event, whereas the amyloid cascade hypothesis focuses on the toxic accumulation of misfolded proteins as the root cause.
Historical Scientific Context
Dr. Gonzalez-Lima has long advocated for examining the combined vascular and metabolic deficits observed in Alzheimer’s disease (AD), rather than concentrating primarily on amyloid pathology. He argues that AD research has failed to advance as expected because it continues to rely on the singular 1900s case described by Alois Alzheimer—a patient who, in hindsight, clearly exhibited early-onset Alzheimer’s disease (EOAD) [7]. This original case may not be representative of the vast majority of AD, since EOAD accounts for only 4–6% of all AD diagnoses [8]. Consequently, Dr. Gonzalez-Lima contends the field’s heavy emphasis on amyloid may reflect an overgeneralization from a relatively rare clinical subgroup.
At the turn of the 20th century, dementia was divided into “senile dementia” (affecting older adults) and “presenile dementia” (manifesting at younger ages) [9]. Alois Alzheimer’s colleague, Emil Kraepelin, renamed presenile dementia Alzheimer’s disease, a label that persisted [9]. Meanwhile, another clinician, Oscar Fischer, focused on senile dementia—an entity more akin to today’s late-onset AD (LOAD) [9]. Although Kraepelin and Fischer were scientific rivals, both contributed critical insights that illuminate AD’s complexity and underscore how multiple subtypes may exist within the broader dementia spectrum.
This historical context highlights a nuanced reality: AD’s etiology has never been monolithic. Just as type 1 and type 2 diabetes arise from distinct origins despite sharing certain features, so, too, might EOAD and LOAD constitute separate diseases with different pathological drivers. By revisiting this century-old distinction, researchers can better appreciate Dr. Gonzalez-Lima’s critique: modern Alzheimer’s investigations, in his view, have fixated on amyloid-centric explanations rooted in an unusual early-onset case, diverting attention from the vascular and metabolic mechanisms likely central to most AD patients.
The discussion below illustrates why early-onset (presenile) and late-onset (senile) AD are increasingly regarded as separate entities with varying pathological drivers. Dr. Gonzalez-Lima’s stance—that an exclusive focus on amyloid may overlook vascular and metabolic dimensions crucial to most AD patients—serves as a backdrop for understanding EOAD’s unique genetic underpinnings versus LOAD’s broader confluence of vascular risk factors.
“Presenile” - Early-Onset Alzheimer’s Disease
Early-onset Alzheimer’s disease (EOAD) is diagnosed before 65 years of age and comprises roughly 5% of all AD cases [8]. Its clinical course differs significantly from late-onset AD (LOAD), often presenting with faster progression, delays in accurate diagnosis, and age-specific psychosocial challenges—such as balancing employment and family responsibilities—thereby heightening the need for tailored interventions [10]. Clinical management for EOAD thus prioritizes the specific cognitive domains most affected and implements psychosocial strategies suited to a younger demographic [8].
EOAD Distinctive Traits:
- Significant delays to diagnosis
- Diagnosis <65 years of age
- Higher prevalence of traumatic brain injury (TBI)
- Greater white matter changes
- Higher burden of neurofibrillary tangles and plaques, especially in posterior neocortex
- Greater tau/neurofibrillary tangle load per stage of dementia
- Lower frequency of the APOE ε4 allele
- Increase hereditary and family risk
- Strongly linked to autosomal dominant mutations in genes like APP, PSEN1, and PSEN2
- Lower incidence of comorbidities including diabetes, obesity, circulatory disorders
It is clear that EOAD is not simply primary or late-onset AD (LOAD) occurring at a younger age. There are many differences and unique characteristics associated with EOAD. The prevailing AD etiology, amyloid cascade hypothesis fits EOAD better where amyloid beta is playing a greater causal role [8]. Patients with EOAD overall have greater parietal atrophy, more white matter abnormalities, and less hippocampal volume loss, compared to those with LOAD [8].
Patients with EOAD also tend to have fewer vascular or metabolic comorbidities—such as diabetes, obesity, and circulatory disorders—underscoring the possibility that different mechanisms drive pathology in EOAD and LOAD [8].
Some researchers and clinicians refer to early-onset AD as familial AD, a terminology that aligns with Dr. Gonzalez-Lima’s assertion that EOAD is a profoundly hereditary condition. Indeed, many of the earliest documented EOAD cases—inclusive of Auguste Deter, the first patient described by Alois Alzheimer—harbored mutations in presenilin genes (PSEN1 or PSEN2) [11, 12]. In the early 1990s, linkage analyses uncovered that three genes—amyloid-beta A4 precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2)—are directly implicated in familial EOAD [11]. Of these, PSEN1 mutations account for approximately 81% of familial AD cases, APP mutations for 14%, and PSEN2 for 6% [13].
Although various susceptibility genes increase the risk of developing any form of AD, the single most influential genetic risk factor in the general population remains the APOE ε4 allele [11,14,15]. APOE plays a critical role in lipoprotein metabolism by binding soluble Aβ, thus modulating its clearance and aggregation [8]. Intriguingly, while APOE ε4 is a strong predictor for EOAD, its presence sometimes correlates with a delayed onset of disease in EOAD compared to LOAD, even though overall risk remains elevated [14]. This paradox highlights the complexity of AD genetics, suggesting that multiple alleles and gene-environment interactions collectively shape disease onset and progression.
Considering the intensity of the challenges associated with EOAD and the immense weight of this diagnosis it is fortunate that EOAD represents a small minority of AD [16, 17, 18]. Even though a very small percentage of all AD is classified as EOAD, the majority of research historically focused on this area [11]. Dr. Gonzalez-Lima suggests there is a belief that if we are able to uncover what’s happening in the early onset cases, then we can easily translate that to addressing the more common LOAD. This may not be the case, considering EOAD and LOAD differ in their etiology, they are effectively two separate diseases.
“Senile” or “Primary” - Late-Onset Alzheimer’s Disease
Late-onset Alzheimer’s disease is by far the more common form of AD. LOAD is diagnosed after age 65, represents at least 90-95% of all AD diagnoses, and differs fundamentally from EOAD [19, 20]. Beyond age, LOAD is less strongly linked to mutations in genes like APP, PSEN1, and PSEN2, which directly disrupt amyloid-beta processing and lead to aggressive plaque formation [13].
In contrast, LOAD is associated with the APOE ε4 allele, a susceptibility gene that impairs lipid metabolism and amyloid clearance but does not guarantee disease development [15]. Relative to the APOE ε3/ε3 genotype, carriers with one copy of the APOE ε4 allele have an ∼3.7-fold increase, while those with two copies have a 12-fold increase in risks for developing AD [15].
LOAD Distinctive Traits:
- Diagnosis follows traditional timeline
- Diagnosis at 65 years or older
- High frequency of the APOE ε4 allele
- Neurofibrillary tangles and plaques often more age-related
- Extremely low hereditary and family risk
- Less associated with mutations in APP, PSEN1, and PSEN2 genes
- Higher incidence of comorbidities diabetes, obesity, especially circulatory disorders
- Impaired brain glucose uptake and poor metabolic function
Building on clinical and postmortem findings, numerous studies have questioned the long-standing view that amyloid plaques and neurofibrillary tangles fully explain the pathogenesis of late-onset Alzheimer’s disease.
Contrary to the classic amyloid hypothesis, data now show that even healthy, cognitively intact individuals can harbor brain pathology that rivals—or even exceeds—the plaque burden seen in mild cognitive impairment (MCI) or early AD dementia [21, 22].
A prominent illustration is the Nun Study, in which a cohort of elderly nuns (aged 77–103) underwent both neurocognitive assessments (e.g., Mini-Mental State Examination) and postmortem brain examinations [22]. While some participants with high plaque loads demonstrated no cognitive impairment, others with fewer lesions displayed pronounced symptoms. This paradox suggests that additional factors besides amyloid drive the clinical manifestations of AD.
Subsequent analyses of the Nun Study, including recent publications from February 2025 [24], have highlighted a high prevalence of “mixed dementia”—a composite of AD and vascular pathology [23]. Notably, a subset of cognitively normal participants exhibited abundant plaques, pointing to a potential resilience mechanism or an alternative disease pathway [22]. These observations reinforce the notion that amyloid accumulation alone does not fully account for AD development.
In fact, LOAD appears to stem from a convergence of genetic, vascular, and lifestyle influences. Comorbidities common in later life, such as hypertension and atherosclerotic disease, can exacerbate reductions in cerebral blood flow, intensifying the risk and severity of AD-related decline [21,25].
Rather than being a mere consequence of neuronal atrophy, decreasing cerebral blood flow and metabolic function may precede structural and symptomatic changes, as mounting evidence suggests [21,25,26]. As a result, an increasing number of clinicians and researchers now regard AD as, at least in part, a vascular and metabolic disorder—one that involves compromised perfusion, impaired glucose utilization, insulin resistance in the brain, and mitochondrial dysfunction. This expanded perspective opens new avenues for diagnosing, preventing, and managing LOAD by targeting these underlying vascular and metabolic processes.
The Vascular Hypothesis of AD
Originally proposed about three decades ago based on rat models subjected to chronic brain hypoperfusion, the vascular hypothesis posits that reduced cerebral blood flow and vascular dysfunction are key drivers of Alzheimer’s disease (AD) [26]. This approach, considered by some to be the second most common etiology of dementia [27], is championed by Gonzalez-Lima for late-onset AD (LOAD), although he acknowledges that early-onset AD (EOAD) may follow a distinct path.
Multiple vascular pathways have been implicated in AD pathogenesis. Dysregulation of neurovascular coupling can compromise oxygen and nutrient delivery, while blood–brain barrier breakdown permits harmful substances to invade the brain. Additionally, impaired clearance of amyloid-β, which acts as toxic metabolic waste, further damages the vasculature and amplifies neuronal stress [28]. A critical illustration of this process is the two-hit hypothesis, suggesting microvascular insults occur before significant amyloid-β buildup, thereby initiating cognitive decline [29]. This early insult can, in turn, create a feed-forward loop in which amyloid-β accumulation exacerbates vascular dysfunction [28].
An analogy can help clarify these interconnected issues: imagine a city’s road network, where blocked or narrowed highways (blood vessels) slow the delivery of essential goods (oxygen and nutrients) and hinder waste removal. As traffic piles up, the city becomes congested, infrastructure falters, and garbage accumulates—much like amyloid-β in the brain. Over time, these cumulative backups compound existing damage, compounding risk of a systemic breakdown akin to cognitive decline.
Large-scale population studies [30] and longitudinal neuroimaging data [31] further reinforce the central role of vascular factors, showing that cerebral blood flow reductions often precede measurable amyloid-β and tau deposits. Identifying reliable vascular biomarkers of AD has therefore become a key research priority, with the ultimate goal of better predicting risk, informing earlier intervention, and potentially preventing or delaying the onset of clinically significant cognitive symptoms.
Vascular-Hypometabolism Hypothesis of AD
In 2001, Dr. Gonzalez-Lima and his team advanced an alternative view of AD by examining in vivo imaging findings alongside postmortem brain tissue from patients with AD and non-AD controls [32]. Crucially, they focused on specific brain areas known to exhibit early and pronounced declines in energy metabolism—regions that, when compromised, often coincide with the onset of cognitive deficits. To quantify these metabolic changes, the researchers used quantitative cytochrome c oxidase (CO) histochemistry, a method that directly measures the capacity of neurons for oxidative energy production [33,34].
Studies have consistently shown that certain cortical areas, such as the posterior cingulate cortex (PCC) and related regions, are among the first to manifest metabolic deficits in AD. The PCC is a critical hub for memory retrieval and is deeply involved in integrative cognitive functions. When its oxygen and energy demands are not met, early symptoms of AD—like subtle memory lapses—can emerge. By homing in on these sites, Gonzalez-Lima’s team aimed to capture the initial metabolic “red flags” that might precede or trigger the hallmark histopathologies (e.g., plaques and tangles).
Significance of Cytochrome c Oxidase (CO)
Cytochrome c oxidase is the terminal enzyme in the mitochondrial electron transport chain, directly responsible for transferring electrons to oxygen and thus driving ATP production. Because CO activity reflects the rate at which neurons can generate energy, it is widely regarded as a precise indicator of mitochondrial health and overall metabolic capacity. A decrement in CO disrupts oxidative phosphorylation, leading to reduced ATP availability, higher oxidative stress, and ultimately the neuronal dysfunction that characterizes AD.
By marrying advanced imaging techniques with robust biochemical markers like CO, Dr. Gonzalez-Lima’s laboratory introduced a framework that ties vascular insufficiency (reduced blood flow, decreased oxygen availability) to mitochondrial failure (diminished CO activity).
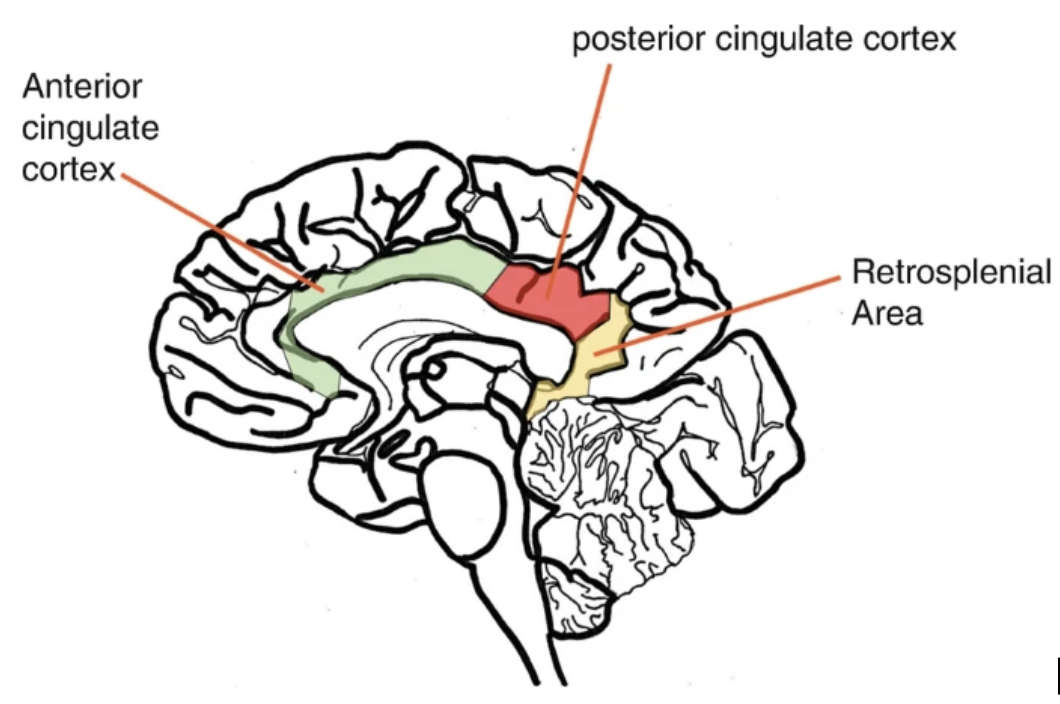
Employing quantitative cytochrome c oxidase (CO) histochemistry, researchers examined the six cortical layers (I–VI) within the posterior cingulate cortex (PCC) and primary motor cortex (PMC)—two regions differing in both function and susceptibility to AD-related damage.By comparing these areas, the investigators aimed to discern whether metabolic deficits in AD are region-specific or more uniformly distributed throughout the cortex.
The results revealed statistically significant (p < 0.05) reductions in CO activity across all PCC layers (I–VI) in AD patients relative to controls, with layers I and II showing especially pronounced decrements—39% and 32%, respectively (p < 0.01) [32]. On average, CO activity in the PCC dropped by 28%, which was nearly double the nonsignificant change observed in the PMC region [32].
This selective vulnerability of the PCC suggests that its high demand for oxygen and glucose—integral to cognitive functions like memory consolidation and information processing—may render it more susceptible to early energy deficiencies. In contrast, the PMC, essential for motor output, appears relatively spared in the disease’s initial stages.
From a translational standpoint, these findings underscore the possibility that energy failure in specific cortical areas central to memory and cognition could drive or exacerbate cognitive decline in AD. Observing larger metabolic deficits in layers I and II is particularly noteworthy because these superficial layers are rich in interconnected neuronal processes and synaptic activity, making them crucial for interregional communication. The pronounced deficit in CO function points to compromised oxidative metabolism—and, by extension, mitochondrial health.
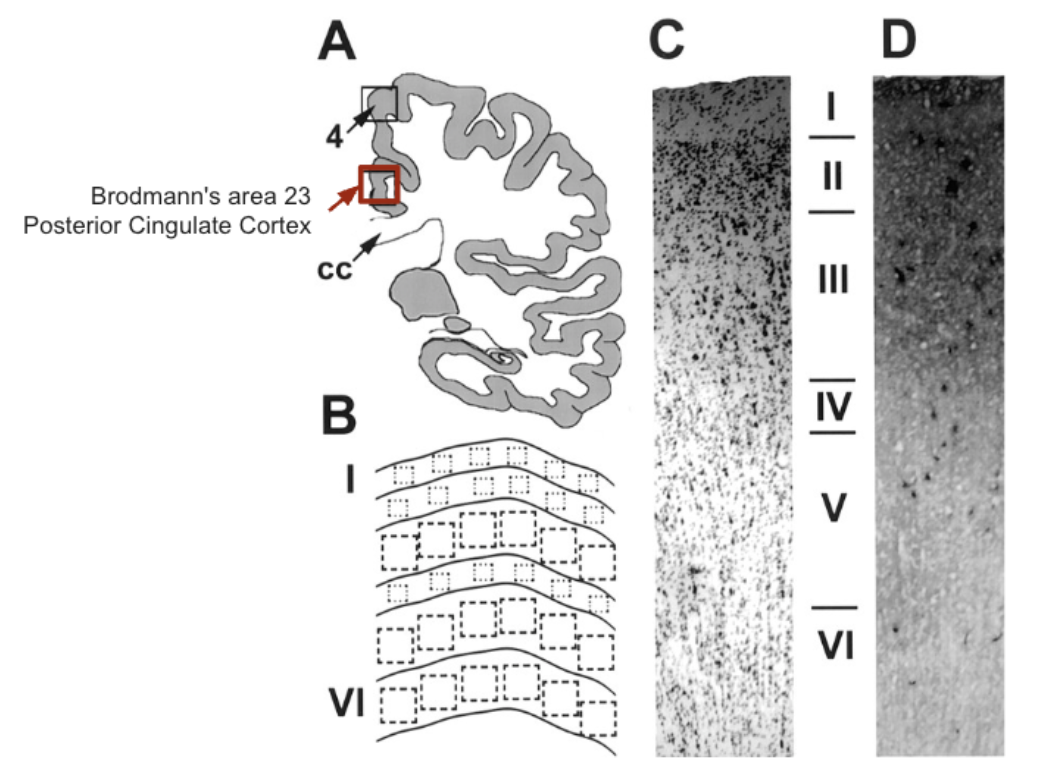
The results of this high-impact research work, in conjunction with previous research [35,36] suggest that the pronounced energy-metabolism deficits often observed in the posterior cingulate cortex (PCC) of probable AD cases may stem from greater CO decrements in the superficial layers (I and II). These layers are densely populated by synapses and are critical for integrating information across cortical regions. Thus, any diminished CO activity here directly compromises oxidative metabolism, leading to a shortfall in ATP and impairing the brain’s capacity to sustain essential functions such as neurotransmission and protein synthesis [32,35,36].
Remarkably, the research also uncovered a lack of correlation between CO activity and the conventional histopathological measures of AD—namely, amyloid plaques and neurofibrillary tangles. This finding implies that CO deficits and broader hypometabolic processes might precede or operate independently of amyloid-driven pathology in the PCC [32].
Reductions in energy metabolism are one of the earliest detectable abnormalities in AD [25, 32] Therefore, early detection may even present a strong prevention opportunity given the extended pre-diagnostic timeframe for AD, sometimes lasting 20 years.
Moreover, regional decreases in metabolic function, as shown through Positron Emission Tomography (PET), may serve as an early biomarker for AD risk. The brain’s high dependence on a continuous, ample supply of oxygen and nutrients underscores how vascular insufficiency and mitochondrial dysfunction could trigger or exacerbate cognitive decline—particularly in late-onset AD (LOAD), where vascular comorbidities often compound the problem [25].
By spotlighting CO deficits rather than plaque buildup, Dr. Gonzalez-Lima’s work helps reshape our fundamental understanding of AD, shifting it toward a hypometabolism disorder with mitochondrial failure at its core. His unified model—integrating vascular pathology, energy deficits, and structural changes—both challenges and refines the long-standing amyloid-centric approach to AD’s etiology, paving the way for more metabolism-focused therapeutic strategies.
Mitochondria as the Metabolic Choke Point
Dr. Gonzalez-Lima's research identified mitochondria, specifically cytochrome c oxidase (CO), in the electron transport chain (ETC) as the metabolic choke point in neuronal energy metabolism. In AD patients there was an astounding 39% reduction in CO activity in the superficial layers of the posterior cingulate cortex (PCC) region of the brain [32]. The PCC is vital for memory and it is among the first regions affected by AD [32]. Mitochondrial dysfunction as measured by CO deficit correlates with disease duration [32] and disrupts oxidative phosphorylation, leading to impaired ATP production, increased oxidative stress, as well as functional uncoupling between cortical layers, exacerbating hypometabolism [5,37]
Cytochrome c oxidase is the mitochondrial enzyme that is responsible for oxygen consumption at the cellular level as it transfers electrons to oxygen, producing a reduction reaction in which oxygen becomes water and generates a proton gradient for ATP synthesis [38]. CO is also known as complex IV in the ETC [38]. Without a fully functioning CO enzyme, the mitochondria have difficulty creating energy in the form of ATP. The brain requires constant energy, therefore complete failure of CO would be deadly, and the impact of degraded CO activity leads to significant neurological diseases such as AD.
Every organism on the planet that uses oxygen to obtain energy relies on CO. If you’re not getting enough oxygen, the process cannot move on to produce electrons, or a proton gradient which significantly reduces oxidative energy production. One common symptom associated with dementia is a drive to eat carbohydrates and simple sugars [39]. This particular symptom might be explained in part by CO degradation which therefore produces a forced reliance on anaerobic metabolism. Given the great need for oxygen at complex IV (aka. CO) this becomes the rate-limiting component of the ETC and is HIGHLY susceptible to ischemia or hypoxia conditions [40].
Research indicates that compromising circulation by partially occluding blood vessels to the brain, causes a reactive downregulation of CO which leads to poor mitochondrial respiration and ATP production [25,32]. The destructive cycle of chronic blood flow and oxygen insufficiency is considered a potent driver of downstream effects including memory loss, cognition, and behavioral changes in AD which originate in the mitochondria.
The brain has a disproportionate energy demand which makes it susceptible to imprecision or insufficiency of blood and oxygen for energy production. The brain’s energy demand is the highest of any organ in the body relative to its weight. Although the brain only represents about 2% of total body weight in humans, it consumes about 20% of daily total energy expenditure so that’s a grossly disproportionate amount of energy [41].
In addition, the brain is severely limited in its ability to leverage anaerobic metabolism for energy production [42]. Population-based research shows definitively that age is the number one predictor of AD [1], but following age, reduced blood flow [29] and hypometabolism [25], due to diminished activity of CO [32], are the second most highly correlated with cognitive decline. Hypoperfusion and metabolic dysfunction are involved as a root cause in producing signs and symptoms of clinical cognitive decline and neurodegenerative diseases. According to the vascular hypometabolism hypothesis LOAD develops very slowly over decades, an extended preclinical stage provides an opportunity for intervention, prevention, and course correction.
Diabetes-like Trajectory for AD?
The term “type 3 diabetes” is a popular moniker for AD, although it is not an officially recognized medical or diagnostic term. Major health organizations such as the World Health Organization (WHO) or the American Diabetes Association (ADA) have not adopted the term, and it is not used in clinical coding [2]. Nonetheless, type 3 diabetes remains a popular theoretical concept, used by some researchers to highlight the overlap in the pathological mechanisms underlying AD and T2DM [2,3].
This topic has attracted interest because some studies indicated that the risk of developing dementia, specifically AD, in patients diagnosed with diabetes may be increased. A meta-analysis of 144 prospective studies found a 1.25- to 1.91-fold increased risk of cognitive disorders (cognitive impairment and dementia) [43]. There is overlap with the vascular hypometabolism hypothesis, for example, a 5-year follow up study found that diabetes is linked to an increased risk of vascular cognitive impairment and vascular dementia, suggesting that diabetes primarily impacts dementia through vascular pathways [44].
Recent research has focused on the strong link between AD and metabolic dysfunction. Dr. Suzanne de la Monte is credited with coining the term type-3 diabetes. According to Dr. de la Monte, AD is a downstream consequence of metabolic dysfunction and insulin resistance in the brain often linked to type 2 diabetes (T2D) [45]. Indeed, research suggests that as many as 8 out of 10 individuals with Alzheimer’s disease also have T2D or abnormal blood glucose levels, highlighting a significant correlation between the two conditions [3].
Like diabetes, less than a decade ago, AD is formally classified as a chronic progressive disorder without a cure. That belief has largely deteriorated with regard to T2D due to continued collective research efforts that demonstrated, targeted lifestyle interventions, and metabolic therapies, can reverse the disease or put T2D patients into remission indefinitely [46]. Tireless work on behalf of renowned AD researchers, such as Francisco Gonzalez-Lima have made tremendous progress in describing the linkage between vascular and metabolic changes that likely contribute to AD progression in the vast majority of patients. Given the optimism among a growing contingency of AD researchers [5], perhaps AD can follow a trajectory similar to T2D in which effective AD prevention and treatment interventions will become within reach in the near future.
Interventions: Methylene Blue, Ketones, and Near-Infrared Light
Current standards of care and medicinal offerings for AD patients lack true effectiveness. There are some promising new drugs being investigated, but overall, treatment options for most AD patients are woefully scarce. While amyloid-targeting therapies like lecanemab or donanemab have brought hope for AD modification through immunoprevention they are incredibly costly at tens of thousands, yearly [47]. Emerging research highlights known interventions including methylene blue, ketones, and near-infrared light for addressing mitochondrial dysfunction and lifestyle modification for improvements in vascular health.
Methylene Blue: A Mitochondrial Rescue Agent
Building on the notion that Alzheimer’s disease (AD) arises from fundamental metabolic deficits, Dr. Francisco Gonzalez-Lima’s research highlights how methylene blue (MB) can counteract hypometabolism at the precise stage where cytochrome c oxidase (CO) activity falters. Previous findings show that CO deficits lead to lower ATP production and impaired neuronal function—particularly in high-demand regions like the posterior cingulate cortex (PCC). By serving as an alternative electron carrier in the mitochondrial electron transport chain (ETC), MB helps bridge the energy gap caused by diminished CO function [48].
Imagine the ETC as a factory assembly line, where each station (Complex I, II, III, IV/CO) relies on receiving and passing along “electron components” to ultimately produce ATP. If the last station, CO (Complex IV), slows down or malfunctions, the entire line bogs down, creating an energy bottleneck. Methylene blue acts like an extra forklift that can carry the electron components directly to where they’re needed, bypassing the inefficiency and keeping production running smoothly. This maintains adequate ATP output—even when CO is underperforming.
In low doses (0.5–4 mg/kg), MB not only donates electrons to the ETC but also increases cellular oxygen consumption by 37–70% and can boost ATP production by up to 30% in human cell lines and animal models [49,50]. Crucially, functional magnetic resonance imaging (fMRI) studies show that low-dose MB helps reduce oxygen to water, supporting metabolic function in both conditions with sufficient oxygen and hypoxic conditions [51].
- Hypoxic conditions indicate reduced or inadequate oxygen availability, which is highly relevant in AD where impaired vascular function and diminished blood flow can deprive neurons of the oxygen they require.
Within the vascular-hypometabolic framework, neurons experiencing poor perfusion operate closer to a hypoxic threshold. When CO underperforms, these cells cannot fully capitalize on what little oxygen is available—further worsening energy deficits. MB’s unique capacity to maintain mitochondrial function in both ample and low-oxygen scenarios means it could play a critical compensatory role in AD, where chronic hypoperfusion is common. By improving oxygen utilization, MB may help prevent the deleterious spiral of failing energy metabolism that underlies cognitive decline and other clinical manifestations of the disease.
Some findings indicate that MB-driven increases in oxygen consumption can prompt a brief drop in local oxygen levels—often termed transient local hypoxia—which triggers cytochrome c oxidase (CO) to shift from reducing oxygen to producing nitric oxide (NO) [52]. NO is a powerful vasodilator that relaxes vascular smooth muscle, boosting cerebral blood flow and glucose uptake [53]. Mechanistically, as CO accelerates electron transport and oxygen use, local tissue first experiences lowered oxygen partial pressure. Sensing this change, CO acts as a signal transducer to generate NO, which then activates downstream pathways (e.g., cGMP), causing vessel dilation to meet the heightened metabolic demand.
This vascular benefit dovetails with the vascular-hypometabolic framework of AD, where chronic reductions in cerebral perfusion and energy deficits perpetuate cognitive decline. By restoring CO function (and thereby ATP production) while also inducing vasodilation via NO, MB addresses two principal obstacles in AD: impaired mitochondrial capacity and inadequate blood flow. Consequently, MB may help alleviate hypoperfusion, improve nutrient delivery, and slow the progressive cascade of neurodegeneration observed in AD and other conditions marked by mitochondrial dysfunction and vascular insufficiency.
Ketones: Alternative Fuel For Closing Glucose Energy Gap
Having established how methylene blue can bolster mitochondrial function by enhancing the electron transport chain (ETC), we now turn to another metabolic strategy that can potentially offset diminished energy production in Alzheimer’s disease (AD): ketone utilization.
Under normal circumstances, the adult brain derives approximately 95% of its energy from glucose through oxidative metabolism. However, in AD, glucose uptake and glycolytic efficiency can be significantly impaired due to factors like insulin resistance and neuronal metabolic dysfunction [59]. When cells cannot efficiently metabolize glucose, ATP production drops, promoting oxidative stress and neuronal damage. In such scenarios, the brain can turn to alternative energy substrates, including ketones (primarily β-hydroxybutyrate and acetoacetate) and, to a lesser extent, lactate [54, 55].
Numerous studies have reported metabolic recovery and neuroprotective effects from ketogenic diets or exogenous ketone supplements [56,57]. By increasing the availability of ketones—often considered a more “efficient” fuel—neurons impacted by glucose hypometabolism can tap into a readily oxidized energy source that demands less oxygen per unit of ATP generated [59].
When glucose metabolism falters due to insulin resistance or other disruptions in the AD brain, glycolysis (the sequence of reactions converting glucose into pyruvate) becomes less effective. This shortfall reduces substrate flow into the tricarboxylic acid (TCA) cycle, compromising ATP generation in the mitochondrial ETC. Ketones, however, are metabolized differently. After crossing the blood-brain barrier, they can be converted to acetyl-CoA in mitochondria, thereby bypassing several enzymatic steps required for glucose breakdown. This direct funneling into the TCA cycle helps sustain ATP production, even under conditions of impaired glycolysis [59].
Dr. Stephen Cunnane has extensively investigated how ketones serve as an alternative energy source for individuals with cognitive impairments, including mild cognitive impairment (MCI) and AD [54,57,58,59]. He characterizes ketones as an “energy rescue” for neuronal cells that can no longer obtain sufficient ATP from glucose metabolism. By bridging this gap, ketones potentially ameliorate hallmark features of neurodegeneration in AD [59]. In addition:
- Ketones can reduce reactive oxygen species (ROS) by improving the efficiency of mitochondrial respiration [54].
- Abundant ketone availability ensures the brain can meet most of its energy demands, effectively compensating for glucose deficits [59].
Given ketones’ ability to supply an alternative fuel and MB’s role in enhancing ETC efficiency, Dr. Gonzalez-Lima hypothesizes complementary or even additive effects when these interventions are combined [60]. While MB tackles enzymatic bottlenecks in the ETC—particularly cytochrome c oxidase (CO) dysfunction—ketones supply alternative substrates that bypass impaired glycolytic pathways. Integrating both approaches could ultimately reduce the high metabolic vulnerability seen in AD neurons by addressing multiple points in the energy-production chain.
Near-Infrared Light a Targeted Approach
Having explored how methylene blue (MB) improves electron transport chain (ETC) function and how ketones offer a vital alternate fuel in AD, we now turn to a third metabolic approach: near-infrared (NIR) light therapy. This technique, commonly delivered through low-power lasers or light-emitting diodes (LEDs), relies on photoneuromodulation—the utilization of specific light wavelengths to activate cytochrome c oxidase (CO) and bolster neuronal ATP production [60,61]. By directly enhancing mitochondrial performance, NIR therapy targets the vascular-hypometabolic deficits characteristic of Alzheimer’s disease.
Mechanism of Photostimulation: How Photon Donation Works
NIR wavelengths between approximately 600 and 1150 nm can traverse the skull and interact directly with CO, the rate-limiting enzyme in mitochondrial oxidative phosphorylation [61, 62]. Unlike MB—which donates electrons—NIR donates photons, or discrete energy packets from the electromagnetic spectrum. When these photons are absorbed by the metal centers (e.g., copper and heme groups) within CO, the enzyme’s conformation changes, lowering the activation energy for converting oxygen to water. This, in turn:
- Enhances Oxygen Consumption – The excited CO more readily binds and reduces oxygen, accelerating electron flow through the ETC.
- Boosts ATP Production – Because oxidative phosphorylation hinges on electron flow, increased activity at CO translates into higher ATP yields.
By bypassing the need for electrons solely derived from food substrates, NIR can photostimulate CO even in cells with partial ETC blockages—a hallmark of the metabolic bottlenecks seen in AD. As Dr. Gonzalez-Lima notes, this heightened enzymatic reaction leads to increased oxygen consumption and upregulated ATP production, potentially reversing or mitigating the energetic deficits at the core of AD’s hypometabolism [62]. Lasting Metabolic Benefits
NIR’s effects extend beyond an immediate boost in oxygen consumption. Studies show that light exposure triggers enzymatic induction, whereby gene expression and protein synthesis elevate CO levels over time [62]. Rodent experiments have demonstrated significantly higher CO concentrations in brain tissue one day after a single session, and human participants can experience behavioral improvements persisting for 2–4 weeks post-treatment. This phenomenon implies that near-infrared not only addresses acute energy shortfalls but also creates a sustained upregulation of the cell’s oxidative capacity and cerebral blood flow [62]
Relevance for AD and Synergy with Other Interventions
NIR therapy directly tackles the hypoperfusion and diminished ATP production central to AD’s vascular-hypometabolic pathology. The potential synergy with MB is especially noteworthy: while MB donates electrons to stabilize the ETC, NIR donates photons, further fueling CO activity and enhancing neuronal energy metabolism [63]. Meanwhile, ketones complement these mechanisms by bypassing impaired glycolysis and offering a readily oxidized substrate for ATP generation. Together, these interventions could help interrupt the downward spiral of insufficient blood flow, oxidative stress, and energy deficits that drives disease progression in AD.
Novel synergistic, evidence-based interventions have potential to outperform many available AD medications. Francisco Gonzalez-Lima remains on the cutting edge contributing potent and unique research data for addressing the root cause of AD. Together MB, ketones, and NIR offer a promising multimodal strategy for addressing the metabolic failures underlying AD progression in mitochondria. There is great potential that some of these strategies will extend beyond AD to several neurological diseases.
Conclusion
Alzheimer’s disease has long been characterized by cellular or structural changes associated with amyloid plaques and neurofibrillary tangles. Alzheimer's disease (AD) has many characterized abnormalities including histopathological, molecular, and biochemical abnormalities. Consequently, research and targeting the root cause of AD to improve human outcomes is a complex field of scientific investigation. Francisco Gonzalez-Lima's research offers a transformative perspective on Alzheimer's disease (AD), adding clarity, focusing on mitochondrial dysfunction and cerebral hypometabolism as critical drivers of neurodegeneration.
Gonzalez-Lima's pioneering work also explores novel interventions like methylene blue, ketones, and near-infrared light therapy, which have been shown to have a restorative impact on mitochondrial function in brain cells. The complementary approaches detailed in this review in combination with additional lifestyle habits such as exercise are likely to gain traction as they increase agency for individuals who aim to address AD directly by improving vascular health and enhancing metabolic function.
- Manly, J. J., Jones, R. N., Langa, K. M., Ryan, L. H., Levine, D. A., McCammon, R., Heeringa, S. G., & Weir, D. (2022). Estimating the Prevalence of Dementia and Mild Cognitive Impairment in the US: The 2016 Health and Retirement Study Harmonized Cognitive Assessment Protocol Project. JAMA neurology, 79(12), 1242–1249. https://doi.org/10.1001/jamaneurol.2022.3543
- Kciuk, M., Kruczkowska, W., Gałęziewska, J., Wanke, K., Kałuzińska-Kołat, Ż., Aleksandrowicz, M., & Kontek, R. (2024). Alzheimer's Disease as Type 3 Diabetes: Understanding the Link and Implications. International journal of molecular sciences, 25(22), 11955. https://doi.org/10.3390/ijms252211955
- Peng, Y., Yao, S. Y., Chen, Q., Jin, H., Du, M. Q., Xue, Y. H., & Liu, S. (2024). True or false? Alzheimer's disease is type 3 diabetes: Evidences from bench to bedside. Ageing research reviews, 99, 102383. https://doi.org/10.1016/j.arr.2024.102383
- 2024 Alzheimer's disease facts and figures. (2024). Alzheimer's & dementia : the journal of the Alzheimer's Association, 20(5), 3708–3821. https://doi.org/10.1002/alz.13809
- Vitali, F., Branigan, G. L., & Brinton, R. D. (2021). Preventing Alzheimer's disease within reach by 2025: Targeted-risk-AD-prevention (TRAP) strategy. Alzheimer's & dementia (New York, N. Y.), 7(1), e12190. https://doi.org/10.1002/trc2.12190
- Ma, C., Hong, F., & Yang, S. (2022). Amyloidosis in Alzheimer's Disease: Pathogeny, Etiology, and Related Therapeutic Directions. Molecules (Basel, Switzerland), 27(4), 1210. https://doi.org/10.3390/molecules27041210
- de la Torre J. (2018). The Vascular Hypothesis of Alzheimer's Disease: A Key to Preclinical Prediction of Dementia Using Neuroimaging. Journal of Alzheimer's disease : JAD, 63(1), 35–52. https://doi.org/10.3233/JAD-180004
- Mendez M. F. (2017). Early-Onset Alzheimer Disease. Neurologic clinics, 35(2), 263–281. https://doi.org/10.1016/j.ncl.2017.01.005
- Goedert M. (2009). Oskar Fischer and the study of dementia. Brain : a journal of neurology, 132(Pt 4), 1102–1111. https://doi.org/10.1093/brain/awn256
- Eriksson, H., Fereshtehnejad, S. M., Falahati, F., Farahmand, B., Religa, D., & Eriksdotter, M. (2014). Differences in routine clinical practice between early and late onset Alzheimer's disease: data from the Swedish Dementia Registry (SveDem). Journal of Alzheimer's disease : JAD, 41(2), 411–419. https://doi.org/10.3233/JAD-132273
- Liu, PP., Xie, Y., Meng, XY. et al. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Sig Transduct Target Ther 4, 29 (2019). https://doi.org/10.1038/s41392-019-0063-8
- Müller, U., Winter, P., & Graeber, M. B. (2013). A presenilin 1 mutation in the first case of Alzheimer's disease. The Lancet. Neurology, 12(2), 129–130. https://doi.org/10.1016/S1474-4422(12)70307-1
- Ertekin-Taner N. (2007). Genetics of Alzheimer's disease: a centennial review. Neurologic clinics, 25(3), 611–v. https://doi.org/10.1016/j.ncl.2007.03.009
- Polsinelli, A. J., Lane, K. A., Manchella, M. K., Logan, P. E., Gao, S., & Apostolova, L. G. (2023). APOE ε4 is associated with earlier symptom onset in LOAD but later symptom onset in EOAD. Alzheimer's & dementia : the journal of the Alzheimer's Association, 19(5), 2212–2217. https://doi.org/10.1002/alz.12955
- Huynh, T. V., Davis, A. A., Ulrich, J. D., & Holtzman, D. M. (2017). Apolipoprotein E and Alzheimer's disease: the influence of apolipoprotein E on amyloid-β and other amyloidogenic proteins. Journal of lipid research, 58(5), 824–836. https://doi.org/10.1194/jlr.R075481
- Clemerson, G., Walsh, S., & Isaac, C. (2014). Towards living well with young onset dementia: An exploration of coping from the perspective of those diagnosed. Dementia (London, England), 13(4), 451–466. https://doi.org/10.1177/1471301212474149
- Ducharme, F., Kergoat, M. J., Antoine, P., Pasquier, F., & Coulombe, R. (2013). The unique experience of spouses in early-onset dementia. American journal of Alzheimer's disease and other dementias, 28(6), 634–641. https://doi.org/10.1177/1533317513494443
- Rosness, T. A., Barca, M. L., & Engedal, K. (2010). Occurrence of depression and its correlates in early onset dementia patients. International journal of geriatric psychiatry, 25(7), 704–711. https://doi.org/10.1002/gps.2411
- Reitz, C., Rogaeva, E., & Beecham, G. W. (2020). Late-onset vs nonmendelian early-onset Alzheimer disease: A distinction without a difference?. Neurology. Genetics, 6(5), e512. https://doi.org/10.1212/NXG.0000000000000512
- Rabinovici G. D. (2019). Late-onset Alzheimer Disease. Continuum (Minneapolis, Minn.), 25(1), 14–33. https://doi.org/10.1212/CON.0000000000000700
- Solis, E., Jr., Hascup, K. N., & Hascup, E. R. (2020). Alzheimer's Disease: The Link Between Amyloid-β and Neurovascular Dysfunction. Journal of Alzheimer's disease : JAD, 76(4), 1179–1198. https://doi.org/10.3233/JAD-200473
- Iacono, D., Markesbery, W. R., Gross, M., Pletnikova, O., Rudow, G., Zandi, P., & Troncoso, J. C. (2009). The Nun study: clinically silent AD, neuronal hypertrophy, and linguistic skills in early life. Neurology, 73(9), 665–673. https://doi.org/10.1212/WNL.0b013e3181b01077
- Snowdon D. A. (1997). Aging and Alzheimer's disease: lessons from the Nun Study. The Gerontologist, 37(2), 150–156. https://doi.org/10.1093/geront/37.2.150
- Clarke, K. M., Etemadmoghadam, S., Danner, B., Corbett, C., Ghaseminejad-Bandpey, A., Dopler, M., Parker-Garza, J., Alhneif, M., Babu, S., Ogunbona, O. B., Gonzalez, A. D., Salardini, A., & Flanagan, M. E. (2025). The Nun Study: Insights from 30 years of aging and dementia research. Alzheimer's & dementia : the journal of the Alzheimer's Association, 21(2), e14626. https://doi.org/10.1002/alz.14626
- Raut, S., Bhalerao, A., Powers, M., Gonzalez, M., Mancuso, S., & Cucullo, L. (2023). Hypometabolism, Alzheimer's Disease, and Possible Therapeutic Targets: An Overview. Cells, 12(16), 2019. https://doi.org/10.3390/cells12162019
- de la Torre J. (2018). The Vascular Hypothesis of Alzheimer's Disease: A Key to Preclinical Prediction of Dementia Using Neuroimaging. Journal of Alzheimer's disease : JAD, 63(1), 35–52. https://doi.org/10.3233/JAD-180004
- Iadecola, C., Duering, M., Hachinski, V., Joutel, A., Pendlebury, S. T., Schneider, J. A., & Dichgans, M. (2019). Vascular Cognitive Impairment and Dementia: JACC Scientific Expert Panel. Journal of the American College of Cardiology, 73(25), 3326–3344. https://doi.org/10.1016/j.jacc.2019.04.034
- Eisenmenger, L. B., Peret, A., Famakin, B. M., Spahic, A., Roberts, G. S., Bockholt, J. H., Johnson, K. M., & Paulsen, J. S. (2023). Vascular contributions to Alzheimer's disease. Translational research : the journal of laboratory and clinical medicine, 254, 41–53. https://doi.org/10.1016/j.trsl.2022.12.003
- Zlokovic B. V. (2011). Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nature reviews. Neuroscience, 12(12), 723–738. https://doi.org/10.1038/nrn3114
- Ruitenberg, A., den Heijer, T., Bakker, S. L., van Swieten, J. C., Koudstaal, P. J., Hofman, A., & Breteler, M. M. (2005). Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Annals of neurology, 57(6), 789–794. https://doi.org/10.1002/ana.20493
- Iturria-Medina, Y., Sotero, R. C., Toussaint, P. J., Mateos-Pérez, J. M., Evans, A. C., & Alzheimer’s Disease Neuroimaging Initiative (2016). Early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis. Nature communications, 7, 11934. https://doi.org/10.1038/ncomms11934
- Valla, J., Berndt, J. D., & Gonzalez-Lima, F. (2001). Energy hypometabolism in posterior cingulate cortex of Alzheimer's patients: superficial laminar cytochrome oxidase associated with disease duration. The Journal of neuroscience : the official journal of the Society for Neuroscience, 21(13), 4923–4930. https://doi.org/10.1523/JNEUROSCI.21-13-04923.2001
- Gonzalez-Lima, F., & Jones, D. (1994). Quantitative mapping of cytochrome oxidase activity in the central auditory system of the gerbil: a study with calibrated activity standards and metal-intensified histochemistry. Brain research, 660(1), 34–49. https://doi.org/10.1016/0006-8993(94)90836-2
- Gonzalez-Lima, F., Valla, J., & Matos-Collazo, S. (1997). Quantitative cytochemistry of cytochrome oxidase and cellular morphometry of the human inferior colliculus in control and Alzheimer's patients. Brain research, 752(1-2), 117–126. https://doi.org/10.1016/s0006-8993(96)01464-3
- Parker, W. D., Jr, Parks, J., Filley, C. M., & Kleinschmidt-DeMasters, B. K. (1994). Electron transport chain defects in Alzheimer's disease brain. Neurology, 44(6), 1090–1096. https://doi.org/10.1212/wnl.44.6.1090
- Parker, W. D., Jr, Filley, C. M., & Parks, J. K. (1990). Cytochrome oxidase deficiency in Alzheimer's disease. Neurology, 40(8), 1302–1303. https://doi.org/10.1212/wnl.40.8.1302
- Wang, W., Zhao, F., Ma, X., Perry, G., & Zhu, X. (2020). Mitochondria dysfunction in the pathogenesis of Alzheimer's disease: recent advances. Molecular neurodegeneration, 15(1), 30. https://doi.org/10.1186/s13024-020-00376-6
- Richter, O. M., & Ludwig, B. (2003). Cytochrome c oxidase--structure, function, and physiology of a redox-driven molecular machine. Reviews of physiology, biochemistry and pharmacology, 147, 47–74. https://doi.org/10.1007/s10254-003-0006-0
- Agarwal, P., Ford, C. N., Leurgans, S. E., Beck, T., Desai, P., Dhana, K., Evans, D. A., Halloway, S., Holland, T. M., Krueger, K. R., Liu, X., Rajan, K. B., & Bennett, D. A. (2023). Dietary Sugar Intake Associated with a Higher Risk of Dementia in Community-Dwelling Older Adults. Journal of Alzheimer's disease : JAD, 95(4), 1417–1425. https://doi.org/10.3233/JAD-230013
- Nalivaeva, N. N., & Rybnikova, E. A. (2023). Editorial: Brain hypoxia and ischemia: New insights into neurodegeneration and neuroprotection, volume II. Frontiers in neuroscience, 16, 1125883. https://doi.org/10.3389/fnins.2022.1125883
- Rae, C. D., Baur, J. A., Borges, K., Dienel, G., Díaz-García, C. M., Douglass, S. R., Drew, K., Duarte, J. M. N., Duran, J., Kann, O., Kristian, T., Lee-Liu, D., Lindquist, B. E., McNay, E. C., Robinson, M. B., Rothman, D. L., Rowlands, B. D., Ryan, T. A., Scafidi, J., Scafidi, S., … McKenna, M. C. (2024). Brain energy metabolism: A roadmap for future research. Journal of neurochemistry, 168(5), 910–954. https://doi.org/10.1111/jnc.16032
- Clarke DD, Sokoloff L. Substrates of Cerebral Metabolism. In: Siegel GJ, Agranoff BW, Albers RW, et al., editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Philadelphia: Lippincott-Raven; 1999. Available from: https://www.ncbi.nlm.nih.gov/books/NBK28048/
- Xue, M., Xu, W., Ou, Y. N., Cao, X. P., Tan, M. S., Tan, L., & Yu, J. T. (2019). Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing research reviews, 55, 100944. https://doi.org/10.1016/j.arr.2019.100944
- MacKnight, C., Rockwood, K., Awalt, E., & McDowell, I. (2002). Diabetes mellitus and the risk of dementia, Alzheimer's disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dementia and geriatric cognitive disorders, 14(2), 77–83. https://doi.org/10.1159/000064928
- de la Monte, S. M., & Wands, J. R. (2008). Alzheimer's disease is type 3 diabetes-evidence reviewed. Journal of diabetes science and technology, 2(6), 1101–1113. https://doi.org/10.1177/193229680800200619
- Hallberg, S. J., Gershuni, V. M., Hazbun, T. L., & Athinarayanan, S. J. (2019). Reversing Type 2 Diabetes: A Narrative Review of the Evidence. Nutrients, 11(4), 766. https://doi.org/10.3390/nu11040766
- Terao, I., & Kodama, W. (2024). Comparative efficacy, tolerability and acceptability of donanemab, lecanemab, aducanumab and lithium on cognitive function in mild cognitive impairment and Alzheimer's disease: A systematic review and network meta-analysis. Ageing research reviews, 94, 102203. https://doi.org/10.1016/j.arr.2024.102203
- Gonzalez-Lima, F., & Auchter, A. (2015). Protection against neurodegeneration with low-dose methylene blue and near-infrared light. Frontiers in cellular neuroscience, 9, 179. https://doi.org/10.3389/fncel.2015.00179
- Xue, H., Thaivalappil, A., & Cao, K. (2021). The Potentials of Methylene Blue as an Anti-Aging Drug. Cells, 10(12), 3379. https://doi.org/10.3390/cells10123379
- Atamna, H., Nguyen, A., Schultz, C., Boyle, K., Newberry, J., Kato, H., & Ames, B. N. (2008). Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 22(3), 703–712. https://doi.org/10.1096/fj.07-9610com
- Huang, S., Du, F., Shih, Y. Y., Shen, Q., Gonzalez-Lima, F., & Duong, T. Q. (2013). Methylene blue potentiates stimulus-evoked fMRI responses and cerebral oxygen consumption during normoxia and hypoxia. NeuroImage, 72, 237–242. https://doi.org/10.1016/j.neuroimage.2013.01.027
- Poyton, R. O., & Ball, K. A. (2011). Therapeutic photobiomodulation: nitric oxide and a novel function of mitochondrial cytochrome c oxidase. Discovery medicine, 11(57), 154–159.
- Lin, A. L., Poteet, E., Du, F., Gourav, R. C., Liu, R., Wen, Y., Bresnen, A., Huang, S., Fox, P. T., Yang, S. H., & Duong, T. Q. (2012). Methylene blue as a cerebral metabolic and hemodynamic enhancer. PloS one, 7(10), e46585. https://doi.org/10.1371/journal.pone.0046585
- Cunnane, S. C., Courchesne-Loyer, A., Vandenberghe, C., St-Pierre, V., Fortier, M., Hennebelle, M., Croteau, E., Bocti, C., Fulop, T., & Castellano, C. A. (2016). Can Ketones Help Rescue Brain Fuel Supply in Later Life? Implications for Cognitive Health during Aging and the Treatment of Alzheimer's Disease. Frontiers in molecular neuroscience, 9, 53. https://doi.org/10.3389/fnmol.2016.00053
- Magistretti, P. J., & Allaman, I. (2018). Lactate in the brain: from metabolic end-product to signalling molecule. Nature reviews. Neuroscience, 19(4), 235–249. https://doi.org/10.1038/nrn.2018.19
- Rusek, M., Pluta, R., Ułamek-Kozioł, M., & Czuczwar, S. J. (2019). Ketogenic Diet in Alzheimer's Disease. International journal of molecular sciences, 20(16), 3892. https://doi.org/10.3390/ijms20163892
- Myette-Côté, É., Soto-Mota, A., & Cunnane, S. C. (2022). Ketones: potential to achieve brain energy rescue and sustain cognitive health during ageing. The British journal of nutrition, 128(3), 407–423. https://doi.org/10.1017/S0007114521003883
- Fortier, M., Castellano, C. A., St-Pierre, V., Myette-Côté, É., Langlois, F., Roy, M., Morin, M. C., Bocti, C., Fulop, T., Godin, J. P., Delannoy, C., Cuenoud, B., & Cunnane, S. C. (2021). A ketogenic drink improves cognition in mild cognitive impairment: Results of a 6-month RCT. Alzheimer's & dementia : the journal of the Alzheimer's Association, 17(3), 543–552. https://doi.org/10.1002/alz.12206
- Cunnane, S. C., Trushina, E., Morland, C., Prigione, A., Casadesus, G., Andrews, Z. B., Beal, M. F., Bergersen, L. H., Brinton, R. D., de la Monte, S., Eckert, A., Harvey, J., Jeggo, R., Jhamandas, J. H., Kann, O., la Cour, C. M., Martin, W. F., Mithieux, G., Moreira, P. I., Murphy, M. P., … Millan, M. J. (2020). Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nature reviews. Drug discovery, 19(9), 609–633. https://doi.org/10.1038/s41573-020-0072-x
- Gonzalez-Lima, F., Barksdale, B. R., & Rojas, J. C. (2014). Mitochondrial respiration as a target for neuroprotection and cognitive enhancement. Biochemical pharmacology, 88(4), 584–593. https://doi.org/10.1016/j.bcp.2013.11.010
- Morse, P. T., Goebel, D. J., Wan, J., Tuck, S., Hakim, L., Hüttemann, C. L., Malek, M. H., Lee, I., Sanderson, T. H., & Hüttemann, M. (2021). Cytochrome c oxidase-modulatory near-infrared light penetration into the human brain: Implications for the noninvasive treatment of ischemia/reperfusion injury. IUBMB life, 73(3), 554–567. https://doi.org/10.1002/iub.2405
- Cardoso, F. D. S., Barrett, D. W., Wade, Z., Gomes da Silva, S., & Gonzalez-Lima, F. (2022). Photobiomodulation of Cytochrome c Oxidase by Chronic Transcranial Laser in Young and Aged Brains. Frontiers in neuroscience, 16, 818005. https://doi.org/10.3389/fnins.2022.818005
- Gonzalez-Lima, F., & Auchter, A. (2015). Protection against neurodegeneration with low-dose methylene blue and near-infrared light. Frontiers in cellular neuroscience, 9, 179. https://doi.org/10.3389/fncel.2015.00179