Short term treatment with a cocktail of rapamycin, acarbose and phenylbutyrate delays aging phenotypes in mice
Abstract
Pharmaceutical interventions otherwise known as the use of drug-based interventions with regards to aging requires targeting multiple pathways, thus there is rationale to test combinations of drugs targeting different but overlapping processes. A multifaceted approach to treatment of aging in order to determine if combining drugs shown to extend lifespan and healthy aging in mice would have greater impact than any individual drug, a cocktail diet containing 14 ppm rapamycin, 1000 ppm acarbose, and 1000 ppm phenylbutyrate was fed to 20-month-old C57BL/6 and HET3 4-way cross mice of both sexes for three months. Mice treated with the cocktail showed a sex and strain-dependent phenotype consistent with healthy aging including decreased body fat, improved cognition, increased strength and endurance, and decreased age-related pathology compared to mice treated with individual drugs or control. The severity of age-related lesions in heart, lungs, liver, and kidney was consistently decreased in mice treated with the cocktail compared to mice treated with individual drugs or control, suggesting an interactive advantage of the three drugs. Please note that lesions are defined as a region in an organ or tissue which has suffered damage through injury or disease, such as a wound, ulcer, abscess, or tumor. Not in so many words a lesion is damage to an organ or tissue.This study shows that a combination of three drugs, each previously shown to enhance lifespan and health span in mice, is able to delay aging phenotypes in middle-aged mice more effectively than any individual drug in the cocktail over a 3-month treatment period.
Introduction
Aging is a complex process generally associated with multiple biological pathways1,2. Some of these pathways have been targeted by individual drugs resulting in increased lifespan or enhanced healthy aging in mice3. Therefore if multiple pathways of aging are involved in the aging process and the development of age-related diseases, then targeting more than one pathway should have additional benefits with regards to an organism's healthspan. One approach to accomplish this would be to develop combinations or cocktails of drugs that individually have been shown to attenuate or stave off the dysfunction and pathology seen with increasing age4. We selected a drug cocktail of rapamycin (Rap), acarbose (Acb), and phenylbutyrate (Pba) to test this concept in aging mice. The rationale for the drug cocktail is based on validated anti-aging effects of the individual drugs chosen with each targeting different but overlapping pathways of aging.
As we know Rap is an antibiotic clinically approved for treating organ transplant patients5,6 and neoplastic conditions in addition to its newly discovered anti-aging properties. Harrison et al paved the way for a fusillade of follow up studies to their groundbreaking work determining Rap’s anti-aging properties. Continuing on Rap is orally bioavailable and readily crosses the blood brain barrier. We know that Rap specifically inhibits mTOR signaling which plays a significant role in integrating signals from growth factors and nutrients to control protein synthesis. The effect of down regulating mTOR on aging was confirmed by the Intervention Testing Program (funded by National Institute on Aging) showing that rapamycin extends lifespan in mice7. It has also been shown to delay age-associated heart disease and other pathologies in mice8,9,10. Transient Rap treatment has been shown to increase lifespan and healthspan in middle age mice11. The second drug in the cocktail, Acb, is a popular type 2 diabetes medication used for glucoregulatory control12. Considering that one of the hallmarks of caloric restriction (CR) is improved glucose homeostasis and insulin sensitivity13, the similarities between CR and Acb treatment underscore the potential metabolic responses that are present in conditions of improved aging. Like CR, Acb reduces body weight and body fat, improves glucose dysregulation associated with aging, and increases mouse lifespan14. The third drug in the cocktail, Pba, is a derivative of the short chain fatty acid butyrate, which occurs naturally in the gut. It is orally bioavailable and can be detected in the blood stream within 15 min of oral administration, and organs within an hour. We have shown that Pba can enhance physical and cognitive performance with increasing age in mice15. So in essence, administration of all three drugs in a cocktail will improve the healthspan of an organism.
Lifespan extension, which involves starting at a young age through end of life, has been a gold standard in testing anti-aging effects of drugs in preclinical animal models. While this approach provides valuable information, it has less translational value compared to studies starting at midlife for short periods of time with age-specific time points. These types of short-term studies have been reported in the mouse, a very popular preclinical model for aging research. Middle-aged C57BL/6 (B6) mice are available from an NIH supported aging rodent colony for grantees funded by NIH so are a ready source of mice to use in short term drug intervention studies. The strain is well characterized in age-related phenotypes using physiological performance tests and geropathological assessment. Please note that phenotype is defined as a set of observable physical characteristics or measurable properties of an individual resulting from the interaction of its genotype/genetic makeup with the environment such as hair color or blood platelet levels. Since testing a three-drug combination is a novel approach, a second strain would help validate drug effects on aging parameters seen in B6 mice. The HET3 4-way cross mouse strain is used exclusively by the Intervention Testing Program to test a large number of drugs, including the three drugs in our cocktail, in lifespan studies. Therefore, it would be a useful strain to help validate the effects of the cocktail on aging parameters in B6 mice.
In addition to using physiological performance tests as endpoints for aging parameters, we used an anatomic pathology-based assessment system designated as geropathology10. The geropathology system allows an in depth look at age-related pathological phenotypes in a quantitative-like manner. A grading platform has been established for mice, which generates lesion scores for individual organs thereby identifying which organs are more or less responsive to short-term drug treatment16. The system also identifies drug-responsive age-related lesions thus providing additional evidence of the impact of drug treatment on aging. A good example is inflammation, which is readily recognized and easily graded using routine stains of thin tissue sections on glass slides. Inflammation is considered one of the pathways of aging1 so would be of interest for further investigation in a drug study for the types of genes involved using transcriptomic and molecular applications.
Jiang et al posited that the drug cocktail would be successful in delaying aging phenotypes because it would target not just inflammation, but more aging processes than any individual drug. For example, Rap targets autophagy and vascular deficits by downregulating mTOR signaling17. Please note that autophagy is the body's way of cleaning out damaged cells, in order to regenerate newer, healthier cells. Additionally vascular deficits lead to vascular cognitive impairment which is a disorder of the mind in which the mental ability to be aware, to think and to feel is progressively inhibited. Vascular cognitive impairment symptoms can range from forgetfulness to more serious problems with attention, memory, language, and executive functions like problem solving. Acb indirectly targets insulin signaling, mitochondrial dysfunction and oxidative stress by blocking breakdown of complex carbohydrates in the small intestine so that less glucose is available for systemic absorption18. Pba targets dysfunctional proteostasis through the endoplasmic reticulum stress response and targets epigenetic function by inhibiting histone deacetylation resulting in upregulation of genes including anti-inflammatory genes19,20. Please note proteostasis is the process that regulates proteins within the cell in order to maintain the health of both the cellular proteome and the organism itself. Additionally epigenetics is the study of how your behaviors and environment can cause changes that affect the way your genes work. Jiang et al will report here that the drug cocktail delays aging phenotypes in B6 and HET3 mice with several strain and sex dependent differences.
Results
Health enhancing effects of the drug cocktail were dose dependent
The main objective of the study was to show that a cocktail of drugs each shown to have anti-aging effects in mice would be more effective in delaying aging phenotypes than any individual drug within the combination. The dose of each drug used in the cocktail was based on published studies7,14,15, but since each drug had not been used in this type of combination, there was a need to compare a different dose. Therefore, we tested a cocktail with a half dose of each drug compared to the cocktail with a full dose and control in C57BL/6 mice for three months starting at 20 months of age to 23 months of age. Mice fed the full dose cocktail or half dose cocktail through the 3-month treatment period showed generally similar biological changes (Fig. S1) suggesting the lower dose had a biological effect. Both males and females performed better in the spatial learning task when fed the full dose cocktail or the half dose cocktail chow compared to mice fed the control chow (Fig. S2A). The half dose cocktail was less effective than the full dose cocktail in both sexes performing rotarod grip strength tasks (Fig. S2B, C). The half dose cocktail was not nearly as effective in decreasing age-related lesions as the full dose cocktail, but there were some sex dependent exceptions (Fig. S3). The data showed a pattern of the half dose cocktail that was similar to the full dose cocktail, but less effective, helping validate an effective dose for this study. All subsequent data in this report are from mice fed full dose cocktail, or full dose for each individual drug, added to the chow.
C57BL/6 mice receiving the drug cocktail showed decreased body weight and improved performance
Over the 3-month treatment period, C57BL/6 mice fed the drug cocktail chow showed a decrease in body weight as early as the second month compared to mice fed the control chow, which continued through the third month for males but not females (Fig. 1A,B). For mice receiving individual drug chow, there were sex differences in the Rap and Pba cohorts, with no weight loss in females. The decrease in body fat mass paralleled the decrease in body weight (Fig. 1C,D). We measured 3-day food intake once a month and there was no significant difference in amount of food ingested among the cohorts in either males or females (Fig. 1E,F) indicating caloric intake was not a likely factor in compromising phenotypes effected by the medications in the chow. There were no significant differences in changes in lean muscle mass or blood glucose levels in any of the cohorts over the three-month treatment period (data not shown).
Figure 1
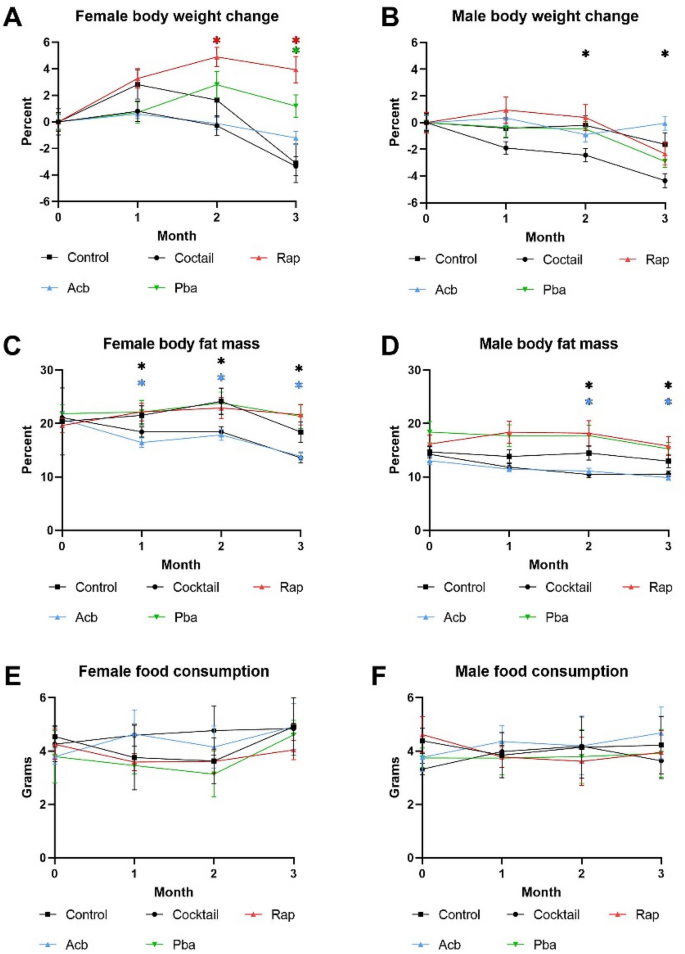
Biological parameters were assessed monthly in C57BL/6 mice fed drug cocktail chow or chow with each individual drug or control for three months starting at 20 months of age. (A) Body weight in females, (B)body weight in males, (C) body fat mass measured by magnetic resonance imaging in females, (D) body fat mass measured by magnetic resonance imaging in males, (E) food intake measured over 3 days each month for females, (F) food intake measured over 3 days each month for males. N = 12–14/cohort. *p ≤ 0.05, ANOVA with Tukey’s post-hoc comparison and two-tailed, unpaired Student’s t-test.
Mice of both sexes fed the cocktail chow were better in finding the escape hole in the spatial learning task compared to mice fed control chow or chow with Acb or Pba (Fig. 2A). However, both male and female mice fed the Rap chow performed just as well as mice fed the cocktail chow. In general, both male and female mice fed the cocktail chow outperformed mice fed individual drug chow in rotarod and grip strength tasks (Fig. 2B,C).
Figure 2
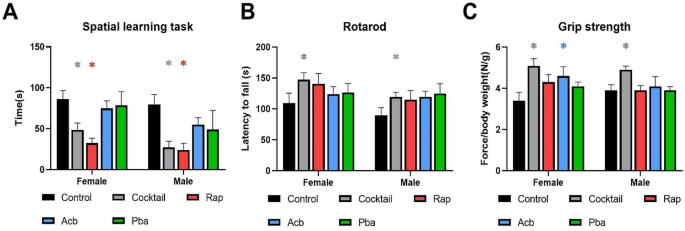
Performance tests were conducted in C57BL/6 mice at 23 months of age, three months after being started on chow containing the drug cocktail or placebo. (A) Values for the box maze were standardized times in seconds to find the escape hole in trial 3. (B) Values for the rotarod were standardized times in seconds staying on the rotating rod. (C) Values for grip strength were standardized force in newtons per body weight measuring the ability to maintain forepaw grip from a parallel meter bar. N = 12–14. *P < 0.05; 2-way ANOVA with Tukey’s post-hoc comparison and two-tailed, unpaired Student’s t-test.
Decreased severity of age-related lesions occurred in C57BL/6 mice fed drug cocktail chow but not consistently in mice fed chow with each individual drug
There was a consistent decrease in lesion severity in both males and females fed chow with the drug cocktail (Fig. 3A,B). Chow with individual Rap, Acb or Pba was in general less effective than cocktail chow in decreasing lesion severity, with some exceptions. Rap treatment was effective in decreasing lesion severity in heart, liver, and kidney in females and kidney in males. All three drugs individually were effective in decreasing lesion severity in the kidneys.
Figure 3
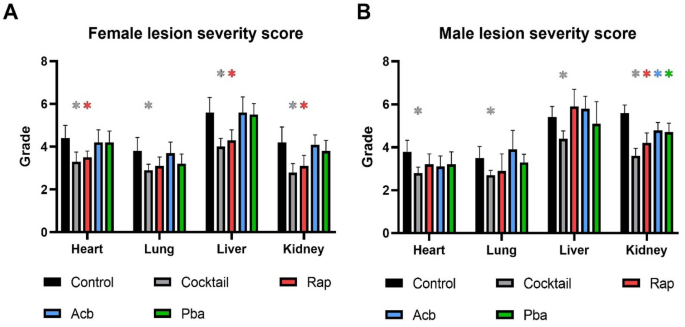
Lesion severity scores were calculated in four major organs from C57BL/6 mice fed chow with a three-drug cocktail or each individual drug for 3 months starting at 20 months of age. (A) Female lesion severity scores. (B) Male lesion severity scores. N = 12–14/cohort. *P < 0.05; 2-way ANOVA with Pearson chi-square test.
The significant decrease in the severity of age-related kidney lesions in mice fed the cocktail chow compared to kidneys from both male and female B6 mice fed the control chow is an example of the impact the drug cocktail had on improving healthy aging in mice. Figure 4A shows a kidney with a moderate to severe geropathology lesion score (grade 3 out of 4) from a B6 female fed the control chow while Fig. 4B shows a kidney with a mild geropathology lesion score (grade 1 out of 4) from a mouse fed the drug cocktail chow.
Figure 4
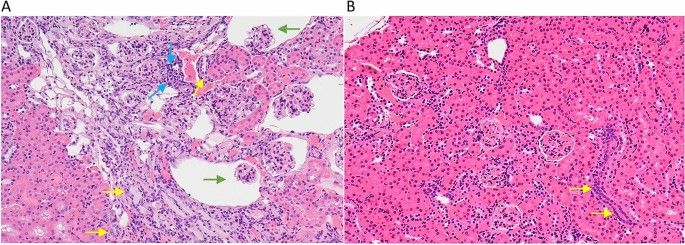
Representative images of H and E-stained kidney sections from mice treated with a drug cocktail or control. (A) The control mouse kidney shows moderate to severe aging lesions (scored as a grade 3). Specific lesions include moderate nephropathy characterized by multifocal tubular basophilia (yellow arrows), glomerular atrophy and dilated Bowman’s space (green arrows) and interstitial inflammatory infiltrates (blue arrows). (B) The cocktail treated mouse kidney shows fewer and less severe aging changes (scored as a grade 1), characterized by focal tubular basophilia (yellow arrows). Magnification ×200.
The drug cocktail lowered transcriptional levels of inflammatory cytokines in the kidney
RNA message levels for TNFa, IL6, and MCP-1 in the kidneys from mice fed the cocktail chow were assessed by rtPCR and shown to be significantly lower than in the kidneys from mice fed the control chow (Fig. 5). The lower expression levels were consistent with low expression levels in the kidneys from young untreated mice. Interestingly, IL1b levels were not affected. In addition, p16 but not p21, was moderately decreased suggesting that some aspects of cell cycle regulation may be effected by the cocktail concurrently with downregulation of inflammatory cytokines.
Figure 5
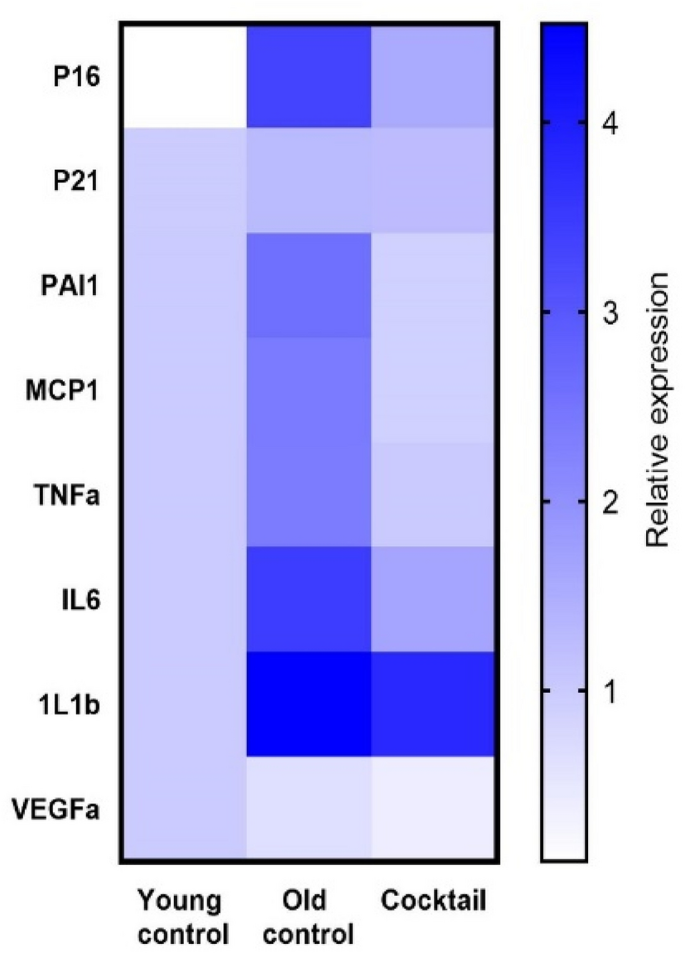
Kidneys from 23-month-old C57BL/6 mice fed the cocktail chow for 3 months showed a general decrease in inflammatory cytokine RNA message by qPCR. Based on the mixed-effect model, which accounts for individual and composite relative expression values, the cocktail diet group showed significantly more anti-inflammatory effect than the control diet group (p < 0.001) and more in line with expression in young mice.
HET3 mice treated with the drug cocktail generally showed responsive phenotypes
We wanted to see if there were any differences in how the drug cocktail affected age-related phenotypes in HET3 mice, a commonly used strain in aging studies, compared to B6 mice. The change for body weight, body fat mass, and food consumption over three months of treatment is shown in Fig. 6. HET3 males treated with the drug cocktail lost a significant amount of weight over the three-month treatment period in contrast to B6 females, but other biological parameters were similar.
Figure 6
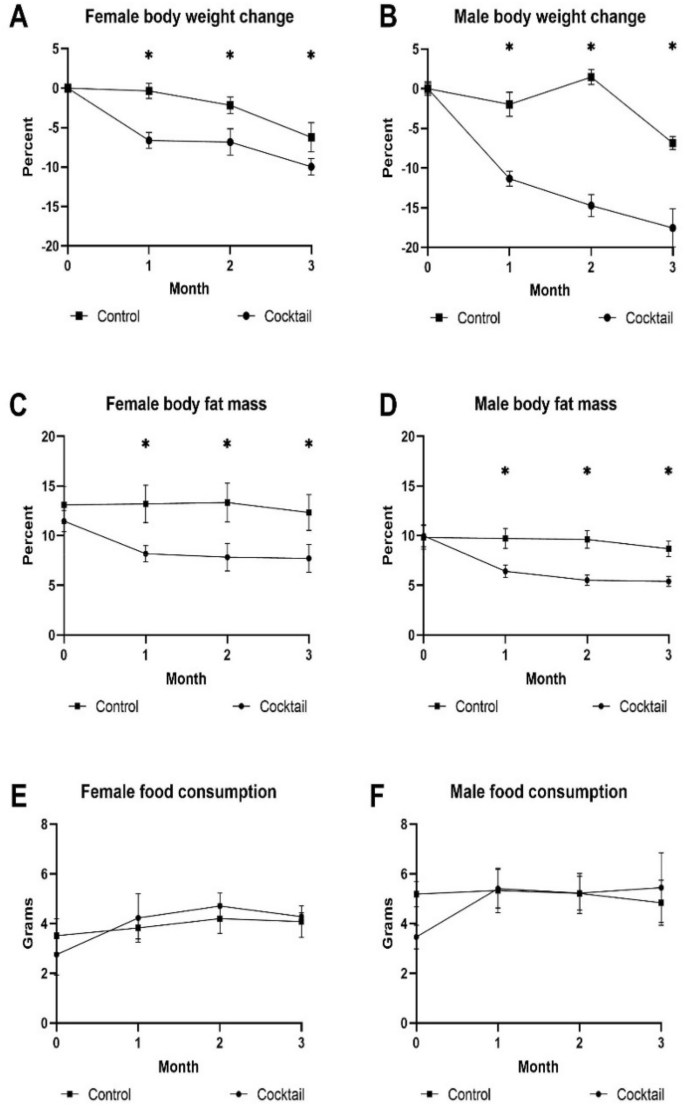
Biological parameters were assessed monthly in HET3 mice fed drug cocktail chow or chow with each individual drug or control for three months starting at 20 months of age. (A) Body weight in females, (B)body weight in males, (C) body fat mass measured by magnetic resonance imaging in females, (D) body fat mass measured by magnetic resonance imaging in males, (E) food intake measured over 3 days each month for females, (F) food intake measured over 3 days each month for males. N = 12–14/cohort. *P < 0.05; two-tailed, unpaired Student’s t-test.
HET3 males fed the cocktail chow showed a trend for improved cognition, and significantly enhanced performance on the rotarod, while HET3 females fed the cocktail chow showed no improvement in cognition (Fig. 7A) but a trend for improvement in the rotarod task (Fig. 7B). Both sexes fed the cocktail chow showed improved grip strength compared to cohorts fed the control chow (Fig. 7C).
Figure 7
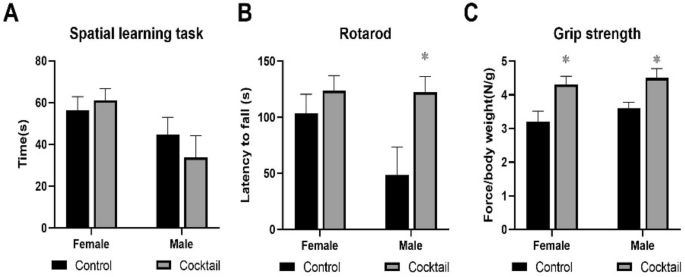
Performance tests were conducted in HET3 mice at 23 months of age, 3 months after being started on chow containing the drug cocktail or placebo. (A) Values for the box maze were standardized times in seconds to find the escape hole in trial 3. (B) Values for the rotarod were standardized times in seconds staying on the rotating rod. (C)Values for grip strength were standardized force in newtons per body weight measuring the ability to maintain forepaw grip from a parallel meter bar. N = 12–14. *P < 0.05; two-tailed, unpaired Student’s t-test.
Age related lesion severity was decreased in organs from HET3 female mice fed the cocktail chow except for liver (Fig. 8A), while HET3 males fed the cocktail chow had decreased lesion severity in organs evaluated except for lungs (Fig. 8B) suggesting there were sex differences in the drug cocktail response to age-related lesions in the HET3 mouse strain.
Figure 8
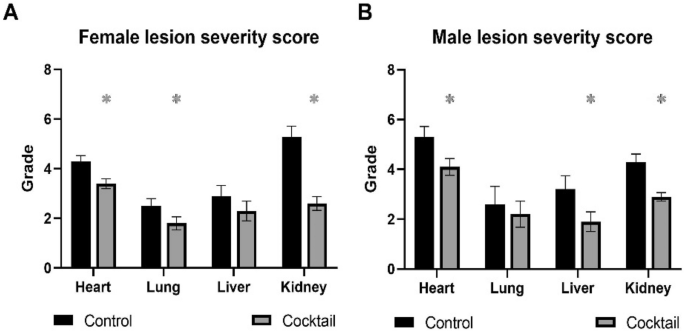
Lesion severity scores were calculated in four major organs from HET3 mice fed chow with a three-drug cocktail or control for 3 months. (A)Female lesion severity scores. (B) Male lesion severity scores. N = 12–14/cohort. *p ≤ 0.05, Pearson chi-square test.
Discussion
The drug cocktail of rapamycin (Rap), acarbose (Acb), and phenylbutyrate (Pba) was studied to test the concept that interventions that extend lifespan in mice will result in improvements in multiple aspects of health span, resulting in significant delays in the appearance of poor physiological performance and progression of organ-based pathology. The rationale for testing the drugs in combination as a single cocktail was based on the different but overlapping anti-aging targeting effects of each drug on processes of aging. C57BL/6 (B6) and HET3 4-way cross (HET3) mice, 23 months of age, fed chow containing the cocktail for three months, showed significant strain and sex-dependent improvements in biological and physiological assessments and suppression of age-related lesions compared to mice fed individual drug or control chow.
In order to confirm that the dose of the drugs in the cocktail would be biologically effective over the three-month treatment period in B6 mice, we tested a different dose. B6 mice were fed a cocktail diet containing one-half the dose of each drug compared to the full dose cocktail diet and the control diet. Interestingly, the half-dose drug cocktail was just as effective as the full dose drug cocktail in preventing age-related cognitive impairment, but was less effective in the other physical performance tests. The half-dose cocktail also had no effect on reducing pathological lesions, suggesting again that there is some specificity in the outcomes based on the dose of drugs used. Higher concentrations of the drugs were not tested, so it is not known whether a higher drug dose cocktail would have had a more comprehensive effect. We concluded that the cocktail drug dose (full dose) used in our study was adequate to show significant age-related phenotypic differences with mice treated with the individual drugs or control (no drugs).
The major biological changes that we measured in B6 mice treated with the drug cocktail for 3 months were in body weight and body fat mass. Body weight was decreased by the cocktail in females after one month of treatment and after 2 months of treatment in males. The trend in females was not seen after three months of treatment but was seen as a significant decrease in males suggesting sex differences in metabolic response to the drug cocktail. This observation was further validated by the concurrent decrease in body fat mass in both sexes. Interestingly, B6 female mice treated with Rap or Acb actually gained weight, but males did not adding evidence of the sex dependent differences in metabolic response to the individual drugs. Acb treatment consistently decreased body fat mass in both females and males similar to the drug cocktail. Since there were no significant changes in lean muscle mass in any of the cohorts, changes in body weight could be explained by changes in body fat mass in most instances. Effect of the cocktail diet on fat mass was striking in both strains and appeared to be due to Acb. One of the explanations for these phenotypic responses might be due to the glucoregulatory control effect by Acb, which selectively inhibits carbohydrate metabolism by allowing caloric compensation in food intake27. This selective inhibition promotes a similar effect as caloric restriction, which reduces calorie consumption provided by all macronutrients. Previous studies showed that Acb treatment led to lifespan extension principally in male mice28. Acb related metabolic pathways were similar in female and castrated males suggesting the sexual dimorphism effect was related to gonadal hormone differences. The sex bias for changes in fat mass were not as apparent in this study where decreases in fat mass occurred in females after one month of treatment with cocktail or Acb and after two months of treatment in males. These decreases continued in both sexes through three months of treatment.
Several tests were conducted to measure physiological performance in B6 mice treated with the drug cocktail or each individual drug. Both males and females fed the cocktail chow showed improved cognitive performance in the spatial learning task. When fed chow with each individual drug, Rap but not Acb or Pba, showed an effect similar to the drug cocktail in both sexes. Rap thus appeared to be the major contributor for the cocktail’s effect on suppressing cognitive impairment. Decreased neuronal activation and imparied cognitive performance during aging occurs in both humans and rodents. Chronic mTOR attenuation by rapamycin has shown the benefits of restoring deficits in neurovascular coupling response and cerebrovascular dysfunction in aging rodent models29. Similar effects may be occurring in the current study.
Additional performance tests in B6 mice included rotating rod and grip strength tasks. Both females and males fed chow with drug cocktail performed better in both tasks compared to females and males fed the control chow. In order to see if a particular drug in the cocktail was a major contributor to effects seen in these two performance tests, mice were fed chow containing each drug, or the cocktail or control. No single drug consistently enhanced performance in both sexes compared to the cocktail. However, female mice fed chow with Acb performed equally well in the grip strength task as females fed chow with the drug cocktail. The fact that this sex-dependent result in strength performance was not seen in the drug cocktail treated mice suggests that the other drugs in the cocktail contributed in some way.
Geropathology assessment of age-related lesions can provide useful information as to how drug treatment can effectively target organs and specific lesions within organs30. Data from this study showed a consistently significant decrease in lesion scores of heart, lungs, liver and kidney from both female and male B6 mice fed chow with drug cocktail compared to lesion scores in these organs from mice fed control chow (no drugs). This type of assessment provides an in depth view of how individual organs responded to drug treatment in B6 mice, and based on the data, we can say the drug cocktail was very effective in delaying the progression of age-related pathology in all organs examined. We view this as a vital component of the study since mice were treated for only three months, which suggests that short term cross-sectional studies designed to test drugs for anti-aging effects in mice would benefit by including geropatholgy assessment. When treatment with individual drugs was compared to treatment with the drug cocktail in B6 mice, there were some unexpected sex dependent results. Rap was almost as effective as the drug cocktail in suppressing age-related lesions in females in heart, liver, and kidney, but much less effective in males (kidney only). The other two drugs, Acb and Pba, were not effective in females at all, and were effective in males only in the kidney. These observations suggest that the adminsitration of the three drugs in combination as a cocktail has a major advantage over any individual drug tested in the study. In addition, the fact that age-related lesions in the kidney seemed to be very responsive to drug treatment suggests that geropathology assessment of the kidney should be included in any short term drug study in aging B6 mice.
Based on geropathology observations in the kidney showing extensive inflammatory activity in mice fed control chow (Fig. 4), it was of interest to explore possible transcriptomic changes associated with suppression of inflammation in mice fed the drug cocktail chow. Using qPCR, RNA message levels were determined for TNFa, IL6, MCP-1, and IL1b in the kidneys from cocktail treated mice and control mice. The lower expression levels of TNFa, IL6, and MCP-1 in cocktail treated mice were consistent with low expression levels in the kidneys from young untreated mice suggesting the drug cocktail delays aging in the kidney partly by anti-inflammatory effects. The fact that RNA message for p16, a gene involved in cell cycle regulation, was also decreased further strengthens this observation. In addition, RNA message for PAI-1 and VEGFa, genes involved in vascular integrity, was decreased in mice fed the drug cocktail chow and is of interest because vascular thrombosis with infarcts (areas of ischemic necrosis) is not uncommon in the kidneys of old B6 mice. These lesions were rarely seen (data not shown) in kidneys from the cocktail treated mice suggesting these genes may be possible anti-aging therapeutic targets in mice.
Observations from this study seen in C57BL/6 (B6) mice are of interest but need to be substantiated in a second mouse strain because of strain variability in drug responsiveness. HET3 4-way cross (HET3) mice are used exclusively by the NIA Intervention Testing Program in lifespan studies to test a large number of drugs7, including the three drugs in our cocktail. Therefore, it was considered a useful strain to help validate the effects of the cocktail on aging parameters in B6 mice. The HET3 mice were tested in the same manner, age, and timing as the B6 mice, but only with the drug cocktail compared to control chow. Overall, HET3 mice were responsive to the drug cocktail. However, there were several differences in the manner of the response, especially depending on sex. First, there was a robust decrease in body weight over the three-month treatment period, especially in males. As in B6 mice, this weight loss was reflected in decreases in body fat mass but not lean muscle mass. There were no differences in amount of food consumed so the metabolic changes were instigated by the drug cocktail. Secondly, both female and male HET3 mice fed drug cocktail chow outperformed mice fed control chow in the grip strength task. However, only male mice on the drug cocktail chow showed improved performance in the rotarod task, while neither female nor male HET3 mice fed cocktail chow showed improved learning ability as assessed by the spatial learning task. Third, severity of heart and kidney lesions were consistently decreased in both females and males fed cocktail chow, while severity of liver lesions was not affected by the cocktail in females and severity of lung lesions was not affected by the cocktail in males. As more therapeutic testing is done in mice, more studies are needed to point out strain differences in drug responsiveness. Because we tested both sexes, HET3 mice were helpful in corroborating the drug cocktail as an effective therapeutic approach to delay aging phenotypes.
In conclusion, the drug cocktail was more effective than each individual drug, and a half dose of the drug cocktail in the diet was overall less effective than the full dose cocktail based on biological, physiological and geropathological endpoints. The sex and strain differences partly agree with previous observations in lifespan studies in mice treated with Rap or Acb7,14. Interestingly, previous studies also suggest that combining pro-longevity treatments may have beneficial effects on longevity and health span beyond that of each drug individually. This study shows that a combination of three drugs previously shown to enhance lifespan and health span in mice is able to delay aging phenotypes more effectively and more robustly than any individual drug in the cocktail when started at middle age and given for a short period of time.
volume
12, Article number: 7300 (2022) Cite this article
1. López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153(6), 1194–1217. https://doi.org/10.1016/j.cell.2013.05.039 (2013). 2. CAS Article PubMed PubMed Central Google Scholar 3. Hara, Y., McKeehan, N. & Fillit, H. M. Translating the biology of aging into novel therapeutics for Alzheimer disease. Neurology92(2), 84–93 (2019). 4. Article Google Scholar 5. Ladiges, W. & Liggitt, D. Testing drug combinations to slow aging. Pathobiol. Aging Age Relat. Dis. 8(1), 1407203. https://doi.org/10.1080/20010001.2017.1407203 (2017). 6. CAS Article PubMed PubMed Central Google Scholar 7. Ladiges, W. The quality control theory of aging. Pathobiol. Aging Age Relat. Dis. https://doi.org/10.3402/pba.v4.24835 (2014). 8. Article PubMed PubMed Central Google Scholar 9. Cowan, P. A. & Heizer, K. E. Sirolimus: Mammalian target of rapamycin inhibitor to prevent kidney rejection. *Nephrol. Nurs. J.*27, 623–625 (2000). 10. CAS PubMed Google Scholar 11. Touzot, M., Soulillou, J. P. & Dantal, J. Mechanistic target of rapamycin inhibitors in solid organ transplantation: from benchside to clinical use. Curr. Opin. Organ. Transplant. 17(6), 626–633 (2012). 12. CAS Article Google Scholar 13. Miller, R. A. et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13(3), 468–477. https://doi.org/10.1111/acel.12194 (2014). 14. CAS Article PubMed PubMed Central Google Scholar 15. Wilkinson, J. E. et al. Rapamycin slows aging in mice. Aging Cell11(4), 675–682. https://doi.org/10.1111/j.1474-9726.2012.00832.x(2012). 16. CAS Article PubMed Google Scholar 17. Flynn, J. M. et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 12(5), 851–862. https://doi.org/10.1111/acel.12109 (2013). 18. CAS Article PubMed Google Scholar 19. Ladiges, W. et al. A new pre-clinical paradigm for testing anti-aging therapeutics. J. Gerontol. A Biol. Sci. Med. Sci. 72(6), 760–762 (2017). 20. Article Google Scholar 21. Bitto, A. et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. Elifehttps://doi.org/10.7554/eLife.16351 (2016). 22. Article PubMed PubMed Central Google Scholar 23. Balfour, J. A. & McTavish, D. Acarbose: An update of its pharmacology and therapeutic use in diabetes mellitus. Drugs46(6), 1025–1054 (1993). 24. CAS Article Google Scholar 25. Anderson, R. M. & Weindruch, R. The caloric restriction paradigm: implications for healthy human aging. *Am J Hum Biol.*24(2), 101–106. https://doi.org/10.1002/ajhb.22243 (2012). 26. Article PubMed PubMed Central Google Scholar 27. Harrison, D. E. et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 13(2), 273–282. https://doi.org/10.1111/acel.12170 (2014). 28. CAS Article PubMed Google Scholar 29. Wiley, J. C., Pettan-Brewer, C. & Ladiges, W. C. Phenylbutyric acid reduces amyloid plaques and rescues cognitive behavior in AD transgenic mice. Aging Cell 10(3), 418–428 (2011). 30. CAS Article Google Scholar 31. Snyder, J. M. et al. Validation of a geropathology grading system for aging mouse studies. Geroscience. 41(4), 455–465 (2019). 32. Article Google Scholar 33. Ren, J. & Zhang, Y. Targeting autophagy in aging and aging-related cardiovascular diseases. Trends Pharmacol. Sci. 39(12), 1064–1076. https://doi.org/10.1016/j.tips.2018.10.005 (2018) ((epub 2018 Oct 26)). 34. CAS Article PubMed PubMed Central Google Scholar 35. Brewer, R. A., Gibbs, V. K. & Smith Jr, D. L. Targeting glucose metabolism for healthy aging. Nutr. Healthy Aging. 4(1), 31–46. https://doi.org/10.3233/NHA-160007 (2016). 36. CAS Article PubMed PubMed Central Google Scholar 37. Kolb, P. S. et al. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int. J. Biochem. Cell Biol. 61, 45–52 (2015). 38. CAS Article Google Scholar 39. Khan, S., Komarya, S. K. & Jena, G. Phenylbutyrate and beta-cell function: Contribution of histone deacetylases and ER stress inhibition. Epigenomics 9(5), 711–720. https://doi.org/10.2217/epi-2016-0160 (2017) ((epub 2017 May 4)). 40. CAS Article PubMed Google Scholar 41. Strong, R. et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15(5), 872–884. https://doi.org/10.1111/acel.12496 (2016). 42. CAS Article PubMed PubMed Central Google Scholar 43. Darvas, M., Mukherjee, K., Lee, A. & Ladiges, W. A novel one-day learning procedure for mice. Curr. Protoc. Mouse Biol. 10(1), e68 (2020). 44. Article Google Scholar 45. Ge, X. et al. Self-motivated and stress-response performance assays in mice are age-dependent. Exp. Gerontol. 91, 1–4. https://doi.org/10.1016/j.exger.2017.02.001 (2017). 46. Article PubMed PubMed Central Google Scholar 47. Phelps, M., Pettan-Brewer, C., Ladiges, W. & Yablonka-Reuveni, Z. Decline in muscle strength and running endurance in klotho deficient C57BL/6 mice. Biogerontology 14(6), 729–739 (2013). 48. CAS Article Google Scholar 49. Ge, X. et al. Grip strength is potentially an early indicator of age-related decline in mice. Pathobiol. Aging Age Relat. Dis. 8(6), 32981. https://doi.org/10.3402/pba.v6.32981 (2016). 50. Article Google Scholar 51. Camell, C. D. et al. Senolytics reduce coronavirus-related mortality in old mice. Science 373, 6552 (2021). 52. Article Google Scholar 53. Brewer, R. A., Gibbs, V. K. & Smith, D. L. Jr. Targeting glucose metabolism for healthy aging. Nutr. Healthy Aging. 4(1), 31–46. https://doi.org/10.3233/NHA-160007 (2016). 54. CAS Article PubMed PubMed Central Google Scholar 55. Garratt, M., Bower, B., Garcia, G. G. & Miller, R. A. Sex differences in lifespan extension with acarbose and 17-α estradiol: Gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell 16(6), 1256–1266 (2017). 56. CAS Article Google Scholar 57. Van Skike, C. E. & Galvan, V. mTOR in cerebrovascular disease. Aging (Albany NY). 11(5), 1331–1332 (2019). 58. Article Google Scholar 59. Klug, J., Christensen, S., Imai, D. M., Snider, T. A. & Ladiges, W. The geropathology of organ-specific aging. J. Transl. Sci. 7(1), 458 (2021). 60. PubMed PubMed Central Google Scholar