Caloric restriction (CR) remains the gold-standard benchmark for delaying age-related decline, yet its translation to everyday life is constrained by adherence, nutritional-adequacy, and quality-of-life concerns. These obstacles have prompted intense interest in pharmacological alternatives that can recapitulate CR’s molecular signature while avoiding sustained dietary deprivation.
SGLT-2 inhibitors create a mild, chronic energy deficit by diverting 60–80 g of glucose per day into the urine, a loss that is metabolically analogous to a small but continuous fast. The resulting fall in blood glucose and insulin initiates the same nutrient-sensing cascade normally triggered by CR or intermittent fasting, but without imposing severe behavioral change.
This glucose siphoning engages four canonical longevity pathways simultaneously: the energy sensors AMPK and SIRT1 are activated, whereas the pro-growth mTOR and insulin/IGF-1 axes are attenuated. The coordinated shift steers cellular priorities away from growth and storage toward maintenance and stress resistance.
AMPK acts as the cell’s fuel-gauge. When ATP falls and AMP rises—conditions reproduced by SGLT-2–induced glucosuria—AMPK phosphorylates downstream targets that boost fatty-acid oxidation, inhibit lipogenesis, and promote mitochondrial biogenesis. In multiple model organisms, persistent AMPK activation aligns with enhanced stress tolerance and extended lifespan.
Sirtuin-1 is an NAD⁺-dependent deacetylase that modulates chromatin structure, inflammatory signaling, and mitochondrial turnover. The energy shortfall imposed by SGLT-2 blockade raises the NAD⁺/NADH ratio, increasing SIRT1 activity. SIRT1 in turn deacetylates PGC-1α, FOXO, and p53, orchestrating antioxidant defenses, improved mitochondrial function, and metabolic flexibility—traits central to healthy aging.
Insulin and IGF-1 convey systemic “nutrient abundance” messages. By lowering circulating glucose, SGLT-2 inhibitors blunt insulin release and secondarily damp IGF-1 signaling. Reduced IIS (insulin/IGF-1 signaling) has been linked to lifespan extension in worms, flies, and mice, likely by reallocating resources from growth to repair and by lowering mitogenic stress that contributes to carcinogenesis.o3
Downstream consequences include enhanced autophagy, mitochondrial biogenesis, and greater metabolic flexibility, all features strongly associated with healthier aging. Together these adaptations improve fuel utilization, limit reactive-oxygen damage, and refresh dysfunctional organelles.
Pre-clinical work—exemplified by empagliflozin in high-fat-fed mice—shows reductions in hepatic steatosis, pro-inflammatory M1 macrophages, and systemic cytokines, alongside increased UCP-1 expression in brown fat, indicating simultaneous benefits for lipid handling, inflammation, and energy expenditure.
Early human studies echo these metabolic shifts with weight loss, lower fasting insulin, and cardiometabolic risk-factor improvements, although trials explicitly powered for healthspan or lifespan outcomes are still lacking. Rigorous, aging-focused studies are needed before SGLT-2 inhibitors can be positioned confidently as longevity therapeutics.
Bacakground
Caloric restriction (CR), the deliberate reduction of caloric intake without malnutrition, remains one of the most robust experimental strategies for extending lifespan across species. Yet, its translation to humans has been anything but straightforward. The strict discipline required, along with potential risks like muscle loss and nutritional imbalances, makes CR a daunting long-term strategy for most people. But what if the body could be coaxed into believing it was fasting, without giving up food?
Recent research has uncovered an alternative approach—one that leverages the body’s own metabolic pathways without continuous dietary restraint. Sodium–glucose cotransporter-2 (SGLT-2) inhibitors, initially developed for diabetes management, have shown potential to replicate many of the molecular effects of CR. By diverting roughly 60–80 g of glucose into the urine each day, these agents create a mild, chronic energy deficit that lowers circulating glucose and insulin levels and engages key nutrient-sensing pathways involved in energy regulation and cellular repair. Consequently, they may offer a pharmacological means to harness the health-extending effects of CR. This review explores the emerging evidence behind SGLT-2 inhibitors as CR mimetics and their relevance in the broader context of metabolic longevity.
The Challenges of Continuous Caloric Restriction
As described in our previous reviews, caloric restriction (CR) is the practice of reducing daily calorie intake without depriving the body of essential nutrients, often to improve health and potentially extend lifespan. [1] Notably, studies have shown that CR can lead to a significant reduction in the incidence of age-associated pathologies and an extension of median lifespan. Seminal papers dating back to the 1940s have demonstrated the longevity potential of CR. In 1949, Tannenbaum & Silverstone conducted a pivotal study examining the effects of varying degrees of CR on the development of skin tumors induced by the carcinogen methylcholanthrene in male mice [2]. The experimental design involved feeding the mice a controlled diet composed of Purina fox chow, skimmed milk powder, and cornstarch. Mice were housed in small groups, with individuals of similar body weights sharing the same amount of restricted food to ensure equal intake within each group. All animals had unrestricted access to water.
After a brief period of adjustment, the mice received seven applications of a 0.3% methylcholanthrene solution in acetone, applied at five-day intervals over 30 days. The findings revealed a clear inverse relationship between CR intake and tumor development:
- 2.3g Daily Intake: Mice developed tumors after an average of 39 weeks.
- 4.0g Daily Intake: Tumors appeared within 25 weeks.
Survival rates also correlated with caloric intake; 38 mice on a 2.9g daily diet survived the study period, while only 27 mice on a 4.0g daily diet survived. These results provide compelling evidence that caloric restriction can delay the onset of carcinogen-induced skin tumors, suggesting a role for CR in promoting anti-aging by reducing cancer incidence, thereby potentially decreasing morbidity and mortality.
While such research is promising, the human application of CR is unfortunately fraught with difficulties. This is because designing and complying with a long-term CR regimen involves exceptional self-discipline and increases the risk of nutritional deficiencies, loss of muscle mass, and a detrimental impact on quality of life. These challenges highlight the pressing need for alternative strategies that can emulate the beneficial effects of CR without necessitating continuous dietary restraint.
The Metabolic "Fasting Switch"
The human body possesses sophisticated mechanisms that respond to nutrient availability, adjusting metabolic processes to either store energy or utilize it efficiently. One such adaptive response is the metabolic "fasting switch," a state wherein the body transitions from using glucose as its primary energy source to relying on fatty acids and ketones. Fatty Acids are long chains of carbon and hydrogen atoms found in fats, which can be broken down to produce energy. Ketones, on the other hand, are energy molecules produced by the liver from fatty acids during periods of low food intake, such as fasting. They serve as an alternative energy source, especially for the brain. [3]
This shift not only conserves glucose but also activates various pathways associated with longevity and cellular repair. It boosts how well the mitochondria, the so-called ‘energy factories’ inside our cells, do their job of harvesting energy from our diet. It also makes the body better at handling stress and reduces damage caused by harmful molecules called free radicals. All of these things can help slow down aging and keep us healthier as we get older. [3]
To achieve the metabolic “fasting switch,” the body needs to reach a state where blood glucose levels are lower than usual. This drop signals to the cells that it is time to switch from using glucose to alternative energy sources, as glucose is already in short supply and cannot be depleted further. Traditionally, humans have used methods like calorie restriction, intermittent fasting, and prolonged exercise to reach this “hypoglycemic” state. While these strategies show promise, they come with challenges, as was discussed in the section above.
However, the advent of SGLT-2 inhibitors, a class of anti-diabetic medications, offers an exciting alternative. These medications help create a metabolic environment similar to that of fasting, without the need for actual food deprivation. In the next section, we will explore the physiological mechanisms by which SGLT-2 inhibitors mimic the effects of CR, such as fasting, and promote the shift towards fatty acids and ketones as the body’s primary energy sources.
SGLT-2 Inhibitors: Catalysts of the Fasting State
SGLT-2 inhibitors exert their effects by blocking the action of a specific protein in the kidneys known as sodium-glucose cotransporter-2 (SGLT-2) [4]. Under normal conditions, this protein acts like a “gatekeeper" that helps reabsorb glucose back into your blood after it is filtered through the kidneys. Usually, when the kidneys filter blood, they remove waste, but they also try to conserve useful molecules, such as proteins, nutrients, and glucose.
When SGLT-2 is inhibited, this reabsorption mechanism is disrupted. Glucose that would normally be reclaimed is instead excreted in the urine, leading to a reduction in circulating blood glucose levels. This persistent glucose loss promotes a mild energy deficit, prompting the body to engage alternative metabolic pathways similar to those activated during caloric restriction. As a result, SGLT-2 inhibitors can induce a metabolic state that mimics fasting, triggering biological processes associated with cellular repair, improved metabolic efficiency, and potentially delayed aging.
Activation of AMP-Activated Protein Kinase (AMPK)
AMPK is one of the key proteins targeted by SGLT-2 inhibitors. [5] AMPK acts as an internal fuel gauge, constantly monitoring the amount of energy a cell has. It does this by sensing the levels of two essential molecules: ATP, which stores energy (often referred to as the “energy currency” of the cell), and AMP, which builds up when energy is in short supply. When the cell’s energy stores are depleted (such as during fasting, exercise, or when using SGLT-2 inhibitors), AMP levels rise, and AMPK becomes activated.
This activation sets off a chain of responses to restore energy balance. It encourages the cells of our body to burn stored fat by boosting a process called fatty acid oxidation. The protein also promotes the creation of new mitochondria via a process known as mitochondrial biogenesis. More and healthier mitochondria mean more efficient energy production across the body. All these effects combined lead to better metabolic health and increased energy efficiency at the cellular level. Crucially, AMPK is also involved in cellular maintenance and repair, and its activation has been associated with slower aging and increased longevity in animal studies.
SGLT-2 inhibitors, by promoting a mild, fasting-like state, help trigger this AMPK pathway without the need for strict dieting or prolonged fasting.
Upregulation of Sirtuin-1 (SIRT1)
Another key protein that is targeted by SGLT-2 inhibitors is Sirtuin-1 (SIRT1). [5] This protein is known for its crucial role in mediating cellular responses to metabolic stress, aging, and changes in nutrient availability. It helps regulate how our cells respond to stress, aging, and metabolism. As a member of the sirtuin family, SIRT1 is intimately involved in longevity-associated processes, including mitochondrial function, inflammation, and metabolic regulation. What makes SIRT1 especially important is that it requires a molecule called NAD+ (nicotinamide adenine dinucleotide) to function. You can think of NAD+ as a sort of ‘helper’ for SIRT1 to mediate its functions, and the key point to note here is that levels of this molecule rise during low-energy states like fasting. [5]
By promoting an energy-deprived state, natural methods like calorie restriction or medications like SGLT-2 inhibitors can increase the pool of NAD+ available, and thus increase the functional capacity of SIRT1.
When SIRT1 is activated, it goes to work by "deacetylating" other proteins. In simpler terms, it modifies these proteins in a way that changes their activity, often turning on beneficial cellular processes. One of its key effects is improving how our cells use energy by enhancing the function of mitochondria. It also promotes fatty acid oxidation, which, as described previously, involves breaking down fat to derive fuel. This process is especially beneficial for longevity, as it helps reduce fat accumulation in the liver, which in turn lowers the risk of fat-related health issues that become more common with age.
To better understand this, Xu et al (2017) conducted a study to investigate the effects of SGLT2 inhibitors on fat breakdown. [6] In the study, the researchers used empagliflozin, a specific SGLT2 inhibitor, in obese mice fed a high-fat diet (HFD). Some of the mice were given the HFD along with empagliflozin for 16 weeks, while others were only given the HFD. The results showed that empagliflozin significantly helped the mice resist weight gain caused by the high-fat diet (HFD).
In particular, the researchers found that the drug helped reduce hepatic steatosis, a condition in which fat builds up in the liver, a common issue among people with obesity. These findings suggest that empagliflozin helps protect against some of the harmful effects of obesity, including high blood sugar and fat accumulation in the liver.
Key proteins, including AMPK (as previously discussed) and acetyl-CoA carboxylase, which is involved in the production and breakdown of fats, were also found to be upregulated. By activating these proteins, empagliflozin helps the body burn more fat, particularly in skeletal muscles and the liver.
Finally, the researchers also found that the drug affected levels of brown fat. Brown fat is a type of fat that burns calories to produce heat, which helps with fat loss. Empagliflozin increased the expression of uncoupling protein 1 (UCP1), a protein found in brown fat that is involved in heat production and fat burning. This increased expression of UCP1 suggested that empagliflozin stimulated fat burning by promoting higher energy expenditure and heat production.
The drug also had anti-inflammatory effects. Obesity is often associated with chronic low-grade inflammation, which can lead to various health problems. In the study, empagliflozin reduced the accumulation of M1 macrophages, a type of immune cell that promotes inflammation, in the liver. At the same time, the drug promoted the shift toward an M2 macrophage phenotype, which is known for its anti-inflammatory effects.
This shift in macrophage activity helped reduce inflammation in the fat tissue and liver, resulting in lower levels of TNF-α (tumor necrosis factor-alpha), a protein that causes inflammation. By reducing inflammation, empagliflozin improved insulin sensitivity and further reduced the risk of metabolic diseases associated with obesity. Overall, the research showed that SGLT2 inhibition not only helps lower blood sugar and promote weight loss by increasing glucose excretion but also improves energy metabolism and reduces inflammation associated with obesity.
In addition to promoting fat breakdown, SGLT-2 inhibitors activate SIRT1, which enhances mitochondrial biogenesis and oxidative metabolism. This is particularly significant because it improves metabolic flexibility and reduces the risk of metabolic diseases that often impact quality of life as we age.
Inhibition of Mechanistic Target of Rapamycin (mTOR)
mTOR is a master regulator in the body that helps cells grow, divide, and manage their metabolism based on the availability of food, energy, and growth signals. You can think of mTOR as a kind of “cellular command center” that tells cells, “We have plenty of resources—go ahead and grow, build, and store energy.” This is great when nutrients are abundant and the body needs to grow or repair. But when mTOR stays active all the time, such as in environments of constant overnutrition, it can lead to problems like chronic inflammation, insulin resistance, and even accelerated aging.
This is where SGLT-2 inhibitors come in. SGLT-2 inhibitors have also been found to gently suppress mTOR activity, essentially sending the body a “low-nutrient” signal, similar to what happens during CR-induced fasting. When mTOR is turned down or inhibited, it triggers a beneficial process called autophagy—a healthy, essential cellular recycling system. During autophagy, cells break down and dispose of damaged or dysfunctional components, such as old proteins or malfunctioning mitochondria, and reuse the parts to create new, healthy structures. It's ike a “deep spring cleaning” for your cells.
By promoting autophagy, the inhibition of mTOR leads to cellular rejuvenation, where cells become more efficient, less damaged, and better equipped to handle stress. It also helps to reduce inflammation, which is a key driver behind many chronic diseases and aging-related conditions. SGLT-2 inhibitors offer a way to inhibit mTOR, thus mimicking the effects of fasting, boosting autophagy, improving mitochondrial health, and ultimately supporting longer, healthier cellular life.
Reduction in Insulin/IGF1 Signaling
One of the more fascinating discoveries in geroscience research has been the role of hormones insulin and IGF-1 in influencing the aging process. Both insulin and IGF-1 are signaling molecules that tell the body it has plenty of nutrients and energy, encouraging cells to grow and divide. This is similar to the way mTOR works. While this is vital for development and healing, elevated and chronic signaling through this pathway is linked to faster aging and a higher risk of age-related diseases like cancer, heart disease, and neurodegenerative conditions (like Alzheimer’s).
One way SGLT-2 inhibitors promote their beneficial effects is by decreasing insulin and IGF1 signaling. This is because the release of insulin in the body is related to the level of glucose in the bloodstream. When blood glucose levels exceed the commonly accepted range, insulin is released from the pancreas, promoting the uptake of glucose from the blood by tissues. This is important because it prevents glucose levels in the bloodstream from spiking too high, which can be dangerous otherwise.
On the contrary, when blood glucose levels are low, insulin is not released, and glucagon, a protein with antagonistic functions, is secreted from the pancreas. Glucagon aims to increase levels of glucose in the bloodstream by mobilizing existing stores in tissue and promoting the production of glucose in liver and kidney cells.
How do SGLT-2 inhibitors fit into all this?
As discussed earlier, SGLT-2 inhibitors work by preventing the kidneys from reabsorbing glucose back into the bloodstream, causing excess sugar to be excreted in the urine. This reduction in blood glucose leads to a drop in insulin levels—an effect that goes beyond just managing diabetes. When insulin levels fall, levels of another growth-related hormone, IGF-1 tend to fall as well. These two hormones share overlapping signaling pathways, and together they send a powerful message to the body: grow, store energy, and build.
But when that message quiets down—when insulin and IGF-1 signaling are reduced—the body enters what scientists sometimes refer to as a “low-nutrient state.” In this mode, biology flips a metabolic switch. Instead of prioritizing growth and energy storage, the body shifts its focus toward preservation: activating repair processes, enhancing cellular cleanup through autophagy, dialing down inflammation, and improving resilience to stress. These are the same kinds of biological changes triggered by calorie restriction, fasting, and certain genetic mutations known to extend lifespan in animal models.
What makes SGLT-2 inhibitors particularly interesting is that they seem to tap into this ancient survival program pharmacologically—without requiring extreme dieting or fasting. By nudging the body into a maintenance-and-repair state, they may do more than regulate blood sugar; they may actually engage cellular pathways that protect against aging and chronic disease. It’s a subtle but profound shift: from managing a symptom to potentially modifying the trajectory of aging itself. [5]
Lower insulin and IGF-1 signaling have been consistently linked to longer life and better health in old age. Van Heemst et al. (2005) explored how genetic differences in the insulin/IGF-1 signaling (IIS) pathway are related to human lifespan, with a particular focus on differences between men and women [7]. The IIS pathway plays a key role in regulating growth, metabolism, and aging. The researchers aimed to determine whether variations in genes that regulate this pathway could influence both body height and survival in older adults. The study involved 1,400 participants, all of whom were 85 years old or older. Each individual underwent genetic analysis to identify variations in key genes related to the IIS pathway. Based on these genetic profiles, the researchers calculated a composite IIS score for each participant, which served as a marker for overall pathway activity: lower scores indicated reduced IIS activity, while higher scores reflected increased activity.
Among women in the study, lower IIS scores were significantly linked to shorter heights but greater longevity profiles. Specifically, women who carried variations in the GH1 gene, which encodes for growth hormone, a key component in promoting tissue growth and a central player in the IIS pathway, were, on average, 2 centimeters shorter than non-carriers and exhibited a 20% lower mortality rate. In contrast, no such associations were observed in the male participants, suggesting that sex-specific effects of IIS pathway modulation on aging may exist.
The study concluded that, at least in females, reduced activity in the IIS pathway, driven by specific genetic variants, was associated with increased survival into old age. These findings support the growing body of evidence that dampening insulin/IGF-1 signaling may play a protective role in aging and longevity, and could represent a promising target for therapeutic strategies aimed at promoting healthy aging.
These findings make SGLT-2 inhibitors an attractive candidate. By lowering insulin levels, SGLT-2 inhibitors not only help manage blood sugar but also tap into one of the most powerful, evolutionarily conserved mechanisms for promoting longevity and reducing the risk of age-related diseases.
Linking It Altogether
The growing body of research on SGLT-2 inhibitors reveals a consistent theme: these medications exert their effects by tipping the body’s internal metabolic scale away from nutrient excess and toward a physiological state resembling caloric restriction. In doing so, they emulate the cellular and systemic effects traditionally achieved through long-term dietary restraint—but without the need for strict caloric reduction.
The figure accompanying this section visualizes this shift as a balance scale for metabolism. On one end lies nutrient overload and accelerated aging, characterized by suppressed AMPK and SIRT1 activity, and heightened mTOR and insulin/IGF-1 signaling. This state promotes energy storage, cellular growth, and chronic inflammation—hallmarks of age-related decline. On the opposite end of the scale is the CR-associated longevity state, defined by activated AMPK and SIRT1, and downregulated mTOR and insulin signaling. This metabolic profile supports energy efficiency, autophagy, mitochondrial health, and cellular repair.
SGLT-2 inhibitors function as a fulcrum-shifting force. By promoting glucose excretion and reducing circulating insulin levels, they activate energy-sensing pathways (AMPK and SIRT1) while dampening pro-growth signals (mTOR and IGF-1). The result is a shift in cellular priorities—from growth and storage to maintenance and resilience. In essence, SGLT-2 inhibitors “flip the scale,” shifting the body’s metabolic state from one of nutrient overload to a CR-like profile associated with improved healthspan and a reduced risk of age-related diseases.
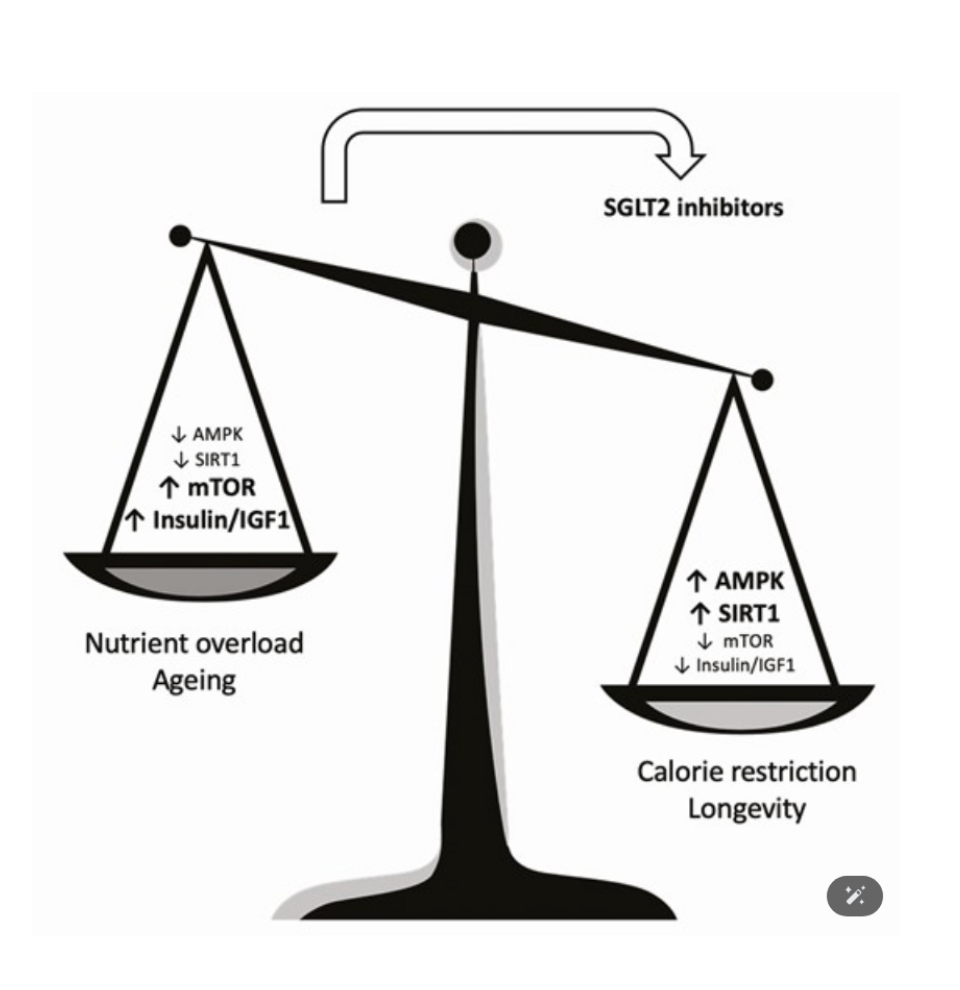
Figure 1. This figure illustrates the metabolic contrast between two physiological states: nutrient overload and aging (left side of the balance scale) versus caloric restriction and longevity (right side of the balance scale). The nutrient overload state is characterized by low AMPK and SIRT1 activity, alongside elevated mTOR and insulin/IGF-1 signaling pathways, which are associated with accelerated aging and chronic disease. In contrast, the CR state features enhanced AMPK and SIRT1 signaling, along with reduced mTOR and insulin/IGF-1 activity, which supports cellular maintenance, stress resistance, and longevity. The curved arrow represents the action of SGLT-2 inhibitors, which shift the metabolic state from one of nutrient excess toward a CR-like profile by modulating the same pathways. This rebalancing highlights their role as pharmacological CR mimetics with potential anti-aging benefits.
Conclusion
The pursuit of interventions that promote longevity has long been shaped by the profound, though often impractical, benefits of caloric restriction. Given the challenges associated with sustained CR adherence, there has been growing interest in identifying alternative strategies that can replicate its favorable metabolic effects. This review highlights the emerging potential of SGLT-2 inhibitors as candidates in this area. Originally developed for glycemic control in diabetes, SGLT-2 inhibitors have demonstrated broader metabolic effects that resemble some of the molecular and physiological shifts induced by CR.
Through a coordinated modulation of key pathways, including AMPK activation, SIRT1 upregulation, mTOR attenuation, and Insulin/IGF-1 signaling reduction, SGLT-2 inhibitors appear to promote a metabolic environment conducive to cellular maintenance, stress resistance, and systemic health. Their ability to induce a mild, fasting-like state through urinary glucose excretion may offer a practical means of engaging nutrient-sensing networks associated with healthy aging.
Future research will be critical to fully elucidate the long-term effects of SGLT-2 inhibitors on aging trajectories and lifespan across diverse populations. Dedicated clinical trials focusing on aging outcomes will be essential to validate and refine their role in longevity medicine. While preliminary evidence is promising, a cautious and rigorous approach will be necessary to determine the extent to which SGLT-2 inhibitors can contribute to healthspan extension beyond their established metabolic benefits.
- Marshall, R. N. (2023, September 30). Eat less, live longer? Insights into the geroprotective effects of calorie restriction and prolonged fasting on Metabolic Health & Longevity. Healthspan.
- TANNENBAUM, A., & SILVERSTONE, H. (1949). The influence of the degree of caloric restriction on the formation of skin tumors and hepatomas in mice. Cancer research, 9(12), 724–727.
- Anton, S. D., Moehl, K., Donahoo, W. T., Marosi, K., Lee, S. A., Mainous, A. G., 3rd, Leeuwenburgh, C., & Mattson, M. P. (2018). Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity (Silver Spring, Md.), 26(2), 254–268.
- Healthspan, G. (n.d.). Doctor-prescribed SGLT-2 inhibitor for longevity and Healthspan. https://gethealthspan.com/protocols/sglt2-metabolic-protocol
- Hoong, C. W. S., & Chua, M. W. J. (2021). SGLT2 Inhibitors as Calorie Restriction Mimetics: Insights on Longevity Pathways and Age-Related Diseases. Endocrinology, 162(8), bqab079.
- Xu, L., Nagata, N., Nagashimada, M., Zhuge, F., Ni, Y., Chen, G., Mayoux, E., Kaneko, S., & Ota, T. (2017). SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine, 20, 137–149.
- van Heemst, D., Beekman, M., Mooijaart, S. P., & Westendorp, R. G. (2005). Reduced insulin/IGF-1 signalling and human longevity. Mechanisms of Ageing and Development, 126(3), 298–302.