When Hormones Shift the Brain’s Set Point: Rewiring of Hypothalamic Circuits in Perimenopause and Evidence-Based Strategies to Counter It
Perimenopause: A Distinct but Overlooked Transitional Phase. Perimenopause often begins in a woman’s late 30s to early 40s and can last anywhere from 4 to 10 years. Despite affecting over 1.3 million women in the U.S. annually, it remains underrepresented in research and clinical training. Symptoms such as disrupted sleep, mood swings, and gradual weight gain frequently appear years before the final menstrual period, reflecting the impact of fluctuating sex hormones—particularly estrogen and progesterone—on multiple systems.
Estrogen in Flux: Metabolic and Appetite Consequences. Estrogen enhances insulin sensitivity, regulates glucose uptake, and promotes fat storage in subcutaneous rather than visceral depots. During perimenopause, estradiol levels can drop by up to 60% within a few years. This hormonal shift contributes to a 5–8% increase in visceral fat and can lower resting energy expenditure by approximately 50–70 kcal/day. Compounding this, reduced estrogen decreases hypothalamic appetite suppression, leading to increased caloric intake and difficulty regulating body weight.
Neural Circuitry and the Hypothalamus: A New Lens on Weight Gain. The hypothalamus integrates hormonal cues to maintain energy homeostasis, and estrogen’s effects are mediated through ERα signaling in regions like the arcuate nucleus and ventromedial hypothalamus. In women, hypothalamic ERα expression declines with age, contributing to impaired appetite regulation and thermogenesis. Functional MRI studies have shown decreased hypothalamic activity in response to satiety cues in perimenopausal women compared to premenopausal controls, further linking hormonal decline to central dysregulation of energy balance.
Experimental Evidence: How ERα Disruption Mimics Perimenopausal Metabolic Shifts. Rodent models confirm the causal role of ERα in regulating metabolism. A recent study demonstrated that deleting ERα in SF1 neurons of the ventromedial hypothalamus reduced energy expenditure and led to a 20% increase in visceral fat without changes in food intake. ERα deletion in POMC neurons of the arcuate nucleus induced hyperphagia but did not affect energy burn. When both pathways were disrupted, mice gained over 15% more weight than controls, mimicking the dual burden of increased appetite and reduced metabolic rate observed in perimenopausal women.
Progesterone’s Decline and Its Reproductive and Neuroendocrine Effects. Anovulatory cycles—common in late reproductive age—result in sharp declines in luteal-phase progesterone, often dropping below 3 ng/mL when ovulation fails. Low progesterone contributes to sleep disturbances, elevated cortisol, and heavy or irregular bleeding due to unopposed estrogen. Studies estimate that up to 30–50% of perimenopausal cycles are anovulatory, and this hormonal imbalance can increase the risk of endometrial hyperplasia by two to threefold if left unregulated.
Evidence-Based Strategies: Nutrition and Exercise Interventions. Caloric needs drop by ~250–300 kcal/day during the menopausal transition, requiring adjustment to prevent weight gain. In a 12-week intervention, women following a DASH-style diet paired with resistance training lost 2.1 kg on average, reduced waist circumference by over 5 cm, and increased trunk muscle mass by 0.14 kg compared to standard care. Additional studies show that consuming 1.0–1.2 g/kg of protein and performing strength training 2–3 times per week helps preserve lean mass and support metabolic health during this stage.
Reframing Perimenopause: From Disruption to Opportunity. Rather than viewing perimenopause as a time of inevitable decline, it can be seen as a critical opportunity for preventive care. Intervening during this stage—when weight gain, insulin resistance, and vasomotor symptoms begin to surface—can improve long-term outcomes. Lifestyle and clinical strategies grounded in emerging neuroendocrine research offer a path to mitigate these changes, empowering women to navigate this life stage with greater agency and support.
Introduction
For decades, the spotlight in women’s health research has rested squarely on menopause, the clear-cut cessation of menstruation. But mounting evidence suggests that the real physiological turbulence often begins much earlier—during a lesser-known but critically important transition called perimenopause. Spanning a few months to more than a decade, perimenopause typically begins in a woman’s late 30s or early 40s. It is a time marked by shifting levels of estrogen and progesterone, which can silently and gradually reshape everything from mood and sleep to fat distribution, metabolic rate, and menstrual regularity.
And yet, perimenopause remains surprisingly under-discussed and under-researched. Unlike menopause, which is defined by a single event—the final menstrual period—perimenopause has no such threshold. Its onset is diffuse and its symptoms often ambiguous, ranging from mild anxiety to stubborn weight gain or disrupted sleep. But beneath these surface-level symptoms lies a complex neuroendocrine story—one increasingly illuminated by cutting-edge research.
A growing body of evidence points to the hypothalamus as a central hub for these changes. This region of the brain, responsible for regulating appetite, energy expenditure, and reproductive function, is exquisitely sensitive to hormonal signals. As estrogen levels fluctuate and decline, the hypothalamic circuits that once maintained metabolic homeostasis begin to misfire. Experimental models, particularly in rodents, have revealed how estrogen receptor alpha (ERα) in specific neuronal populations—such as SF1 neurons in the ventromedial hypothalamus and POMC neurons in the arcuate nucleus—regulate energy output and appetite suppression, respectively. When these pathways lose their estrogenic input, mice show increased hunger, reduced metabolic rate, and significant visceral fat accumulation, mirroring the physiological changes many women experience in perimenopause.
These findings offer critical insight into why weight gain during perimenopause often feels resistant to lifestyle change. It’s not merely a matter of willpower or calories—it’s a rewiring of hormonal control centers in the brain. And estrogen’s influence doesn't stop at metabolism; it extends to the cardiovascular, immune, and skeletal systems as well.
This review synthesizes what we now understand about perimenopause: from estrogen’s neural circuitry to progesterone’s role in stabilizing the uterine lining, and from clinical hormone interventions to nutritional and exercise-based strategies. Drawing from animal studies and clinical trials alike, we’ll examine how to navigate this transition with greater scientific understanding—and how targeted, evidence-based interventions can ease its most disruptive symptoms. When properly addressed, perimenopause is not just a stage to endure, but a pivotal opportunity to optimize long-term health and well-being.
Defining Perimenopause
Perimenopause is the transitional phase leading up to menopause. Historically overlooked and often categorized under the broader umbrella of menopause, perimenopause has recently gained recognition as a distinct stage with its own unique challenges. Typically beginning in a woman’s late 30s to early 40s, perimenopause can last anywhere from a few months to over a decade. It concludes once a woman has gone 12 consecutive months without a menstrual period, marking the onset of menopause. [1]
During this phase, fluctuating levels of estrogen and progesterone can bring about a wide range of physical and emotional changes. In addition to shifts in metabolism, fat distribution, and muscle mass, women may experience mood swings, sleep disturbances, irregular menstrual cycles, hot flashes, and changes in libido. Weight gain—particularly around the abdomen—is a common concern, but it is just one of many symptoms that can affect overall well-being.
Estrogen in Flux During Perimenopause
Estrogen is fundamental to metabolic regulation in premenopausal women. By promoting fat storage in the hips and thighs—a pattern associated with better cardiovascular outcomes—it offers a degree of protection against heart disease. Equally important, estrogen enhances insulin sensitivity, helping maintain stable blood glucose levels and reducing the risk of metabolic disorders. [2]
However, as women transition into perimenopause, estrogen levels begin to fluctuate erratically and gradually decline. These hormonal shifts trigger several notable changes in the body. Fat storage moves from the hips and thighs to the abdominal region, raising the likelihood of metabolic syndrome and cardiovascular disease. Meanwhile, lower estrogen levels contribute to reduced insulin sensitivity, making it easier to store fat and more difficult to regulate blood sugar. Compounding these issues is the fact that estrogen partially suppresses appetite. Its decline often translates into increased hunger and cravings, factors that can intensify weight gain during this period. [2]
Research points to the hypothalamus as a key mediator of these processes. This brain region governs food intake, energy balance, and numerous aspects of the body’s internal balance. However, during perimenopause, the loss of estrogen as a signal leads to disruption in rhythm, tempo, and cohesion across the system.
Within the hypothalamus are specialized nuclei that serve as control hubs for energy balance. Among these, the arcuate nucleus, ventromedial hypothalamus (VMH), and paraventricular nucleus (PVN) are critical players. These areas house neurons that integrate hormonal signals and send commands that influence how much we eat, how much energy we expend, and where our body stores fat.
Estrogen communicates with these brain regions by binding to estrogen receptors, particularly estrogen receptor alpha (ERα). These receptors act like molecular antennas, receiving estrogen’s signals and triggering changes in gene expression that influence metabolism and behavior. In animal models, activating ERα in the arcuate nucleus has been shown to suppress food intake, increase thermogenesis, and even regulate reproductive hormone signaling—demonstrating just how far-reaching this single pathway can be.
During perimenopause, however, the hormonal inputs to this system become erratic. Estrogen levels fluctuate unpredictably, and over time, begin to decline. ERα expression follows suit. When estrogen is abundant, ERα receptors are upregulated and responsive; when estrogen wanes, these receptors diminish in number and sensitivity. This dual loss—of both the signaling molecule and its receptor—reduces the hypothalamus’s ability to suppress appetite and maintain metabolic efficiency.
The result? A brain that becomes less adept at regulating food intake and energy use, leading to increased hunger, diminished satiety, and shifts in body composition. In this way, the hypothalamus—once a tightly controlled command center—begins to lose its ability to orchestrate metabolic harmony, contributing to many of the changes women experience during the menopausal transition. [3]
Experimental Insights: How Hypothalamic Estrogen Receptors Influence Metabolism and Appetite
A foundational study by Xu and colleagues (2011) helped illuminate how estrogen’s metabolic influence is not monolithic but rather distributed across discrete neural circuits in the hypothalamus. Using a genetic approach to selectively delete estrogen receptor alpha (ERα) from specific neurons in the brains of female mice, the researchers effectively simulated the low-estrogen environment of perimenopause and menopause. The goal was to map out how estrogen orchestrates metabolism and appetite—and to determine whether these functions rely on shared or distinct neural pathways. [3]
Their first focus was the ventromedial nucleus (VMN) of the hypothalamus, a region historically linked to thermogenesis and energy output. Within this nucleus lies a population of neurons expressing steroidogenic factor-1 (SF1), a transcription factor that defines cells involved in the regulation of energy balance. When ERα was selectively deleted in these SF1-expressing neurons, the mice did not eat more—but they gained significant visceral fat and exhibited a marked reduction in energy expenditure. This decoupling of food intake from weight gain revealed a critical insight: estrogen modulates basal metabolic rate independently of its effects on appetite, and it does so via ERα signaling in SF1 neurons.
You can think of these hypothalamic neurons as part of a dual control system, akin to climate control in a smart building. Some neurons (like the SF1 group in the VMN) manage the “thermostat”—influencing how many calories the body burns at rest—while others (as explored later in the study) adjust the “supply valve,” regulating food intake. The loss of ERα in the VMN leaves the thermostat turned down, so even without increasing caloric intake, the body begins to store more fat, especially in the abdominal region—mirroring the metabolic phenotype often seen in perimenopausal women.
Next, the researchers turned their attention to another key player in hypothalamic energy regulation: the pro-opiomelanocortin (POMC) neurons of the arcuate nucleus (ARC). These neurons serve as a central node in the brain’s appetite-suppressing network, producing signals like α-MSH (alpha-melanocyte-stimulating hormone) that reduce food intake and promote satiety. When ERα was selectively removed from POMC neurons, the mice developed hyperphagia, or excessive eating, despite no significant changes in resting metabolism or fat distribution.
This outcome draws a clear boundary between two major arms of estrogenic regulation in the hypothalamus: POMC neurons modulate appetite, while SF1 neurons govern energy expenditure. The distinction suggests that estrogen’s protective effects against weight gain are not the result of a single, centralized command but rather a division of labor across specialized neural circuits—each responsible for maintaining a different dimension of metabolic homeostasis.
If the hypothalamus is a regulatory “control tower,” then POMC and SF1 neurons are like different runways—one managing intake (how much energy is brought in), the other managing output (how much energy is spent). When ERα signaling is intact, both systems operate smoothly under estrogen’s direction. But when estrogen wanes during perimenopause, both systems can begin to fail—one leading to increased hunger, the other to reduced energy burn.
The researchers confirmed this by performing a dual knockout, deleting ERα from both POMC and SF1 neurons. The result was a synergistic disruption: mice not only ate more but also burned fewer calories, resulting in pronounced visceral fat accumulation and total body weight gain. This combined phenotype serves as a striking analog to the human perimenopausal transition, when women often experience both increased appetite and declining metabolic efficiency—despite no overt changes in behavior.
The data visuals from Xu et al.'s study provide a compelling window into how disrupted estrogen signaling alters body weight trajectories. In one key figure, the researchers tracked weekly body weights of female mice lacking ERα specifically in POMC neurons (ERα^lox/lox/POMC-Cre) and compared them to genetically identical control mice with intact estrogen signaling (ERα^lox/lox). Over time, the POMC-ERα knockout mice consistently weighed more, highlighting the cumulative metabolic consequences of even a targeted disruption in hypothalamic estrogen signaling.
Interestingly, the observed weight gain was driven not only by increased fat mass but also by changes in lean mass—a shift that suggests a broader metabolic remodeling beyond simple fat accumulation. This distinction matters, as it underscores that the loss of ERα impacts multiple facets of body composition, not merely fat storage. Estrogen’s absence in POMC neurons appears to tip the internal scales toward increased food intake and altered energy allocation, even in the absence of gross caloric excess or reduced physical activity.
These findings help explain a frustrating phenomenon familiar to many women during perimenopause: the sensation of gaining weight despite maintaining consistent dietary and exercise habits. As estrogen levels decline, the brain’s neural circuits that normally keep appetite and energy expenditure in equilibrium begin to lose their regulatory grip. What once was a tightly tuned system becomes noisier and less responsive, allowing gradual, seemingly inexplicable weight shifts to take hold. [3]
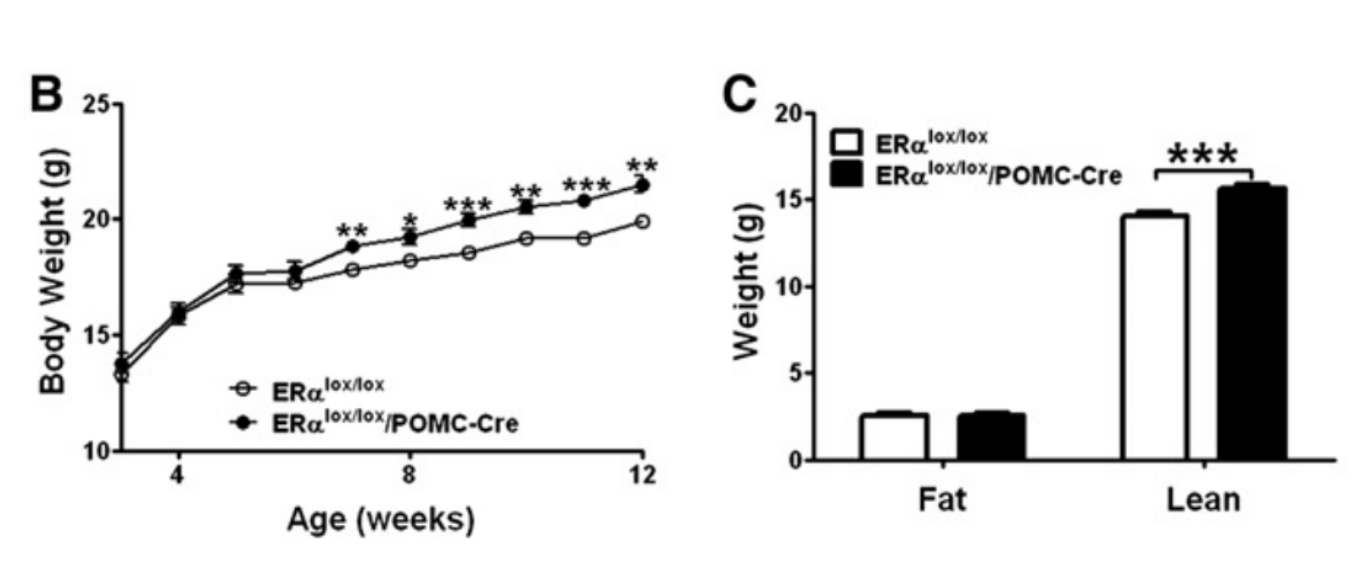
Figure 1. Graph B provides a pictorial demonstration of the effects of ERα receptor depletion in the POMC neurons. The first figure shows the weekly body weight (in g) of female rodents with deleted ERα receptors in the POMC region (ERαlox/lox/POMC-Cre) vs the control group who did not have these receptors deleted (ERαlox/lox). As can be seen, the body weight was consistently higher across the weeks in the experimental group than in the control group. Additionally, Graph C demonstrates that these discrepancies in weight were related to variations in lean mass rather than fat between the two groups. [3]
The Role of Progesterone and Its Changes During Perimenopause
Although perimenopause is often viewed through the lens of declining estrogen, progesterone—another key hormone—also undergoes significant fluctuations during this life stage. Progesterone works with estrogen in a healthy reproductive cycle to help regulate fluid balance, mood stability, and the menstrual cycle. As perimenopause progresses, progesterone levels steadily decrease, a change that can amplify water retention, bloating, and stress responses. Because progesterone exerts a calming, sleep-promoting effect, its decline can also disrupt sleep patterns, contributing to elevated cortisol levels and subsequent weight gain.
One of the most direct consequences of low progesterone arises in the endometrium, the lining of the uterus. Under normal conditions, estrogen helps build up the uterine lining, while progesterone ensures that it is shed regularly. When progesterone levels are too low, estrogen’s growth-promoting influence on the endometrium becomes unopposed. The result can be a thickened uterine lining that sheds at irregular intervals, leading to heavy or prolonged menstrual bleeding. Over time, repeated episodes of unregulated endometrial growth may heighten the risk of endometrial hyperplasia and potentially endometrial cancer. Another hallmark of fluctuating progesterone levels is irregular shedding of the uterine lining, which can manifest as spotting between periods, skipped cycles, or bouts of uncommonly heavy bleeding. In some instances, ovulation does not occur at all, further exacerbating menstrual irregularities. [4]
The protective role of progesterone becomes particularly evident when examined through the lens of clinical intervention. A notable study by Pelisser et al. (2001) investigated how different forms of progestogens affect menstrual stability and endometrial health in women undergoing early menopause. The researchers followed 336 women, all in the early stages of the menopausal transition, and assigned them to one of two hormone therapy regimens: one group received estrogen combined with chlormadinone acetate (CMA)—a synthetic progestin—while the other was given estrogen plus micronized progesterone, a formulation bioidentical to the progesterone naturally produced by the ovaries.
Over a period of 6 to 18 months, a clear pattern emerged. Approximately one-third of the women receiving micronized progesterone achieved amenorrhea—the absence of menstrual bleeding—a clinical marker often associated with stabilized hormonal cycling and successful endometrial regulation. Moreover, this group exhibited more consistent and predictable bleeding patterns compared to those receiving CMA, whose cycles remained more erratic.
These findings highlight a critical therapeutic insight: micronized progesterone not only complements estrogen in managing perimenopausal symptoms but also plays a key role in maintaining uterine health and cycle regularity. Where synthetic progestins like CMA may blunt some estrogenic effects, they often do so with a different pharmacological profile and less predictable outcomes. In contrast, micronized progesterone appears to synchronize more naturally with estrogen, restoring a rhythm that mirrors the body’s original hormonal cadence.
You might think of estrogen as a builder—stimulating endometrial growth—and progesterone as the architect that ensures the structure develops properly and is eventually dismantled in a timely fashion. Without that architectural guidance, estrogen can build unchecked, resulting in endometrial overgrowth and unpredictable shedding. The Pelisser study provides compelling evidence that reintroducing bioidentical progesterone can reinstate architectural order, reducing the likelihood of irregular bleeding and lowering the long-term risk of hyperplasia. [4]
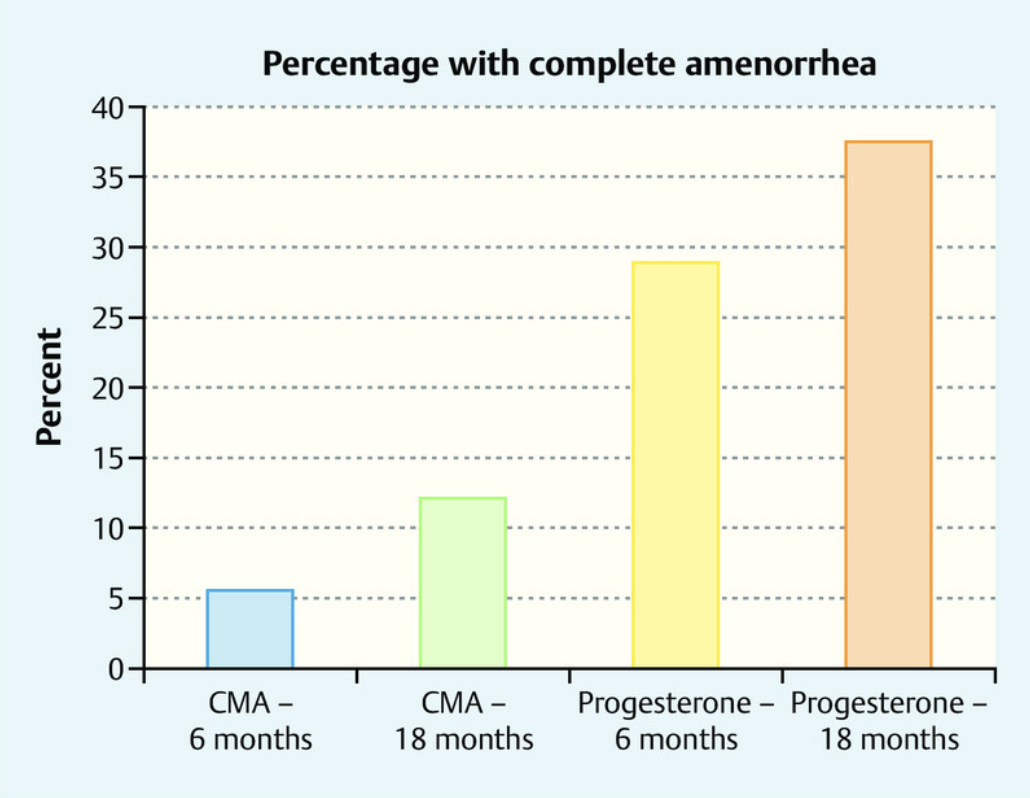
Figure 2. This chart compares the percentage of women who experienced amenorrhea after 6 and 18 months of treatment with either chlormadinone acetate (CMA) or micronized progesterone. The progesterone group showed a higher rate of amenorrhea than the CMA group, illustrating progesterone’s key role in stabilizing the uterine lining. During perimenopause, when progesterone levels decline, estrogen’s influence can dominate, often causing irregular or heavy menstrual bleeding. [4]
Ultimately, while declining estrogen is often the focal point of perimenopausal discussions, the parallel drop in progesterone plays an equally important role in shaping the hormonal landscape. The combined reduction of both hormones has far-reaching implications for weight, mood, and menstrual regularity.
Etiological Bases of Declining Estrogen and Progesterone Levels
From the studies discussed thus far, it is clear that both estrogen and progesterone undergo substantial declines during perimenopause and menopause, raising the question: why do these levels diminish so dramatically?
The answer lies in the natural aging process of the ovaries. Women enter life with a finite number of ovarian follicles, each harboring an egg. Over decades, the follicles steadily decrease in number, and as perimenopause approaches, dwindling follicular reserves lead to erratic and ultimately lower estrogen production. This shift disrupts the menstrual cycle and ripples through metabolism, mood regulation, and a myriad of other physiological processes. After menopause, ovarian estrogen production halts almost entirely, replaced primarily by estrone, a weaker form of estrogen synthesized in adipose (fat) tissue.
Progesterone, meanwhile, follows a similar downward trajectory—albeit for a different reason. Ovulation, which releases an egg monthly, is the main trigger for progesterone secretion. When fertilization does not occur, the egg and the uterine lining are shed during menstruation. As perimenopause advances, however, ovulation grows increasingly sporadic; in some cycles, it does not happen at all. Fewer ovulatory events mean less progesterone is produced, contributing to erratic or heavier menstrual bleeding, sleep disturbances, and mood fluctuations.
Together, these declining hormone levels help explain why so many women encounter weight gain, shifting fat distribution, and emotional ups and downs during perimenopause. Metabolism, appetite signals, and even the brain’s mood and energy regulation all hinge on the complex interplay between estrogen and progesterone. When these hormones drop, the body must recalibrate, leading to transitional symptoms.
Fortunately, a strategic approach to nutrition can help ease the impact of hormonal changes, supporting weight management and overall health. In the next section, we will explore how evidence-based dietary interventions can mitigate some of the most common challenges of perimenopause and facilitate a smoother transition into this new life stage.
Nutritional Approaches for Perimenopausal Weight Loss and Health
One of the most notable shifts during perimenopause and menopause is a slowing of metabolism, as declining hormone levels reduce the number of calories the body burns at rest—sometimes by 250–300 calories per day.
If a woman’s eating and exercise habits remain unchanged, this subtle drop in daily energy expenditure can lead to a gradual weight gain of roughly two kilograms per year. Compounding the issue, changes in body composition often favor increased abdominal fat, placing many women at greater risk of becoming overweight or obese. [1]
In response to this metabolic slowdown, a calorie deficit remains the cornerstone of weight management. However, extreme or overly restrictive diets carry significant drawbacks. Consuming fewer calories than the body’s baseline needs—its basal metabolic rate (BMR)—can make long-term weight loss nearly impossible.
Diets under 1200 kcal per day raise the likelihood of nutrient deficiencies, while very low-calorie diets (under 800 kcal per day) increase the risk of gallstones. In fact, nearly half of individuals following these extreme restrictions regain weight within a year. Furthermore, diets that fall between 800–1000 kcal per day rarely produce sustainable weight loss or promote healthy lifestyle habits.
A safer and more effective strategy is to reduce daily intake by 500–1000 calories—enough to lose about 0.5–1 kilogram per week. This typically translates to consuming around 25 kcal per kilogram of current body weight each day, although exact requirements vary from person to person. Equally important is preserving muscle mass throughout this process. Experts recommend a protein intake of 1–1.2 grams per kilogram of body weight each day (roughly 20% of total calories), combined with regular strength training. While higher-protein diets can be beneficial, they only aid weight loss if total calorie intake remains appropriately controlled. [5]
Over the course of a weight-loss plan, progress typically slows after about 12 weeks as the body adapts. At this stage, shifting focus to weight maintenance helps solidify results. Many clinicians suggest a cyclical approach: shed a set amount of weight, work to maintain it, and then repeat until achieving an overall 5–10% reduction in body weight. This gradual, targeted strategy tends to foster healthier habits that endure long after initial weight loss. [5]
Ultimately, a balanced diet is the most reliable way to foster sustainable weight management. Research indicates that low-carb and low-fat approaches can produce similar results, though their effects on cardiovascular health may differ. The most straightforward tactic for cutting 500–700 calories a day is to reduce portion sizes, skip high-calorie snacks between meals, and limit sugary beverages and alcohol. Taken together, these measures offer a practical way to navigate the metabolic shifts of perimenopause while supporting overall well-being.
Research Spotlight: The Impact of DASH-Based Interventions on Perimenopausal Health
Hao et al. (2021) set out to determine how different lifestyle interventions could improve key health markers in perimenopausal women. A total of 78 participants took part in the study, each randomly assigned to one of three groups for three months of guidance in nutrition, exercise, or both. By comparing the outcomes of these groups, the researchers aimed to pinpoint which combination of strategies most effectively addresses the metabolic and body composition shifts commonly experienced during perimenopause. [5]
Group A received the baseline intervention, consisting of standard gynecological diagnoses and treatments, as well as general health lectures led by a multidisciplinary team of nutritionists, pharmacists, and nurses. These sessions covered fundamental topics, including menopausal nutrition and metabolism, recommended exercise practices, and medication considerations. The goal was to provide women with a broad overview of self-care during the menopausal transition, emphasizing awareness of hormonal changes, potential side effects of medications, and basic dietary principles. However, personalized dietary or exercise coaching was not included in this intervention.
Participants in Group B were offered all of the resources given to Group A—gynecological consultations and general health lectures—but also received a more targeted nutritional intervention using the DASH (Dietary Approaches to Stop Hypertension) framework. Each participant maintained a weekly food diary, documenting their meals for three days every week. These logs were uploaded via WeChat, allowing healthcare professionals to identify and correct problematic eating habits promptly. In addition, participants attended monthly meal demonstrations that modeled balanced portion sizes and offered practical strategies for adhering to the DASH guidelines. This layered approach sought to reinforce dietary education through real-time feedback, ensuring that any misconceptions could be addressed quickly.
Building on the group-based education and personalized DASH instruction offered to Group B, Group C added a structured exercise component led by professional sports coaches. Alongside dietary counseling, participants in Group C engaged in hands-on training sessions that showcased safe and effective workout techniques tailored to perimenopausal needs. These on-site trainings covered everything from aerobic activities to resistance exercises, with coaches providing individualized modifications to accommodate different fitness levels and health conditions. By pairing specialized nutritional advice with supervised physical activity, the Group C intervention aimed to provide a comprehensive framework for maintaining a healthy body composition.
Central to the interventions in Groups B and C was the DASH diet. Originally developed through NIH-funded research in the 1990s, DASH quickly gained recognition for its ability to lower blood pressure and improve cardiovascular health. The diet prioritizes fruits, vegetables, whole grains (such as brown rice and quinoa), and lean proteins, including poultry, fish, beans, and nuts. Low-fat or fat-free dairy products supply calcium and vitamin D without an overabundance of saturated fat, while sodium intake is kept to a minimum to support blood pressure control. Sources of healthy fats—olive oil, avocados, and nuts—are emphasized in place of saturated and trans fats, further bolstering heart health and metabolic function.
At the conclusion of the three-month period, all groups showed improvements in outcomes such as waist circumference, body fat, and muscle mass (see Figure 3). However, Groups B and C generally experienced more pronounced benefits than Group A. For example, Group B reported an average waist circumference decrease of 5.52 cm, while Group C recorded 5.53 cm. Group A, by comparison, saw a smaller reduction of 4.72 cm. Likewise, weight loss was most substantial in Groups B (2.12 kg) and C (1.35 kg), whereas Group A lost only 0.63 kg. Notably, Group C also demonstrated a significant increase in trunk muscle mass—an indicator of the added advantage that structured exercise can provide. [5]
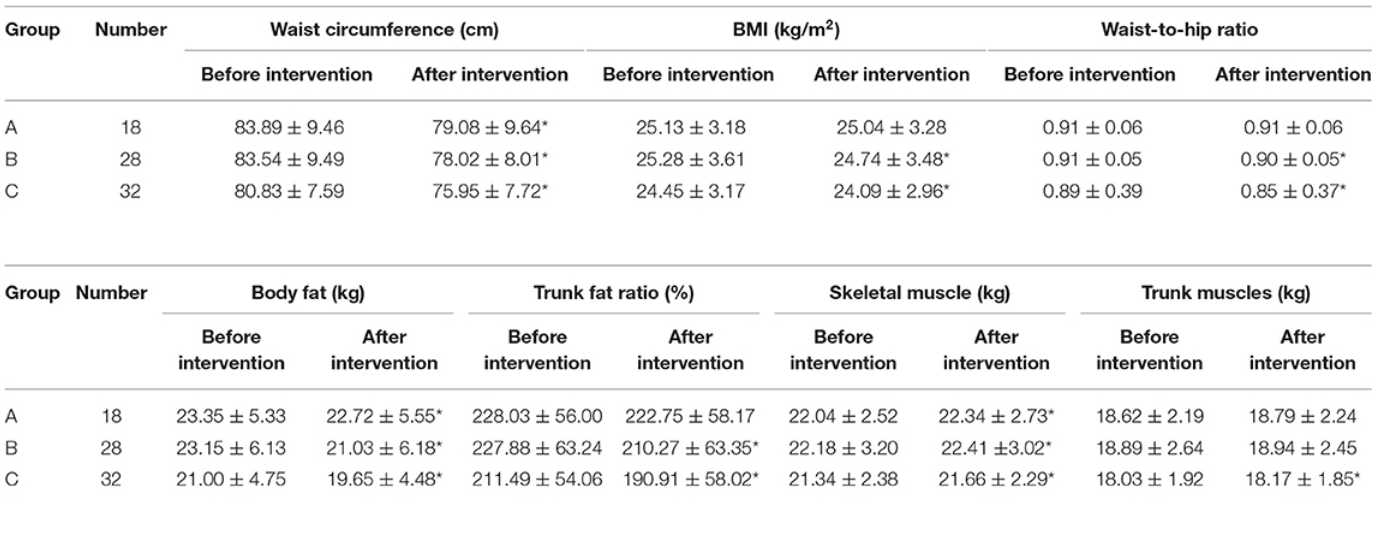
Figure 3. Figure 3. This table presents the pre-and post-intervention values for waist circumference, BMI, waist-to-hip ratio, body fat, trunk fat ratio, skeletal muscle, and trunk muscle mass in three groups of perimenopausal women. Group A received standard care plus general health education, Group B added personalized DASH-based dietary guidance, and Group C combined those interventions with structured exercise coaching. Groups B and C achieved more substantial improvements in weight-related and body composition measures than Group A.
This study underscores the effectiveness of combining a DASH-based diet with personalized coaching and physical activity for managing weight and body composition during perimenopause. The superior results in Groups B and C illustrate how individual guidance can translate nutritional principles into actionable daily habits and how regular exercise further enhances metabolic regulation. With these findings in mind, we now examine the role of exercise alone—or in tandem with dietary changes—in mitigating perimenopausal symptoms and supporting long-term health.
Exercise Training for Perimenopausal Weight Loss and Health
One of the emerging themes in perimenopausal research is the pivotal role of structured exercise programs in managing body composition and overall well-being. As hormonal levels fluctuate, many women experience challenges such as central weight gain, loss of muscle mass, and diminished energy. Exercise—particularly when combined with targeted dietary interventions—can help counter these effects by boosting metabolic rate, preserving muscle, and enhancing mood and sleep quality.
A compelling example is provided by Hao et al. (2022), whose study evaluated how a multifaceted regimen affects trunk muscle mass in perimenopausal women. The participants in Group C received not only personalized dietary support but also a carefully designed workout plan. Twice weekly, they engaged in 30 to 40 minutes of resistance exercises—beginning with a five-minute warm-up, transitioning to 15 minutes of upper-limb dumbbell routines, then 15 minutes of lower-limb squats and squat walking, and concluding with a five-minute cool-down. In addition, they were encouraged to meet a daily step count of 8,000 to 10,000 through brisk walking or jogging, helping to ensure regular aerobic activity. This combined approach significantly increased trunk muscle mass (from an average of 18.03 kg to 18.17 kg), highlighting how a dual focus on strength and cardiovascular training can help maintain muscle integrity during perimenopause. [5]
The value of exercise extends well beyond body composition, as illustrated by a randomized controlled trial by Moilanen et al. (2012). This study explored how aerobic exercise might influence menopausal symptoms such as hot flashes, mood swings, and irritability. The randomized controlled trial enrolled 176 women aged 45 to 63 who were 3 to 36 months post-menopause—often a period characterized by frequent vasomotor and emotional symptoms. Participants in the intervention group engaged in unsupervised aerobic workouts (e.g., brisk walking, jogging, or cycling) for approximately 50 minutes four times a week over 24 weeks. The control group, by contrast, attended bi-monthly health lectures but did not alter their usual activity level. [6]
To gauge daily symptom fluctuations, participants used mobile phone diaries, recording occurrences of hot flashes, night sweats, and mood-related issues. At the end of the study period, the aerobic group reported significant reductions in these complaints compared to the control group. These results may be scientifically explained by improved thermoregulatory control—regular exercise can help stabilize the body’s temperature regulation—and by increases in neurotransmitters like endorphins and serotonin, which can elevate mood and reduce perceived stress. Frequent cardiovascular activity appears to ease some of the most common discomforts of menopause, making day-to-day life more manageable. [6]
Another study conducted by Javadivala et al. (2020) took a more gradual, progressive approach in their 12-week program for perimenopausal women, focusing not only on symptom relief but also on the feasibility of increasing physical activity levels over time. Their intervention group started with short, low-intensity exercise sessions—about 30 minutes per day—and gradually ramped up both the duration and intensity until participants were completing 60-minute workouts at a moderate intensity by the program’s end (See Figure 4). This staged progression was designed to align with each woman’s baseline fitness level and to respect the potential for joint issues or other age-related constraints. [7]
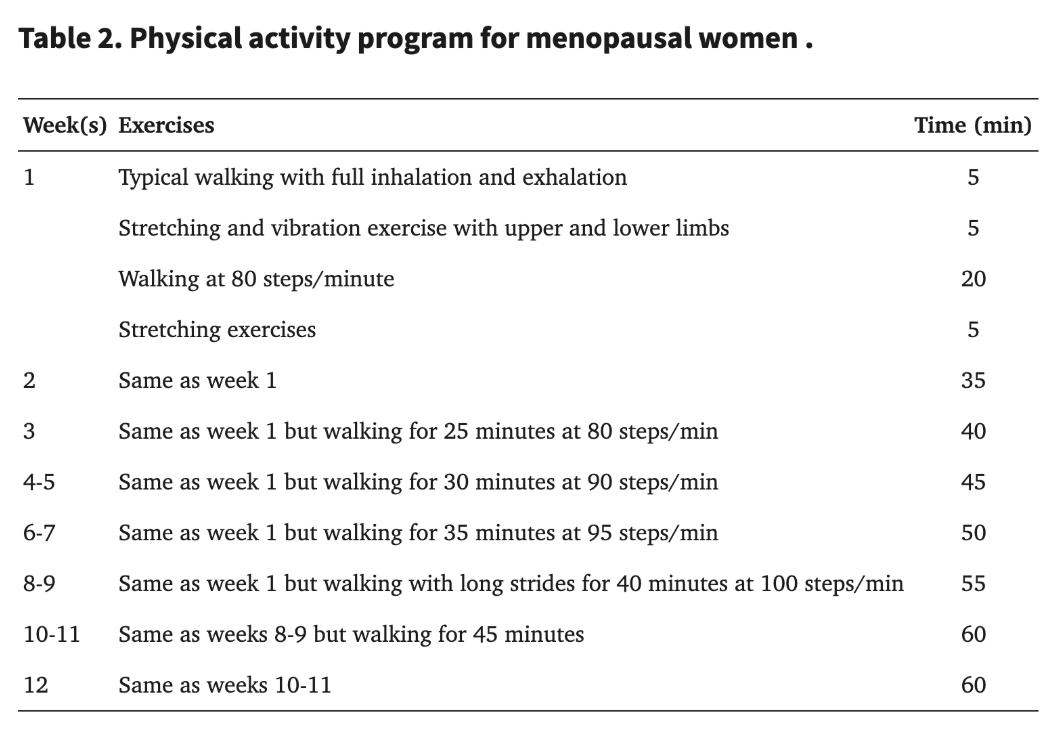
Figure 4. The pictorial above outlines the exercise strategies designed by Javadivala et al. (2021) for each of the 12 weeks for the women in the intervention group. As illustrated, the intensity of the exercises was progressively increased to facilitate acclimatization among the participants. [7]
The researchers assessed symptoms such as hot flashes, sleep disturbances, and joint discomfort at baseline and again at the 12-week mark. Their data collection involved questionnaires and possibly clinical interviews to capture both the frequency and severity of each symptom. By the conclusion of the intervention, the proportion of women experiencing severe hot flashes had dropped from 30.1% to 11.8%, while sleep problems plunged from 28% to 6.5%, and joint pain from 52.7% to 4.4%. Meanwhile, the control group—women continuing their usual care without added physical activity—reported an overall increase in these same symptoms. These striking improvements speak to the power of a tailored, incremental exercise program that respects individual capacity while steadily building endurance and strength. [7]
Collectively, these three studies illuminate how different exercise protocols—be they resistance-focused, aerobic-centered, or progressively staged—can significantly improve outcomes for perimenopausal and postmenopausal women. From increasing trunk muscle mass to reducing vasomotor disturbances and enhancing sleep quality, physical activity emerges as a potent, non-pharmacological strategy for navigating this transitional life stage. At the same time, it is important to remember that individual preferences, baseline fitness levels, and potential health conditions must guide exercise prescriptions.
Concluding Thoughts and Key Takeaways
Perimenopause may once have been overshadowed by menopause itself, but it is increasingly recognized as a dynamic and influential life stage in its own right. Shifting hormone levels—particularly fluctuating estrogen and declining progesterone—can set off a cascade of metabolic changes, from altered fat distribution to disrupted appetite regulation. Yet this transition is anything but one-dimensional: mood swings, changes in menstrual patterns, and sleep disturbances all play a part in reshaping a woman’s daily experience.
The studies and insights outlined here illustrate that these challenges, though complex, are far from insurmountable. Scientific advances have clarified the mechanisms by which estrogen and progesterone interact with hypothalamic pathways, exerting both direct and indirect effects on metabolism, body composition, and even emotional stability.
Encouragingly, researchers are also revealing a roadmap for managing these shifts more effectively. Balanced, nutrient-rich diets—such as DASH or Mediterranean-style eating—offer a foundation for addressing the metabolic slowdown that often accompanies perimenopause. Whether via resistance training or progressive aerobic regimens, layering in structured exercise can preserve muscle mass, reduce visceral fat, and diminish vasomotor symptoms like hot flashes. Notably, personalized approaches—where women receive customized dietary advice or work with professional trainers—tend to yield the most pronounced improvements in both physical and emotional well-being.
Ultimately, current findings suggest that perimenopause need not be seen as a period of inevitable decline. Although hormonal levels do wane over time, informed nutrition, exercise, and self-care choices can substantially ease the transition and support overall health.
- Erdélyi, A., Pálfi, E., Tűű, L., Nas, K., Szűcs, Z., Török, M., Jakab, A., & Várbíró, S. (2023). The Importance of Nutrition in Menopause and Perimenopause-A Review. Nutrients, 16(1), 27.
- Foryst-Ludwig, A.; Kintscher, U. Metabolic impact of estrogen signaling through ERalpha and ERbeta. J. Steroid. Biochem. Mol. Biol. 2010, 122, 74–81.
- Xu, Y., Nedungadi, T. P., Zhu, L., Sobhani, N., Irani, B. G., Davis, K. E., Zhang, X., Zou, F., Gent, L. M., Hahner, L. D., Khan, S. A., Elias, C. F., Elmquist, J. K., & Clegg, D. J. (2011). Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell metabolism, 14(4), 453–465.
- Pélissier C, Maroni M, Yaneva H. et al. Chlormadinone acetate versus micronized progesterone in the sequential combined hormone replacement therapy of the menopause. Maturitas. 2001;40:85–94. doi: 10.1016/s0378-5122(01)00170-0.
- Hao, S., Tan, S., Li, J., Li, W., Li, J., Cai, X., & Hong, Z. (2022). Dietary and Exercise Interventions for Perimenopausal Women: A Health Status Impact Study. Frontiers in nutrition, 8, 752500.
- Moilanen, J. M., Mikkola, T. S., Raitanen, J. A., Heinonen, R. H., Tomas, E. I., Nygård, C. H., & Luoto, R. M. (2012). Effect of aerobic training on menopausal symptoms--a randomized controlled trial. Menopause (New York, N.Y.), 19(6), 691–696.
- Javadivala Z, Allahverdipour H, Asghari Jafarabadi M, Emami A. An Interventional strategy of physical activity promotion for reduction of menopause symptoms. Health Promot Perspect. 2020 Nov 7;10(4):383-392. doi: 10.34172/hpp.2020.57. PMID: 33312934; PMCID: PMC7722991.