Rapamycin for Longevity: series 3
What this study provides is a foundation in which further studies into anti-aging can be used as a basis to start from as well as confirmation that rapamycin is indeed capable of extending both medial and maximal lifespan in mammalian species, i.e. us. This study truly provides a foundation in which subsequent anti-aging studies and endeavors will and already have used to build off of and further progress the field. Harrison et al demonstrated that Inhibition of the mTOR signaling pathway by genetic or pharmacological intervention extends lifespan in invertebrates, including yeast, nematodes and fruit flies [1–5]. However, Harrison et al went a step further and demonstrated that inhibition of mTOR signaling can extend life in a mammalian species, with the previously “untested” drug, rapamycin. The study demonstrated that rapamycin, an inhibitor of the mTOR pathway, extends median and maximal lifespan of both male and female mice with treatment beginning at 600 days of age. Based on age at 90% mortality of the subject population, rapamycin led to an increase of 14% for females and 9% for males. The effect was seen at three independent test sites in genetically heterogeneous mice, chosen to avoid genotype-specific effects on disease susceptibility. Please note, the genetic specific definition of heterogeneity is used with the definition of a non-uniform genetic make-up of the mice used in this study that in effect provide a varied genetic pool to utilize and study, without the risk of possible genetic-based health issues caused by homogenetic mouse populations. Furthermore, disease patterns, or the occurrence of health-related phenomena of rapamycin-treated mice did not differ from those of control mice. A separate study in which rapamycin treatment began at 270 days of age also increased survival in both males and females, based on an interim analysis conducted near the median survival point. Harrison et al explains these findings with the theory that rapamycin may extend lifespan by postponing death from cancer, by retarding mechanisms of aging, or both simultaneously. Most excitingly, these are the first results to demonstrate a role for mTOR signaling in the regulation of mammalian lifespan, as well as pharmacological extension of lifespan in both genders. Harrison et al’s findings have implications for further development of interventions targeting mTOR for the treatment and prevention of age-related diseases and aging process itself.
Because incidences of most diseases rise rapidly with age [6], interventions that delay aging will greatly benefit your general health [7–8]. To date, dietary additives that delay aging and increase lifespan in rodent models have shown only weak effects [9–11]. Before clinical studies are considered, anti-ageing interventions must be repeatable and effective in many mouse genotypes, and not merely postpone strain-specific diseases [12–14].
The National Institute on Aging Interventions Testing Program (ITP) evaluates agents that may delay aging and increase lifespan in genetically heterogeneous mice [15–17]. Agents are chosen as summarized at www.nia.nih.gov. The Study was simultaneously replicated at three separate test sites: The Jackson Laboratory (TJL), the University of Michigan (UM), and the University of Texas Health Science Center (UT). The mouse models used are as follows, BALB/cByJ × C57BL/6J F1 (CB6F1) females and C3H/HeJ × DBA/2J F1 (C3D2F1) males which, just means they used genetically heterogeneous mice which were supplied to each testing site by The Jackson Laboratory, and mated to produce genetically heterogeneous populations in which each animal is genetically unique, but a full sibling of all other mice in the population [18]. Sufficient mice are used to provide 80% power to detect a 10% increase or decrease in mean lifespan with respect to the unmanipulated control populations of the same sex, even if data from one of the three test sites were to be unavailable. Now, a quick biostatistics recap, essentially “power” or more correctly called “statistical power” is defined as the probability that the “test” correctly rejects the null hypothesis when a specific alternative hypothesis is true. The statistical power of a test is representative of the chances of a “true positive result” whose detection is conditional on the actual existence of a quantifiable effect. Additionally, power is usually set at around 80% as statistical power ranges from 0 to 1, hence the high percentages. Furthermore, this means that if there are true effects to be found within 100 different studies with 80% power, only 80 out of 100 statistical tests will actually detect them. So if the study fails to ensure sufficient power, it may not be able to detect a true effect caused by rapamycin treatment at all. Continuing on, the study reports that dietary encapsulated rapamycin increases mouse survival, including survival to the last decile, a measure of maximal lifespan, which are quite the results for a drug labeled and limited as an immunosuppressant for almost 50 years.
Rapamycin reduces function of the rapamycin target kinase TOR and has anti-neoplastic activities, and genetic inhibition of TOR extends lifespan in short-lived model organisms. In male and female mice at each of three collaborating research sites, median and maximum lifespan were extended by feeding encapsulated rapamycin starting at 600 days of age (Figure 1). We analyzed the dataset as of February 1, 2009, with 2% (38 of 1901) of mice still alive. For data pooled across sites, a log-rank test rejected the null hypothesis that treatment and control groups did not differ (p < 0.0001); mice fed rapamycin were longer lived than controls (p < 0.0001) in both males and females. Expressed as mean lifespan, the effect sizes were 9% for males and 13% for females in the pooled dataset. Expressed as life expectancy at 600 days (the age of first exposure to rapamycin), the effect sizes were 28% for males and 38% for females. Mice treated with other agents (enalapril and CAPE) evaluated in parallel did not differ from controls at the doses used (Supplemental Figure 1).
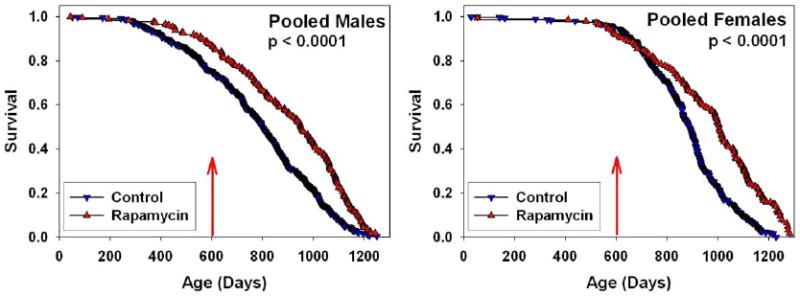
Figure 1. Survival plots for male (left) and female (right) mice, comparing control mice to those fed rapamycin in the diet starting at 600 days of age, pooling across the three test sites. P-values were calculated by the log-rank test. 4% of the control mice, and 3% of rapamycin-treated mice were removed for technical reasons. Only 5 animals (3 controls, 2 rapamycin treated mice) were removed after the start of rapamycin treatment at 600 days. Thus there were no significant differences between the groups with regards to censoring.
Rapamycin-fed and control mice were then compared separately for each combination of site and gender. Rapamycin had a consistent benefit, compared to controls, with p-values ranging from 0.03 to 0.0001 (Figure 2).
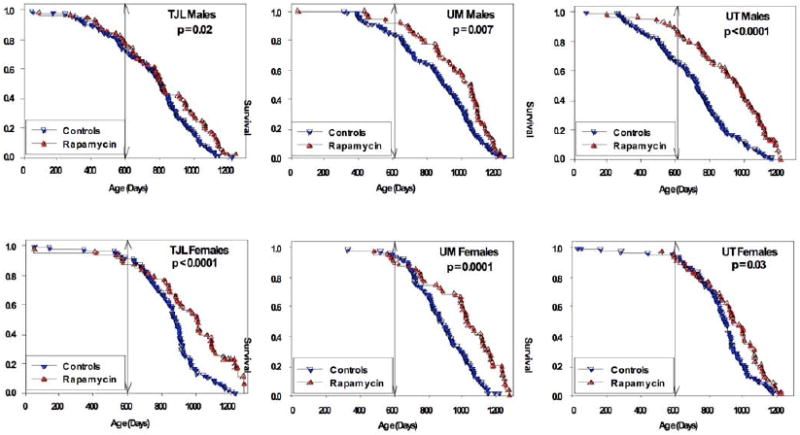
__Figure 2. Survival of control and rapamycin-treated mice for males (top) and females (bottom) for each of the three test sites separately. P-values represent results of log-rank calculations. Vertical lines at age 600 days indicate the age at which rapamycin treatment begins. __
Female mice at all three sites had improved survival after rapamycin feeding (Figure 2). Mean lifespan increases for females were 15%, 16%, and 7% (TJL, UM, UT), and life expectancy at 600 days increased by 45%, 48%, and 22% for females at the three sites. Median lifespan estimates of control females were consistent across sites (881–895 days), and were similar to values noted in Cohort 2004, which ranged from 858 to 909 days [15]. Thus the improvement in survival seen in the rapamycin-fed females is not an artifact of low survival for the control females.
Male mice at all three sites also had improved survival after rapamycin feeding (Figure 2). Mean lifespan increases for males were 5%, 8%, and 15% of TJL, UM, and UT, respectively. Furthermore, male life expectancy at 600 days increased by 16%, 23% and 52%, once again, in TJL, UM, and UT, respectively. Interpretation is somewhat complicated by differences among sites in survival of control males, and because mice assigned to the rapamycin-fed group at UT and perhaps at UM had lower mortality prior to 600 days than controls. Control mice at UT and UM differed from those fed rapamycin, not only in exposure to rapamycin from 600 days of age, but also in specific formulation of the mouse food which is based on the NIH-31 standard, which, as a side note, is food that is formulated for maintenance, growth, reproduction, and lactation in both rats and mice. It contains additional amounts of vitamins to compensate for losses that occur during steam sterilization of said food. It is used between weaning and 600 days and is basically the gold standard or more formally known as the standard reference diet for biological and biomedical research. Continuing on, we thus cannot rule out the possibility that improved survival among males in the rapamycin group, at UT and at UM, might be a reflection of differences in nutritional or health status between control and rapamycin groups prior to 600 days, rather than solely due to the effects of rapamycin treatment. Importantly, the significant benefits of rapamycin on male and female survival at TJL could not have been affected by diet prior to drug administration, because at TJL both control and rapamycin-fed mice received the same food, Purina 5LG6, throughout this period, thus dispelling any doubts of rapamycin’s efficacy in this case.
Harrison et al’s study clearly and plainly demonstrated that maximum lifespan was increased by rapamycin feeding. Table 1 shows the ages at the 90th percentile for control and rapamycin-treated mice, along with the 95% upper confidence bound for the controls. For each site and sex, the 90th percentile age for rapamycin-treated mice is higher than the upper limit for the corresponding control group, showing that rapamycin increases the age for 90th percentile survival.
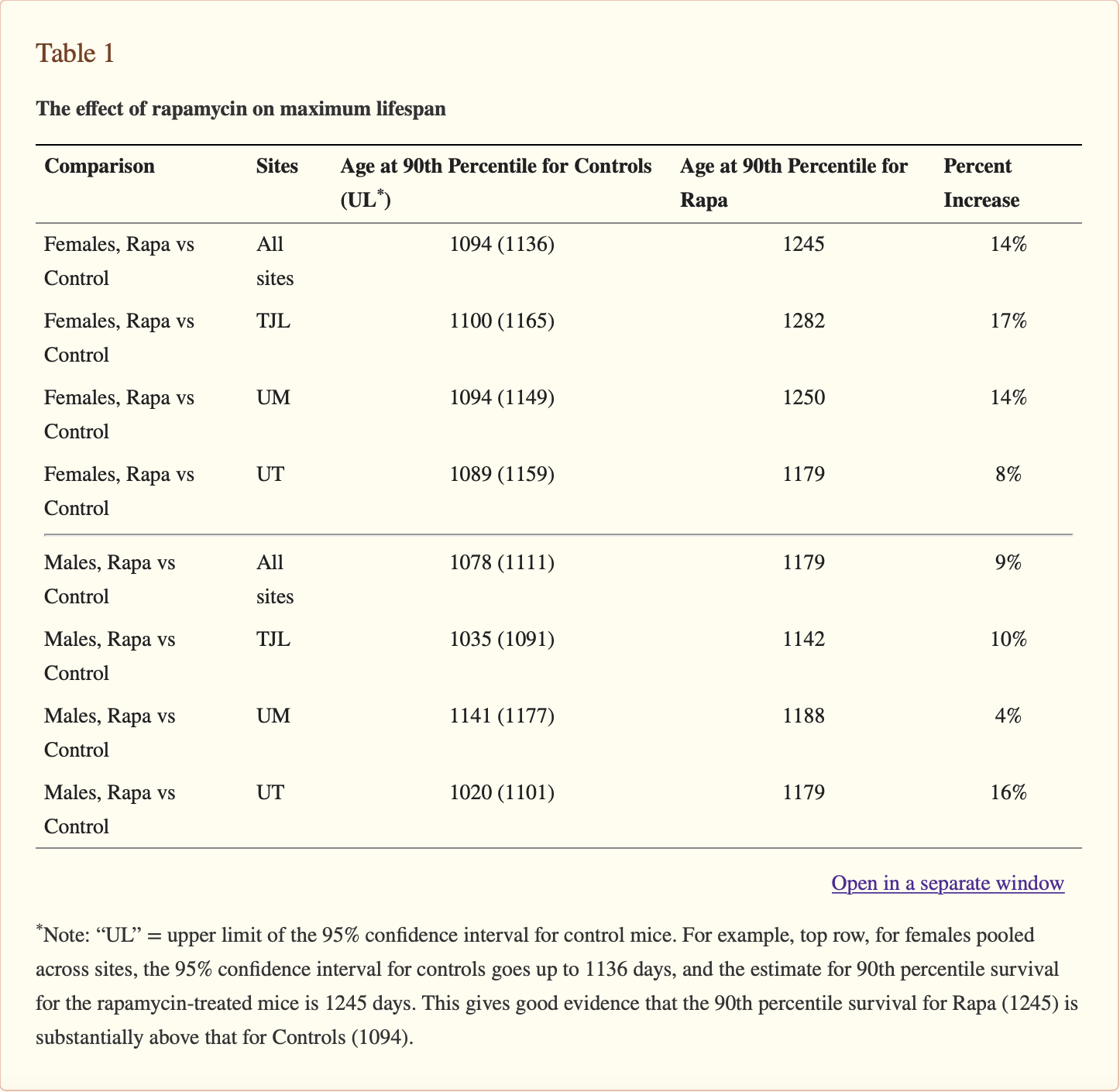
__Table 1. The effect of rapamycin on maximum lifespan Note: “UL” = upper limit of the 95% confidence interval for control mice. For example, top row, for females pooled across sites, the 95% confidence interval for controls goes up to 1136 days, and the estimate for 90th percentile survival for the rapamycin-treated mice is 1245 days. This gives good evidence that the 90th percentile survival for Rapa (1245) is substantially above that for Controls (1094).
In order to determine if increases in maximal lifespan due to rapamycin treatment are statistically significant, Harrison et al compared the proportion of living mice in each group when 90% had died in the joint life table [19] with a more in depth explanation of the details in Supplementary Table S1. Continuing on, summing across the three sites, 4.8% of the female control mice were alive at these ages, compared to 21.5% of the rapamycin-treated females (p < 0.0001). For males, the corresponding values were 5.9% of controls and 20.2% of rapamycin mice (p < 0.0001). The site-specific calculations documented a significant effect on females at both TJL (p = 0.0006) and UM (p = 0.0001); for males it was noted a significant effect at both TJL (p = 0.008) and UT (p = 0.0001), with a marginal effect at UM (p = 0.07). The essential point garnered from this being that rapamycin initiated at 600 days of age leads to a significant increase in maximal lifespan.
Next in line, Harrison et al needed to test whether the spectrum of lesions was indeed altered by dietary rapamycin treatment. Please note the term lesions incorporates any tissues and/or organs that have suffered damage via injury or disease, such as wounds, ulcers, and tumors. Achieving said test results was attained through complete necropsies that were conducted on 31 control mice and 40 rapamycin-fed mice that were either found dead or euthanized when moribound; please note a more in depth explanation of the details can be found in Supplemental Table S2. Although rapamycin postpones death, it did not change the distribution of presumptive causes of death within the sample mouse populations.
A separate group of mice was used to evaluate the effects of encapsulated rapamycin initiated at 270 days of age (Figure 3A). At the time of this analysis, 51% of the females and 68% of the males had died, and a stratified log-rank test showed significantly lower mortality risk in the rapamycin-treated mice compared to controls, pooling across the three test sites (p = 0.0002 for males and p < 0.0001 for females). Sidenote, a stratified log test is a statistical test used to compare survival distributions from two samples, ours being the rapamycin treated mice and the non-treated control mice populations. Continuing on, when each site was evaluated separately, the beneficial effect of rapamycin for females was significant at each site (p < 0.005); while for males, the effect was significant (p < 0.025) at UM and UT, but the results at TJL lacked the same significance. Consequently, rapamycin appears to reduce mid-life mortality risk when treatment starts at 270 days of age, but additional data is needed to provide an accurate estimate of effect size, and to evaluate effects on maximal longevity.
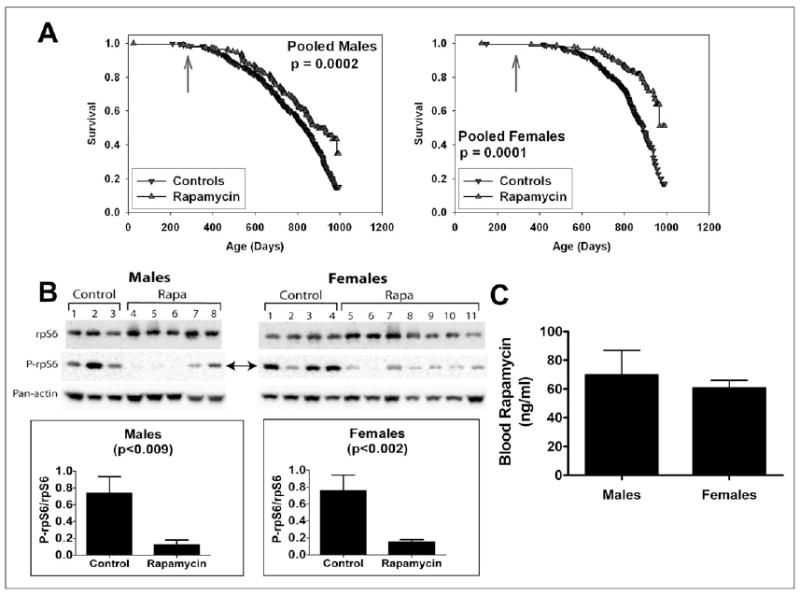
__Figure 3. A. Survival plots for male (left) and female (right) mice, comparing control mice to rapamycin-treated mice of a separate (“Cohort 2006”) population, in which mice were treated with rapamycin from 270 days of age. Because at the time of the interim analysis all live mice were between 800 and 995 days of age, we have only limited information about the shape of the survival curve at ages above 900 days, and the apparent change in slope at the oldest ages (> 990 days) reflects this experimental uncertainty. P-values calculated by the log-rank test. B. Effects of dietary rapamycin on an mTORC1 effector in the visceral fat pads from 750- to 880-day-old male and female mice. Ribosomal subunit protein S6 (rpS6) and its phosphorylation status (P-rpS6 - double arrow) were immunoassayed in tissue lysates prepared from mice consuming microencapsulated rapamycin-containing (Rapa) or control diets. Antibodies used are shown to the left. The ratio of intensity values for P-rpS6: rpS6 are shown in the graphs for females and males. Pan-actin was also immunoassayed in the blots to provide an indication of protein loading for each lane. C. Whole blood rapamycin content in 750- to 880-day-old male and female mice. __
To document biochemical effects of rapamycin at the dosage commonly used for lifespan studies, Harrison et al evaluated the phosphorylation status of the ribosomal protein subunit S6 (rpS6). rpS6 is a target substrate of S6 kinase 1 in the mTOR signaling pathway which is specific to visceral white adipose tissue, and which acts as a sensitive indicator of mTOR inhibition by rapamycin treatment in vivo [20]. In other words, following rpS6 allows Harrison et al to “see” the biochemical effects of rapamycin treatment. Figure 3B pictates that rapamycin reduced phosphorylated rpS6 4–5 fold when administered treatment occurred from 270 to about 800 days of age. Blood levels of rapamycin in the treated mice were equivalent in males and females, ranging between 60 and 70 ng/ml.
Initial evidence that a reduction of TOR function can extend longevity came primarily from studies in yeast and invertebrates [1–5.] Beneficial effects of diet restriction (DR) and dwarf mutations, both of which extend lifespan in rodents, may to some degree result from repression of the mTOR complex 1 (mTORC1) pathway [21-23]. It is not yet known to what extent inhibition of mTOR will recapitulate other aspects of the phenotypes associated with DR or dwarf mutations. Harrison et al’s demonstration that rapamycin feeding increases lifespan even when started late in life, not to mention the absence of changes in body weight (data not shown), and as such, distinguish these results from studies using DR; in all cases DR reduces body weight, and in most reports though not all, DR produces little if any benefit if started after about 550 days of age [21,24].
Rapamycin may extend lifespan in old genetically heterogeneous mice through a combination of anti-neoplastic effects and effects on cellular stress resistance and response to nutrient dynamics [25–29]. The increase in both median and maximum lifespan seen in rapamycin-fed mice is consistent with the hypothesis that inhibiting the mTORC1 pathway retards mammalian ageing, but is not compelling proof that ageing rates are altered, which would require further testing on whether the treatment of rapamycin decelerates age-dependent changes in multiple organs, cell types, and intra-cellular and extra-cellular processes [14]. Comparing effects of rapamycin treatment and other models of decelerated aging will help narrow the list of possible mechanisms for longevity extension.
At the cellular level, mTORC1 helps to coordinate growth and survival responses induced by alterations in nutrient availability, energy status, growth factor stimuli and exposure to potentially lethal cell stresses [27–29]; as a key regulator of nutrient/stress sensing pathways, the importance of TOR function in regulating lifespan in invertebrates and in mammals as well. It is especially noteworthy that rapamycin feeding can extend mouse lifespan even when started late in life; in terms of the percentage of the maximal lifespan, a 600-day-old mouse is roughly the equivalent of a 60-year-old person [14]. An effective anti-ageing intervention that could be initiated later than the midpoint of the lifespan could prove to be especially relevant to clinical situations, in which the efficacy of anti-ageing interventions would be particularly difficult to test in younger volunteers. Data garnered by Harrison et al’s study further justifies spending special attention and further research into the role of the TOR pathway in control of aging in mammals and in the pathogenesis of late-life illnesses.
1. Kaeberlein M, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. [PubMed] [Google Scholar]
2. Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. [PMC free article] [PubMed] [Google Scholar]
3. Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. [PubMed] [Google Scholar]
4. Kapahi P, et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. [PMC free article] [PubMed] [Google Scholar]
5. Vellai T, et al. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620.[PubMed] [Google Scholar]
6. Kohn RR. Principles of Mammalian Aging. 2nd. Prentice-Hall; NJ: 1978. p. 151. 1978 Fig. 6.6. [Google Scholar]
7. Miller RA. Extending life: scientific prospects and political obstacles. Milbank Quarterly. 2002;80:155–174. [PMC free article] [PubMed] [Google Scholar]
8. Olshansky SJ, Perry D, Miller RA, Butler RN. In pursuit of the longevity dividend. The Scientist. 2006;20:28–35. [Google Scholar]
9. Schneider EL, Miller RA. Anti-Aging Interventions. In: Tallis R, Fillit H, Brockelhurst JC, editors. Brockelhurst's Textbook of Geriatric Medicine. Churchill Livingstone; New York: 1998. pp. 193–199. [Google Scholar]
10. Archer JR, Harrison DE. L-deprenyl treatment in aged mice slightly increases lifespans, and greatly reduces fecundity by aged males. J Gerontol Biol Sci. 1996;51A:B448–453. [PubMed] [Google Scholar]
11. Schneider EL, Reed JD., Jr Life extension. New Engl J Med. 1985;312:1159–1168. [PubMed] [Google Scholar]
12. Phelan JP, Austad SN. Selecting animal models of human aging. Inbred strains often exhibit less biological uniformity than F1 hybrids. J Gerontol. 1994;49:B1–11. [PubMed] [Google Scholar]
13. Klebanov SE, et al. Maximum life spans in mice are extended by wild strain alleles. Exp Biol Med. 2001;226(9):854–859. [PubMed] [Google Scholar]
14. Flurkey K, Currer JM, Harrison DE. The Mouse in Aging Research. In: Fox JG, et al., editors. The Mouse in Biomedical Research. Second. III. Academic Press; 2007. pp. 637–672. [Google Scholar]
15. Miller RA, et al. An Aging Interventions Testing Program: study design and interim report. Aging Cell. 2007;6:565–575. [PubMed] [Google Scholar]
16. Nadon NL, et al. Design of Aging Intervention Studies: the NIA Interventions Testing Program. 2008. AGE online - http://dx.doi.org/10.1007/s11357-008-9048-1. [PMC free article] [PubMed]
17. Strong R, et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7:641–650. [PMC free article] [PubMed] [Google Scholar]
18. Roderick TH. Selection for radiation resistance in mice. Genetics. 1963;48:205–216. [PMC free article][PubMed] [Google Scholar]
19. Wang C, Li Q, Redden DT, Weindruch RD, Allison B. Statistical methods for testing effects on “maximum lifespan” Mech Ageing Dev. 2004;125(9):629–632. [PubMed] [Google Scholar]
20. Petroulakis E, Mamane Y, Le Bacquer O, Shahbazian D, Sonenberg N. mTOR signaling: implications for cancer and anticancer therapy. Br J Cancer. 2007;96(Suppl):R11–15. [PubMed] [Google Scholar]
21. Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. [PubMed] [Google Scholar]
22. Sharp ZD, Bartke A. Evidence for down-regulation of phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory signaling pathways in Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2005;60:293–300. [PubMed] [Google Scholar]
23. Hsieh CC, Papaconstantinou J. Akt/PKB and p38 MAPK signaling, translational initiation and longevity in Snell dwarf mouse livers. Mech Ageing Dev. 2004;125:785–798. [PubMed] [Google Scholar]
24. Dhahbi JM, et al. Temporal linkage between the phenotypic and genomic responses to caloric restriction. PNAS. 2004;101:5524–5529. [PMC free article] [PubMed] [Google Scholar]
25. Garber K. Rapamycin's resurrection: a new way to target the cancer cell cycle. J Natl Cancer Inst. 2001;93:1517–1519. [PubMed] [Google Scholar]
26. Lorberg A, Hall MN. TOR: the first 10 years. Curr Top Microbiol Immunol. 2004;279:1–18. [PubMed] [Google Scholar]
27. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. [PubMed] [Google Scholar]
28. Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25:6373–6383. [PubMed] [Google Scholar]
29. Sonenberg N, Hinnebusch AG. New modes of translational control in development, behavior, and disease. Mol Cell. 2007;28:721–729. [PubMed] [Google Scholar]
30. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. [PubMed] [Google Scholar]