Targeting Inflammation and Bone Loss in Periodontal Disease with Low-Dose Rapamycin: A Review of Mechanisms and Clinical Potential
mTORC1 Activation and Inflammation: mTORC1, when chronically activated due to high nutrient levels, can lead to an overproduction of pro-inflammatory cytokines, contributing to chronic inflammation and various age-related diseases. This persistent inflammation, referred to as "inflammaging," can trigger and exacerbate autoimmune conditions, as the immune system remains in a constant state of alert due to accumulated cellular debris and stress signals.
Role of Rapamycin: Rapamycin selectively inhibits mTORC1, which helps reduce excessive inflammation by allowing autophagy to resume, clearing damaged cellular components and lowering the release of damage-associated molecular patterns (DAMPs). This action not only mitigates chronic inflammation but also preserves immune function by using low doses that primarily target mTORC1, minimizing the risk of immunosuppression associated with higher doses.
Chronic inflammation driven by bacterial overgrowth is a key factor in the development of periodontal disease, leading to conditions such as gingivitis and periodontitis. This dysregulated immune response not only targets harmful bacteria but also damages beneficial bacteria and gum tissue, creating a destructive cycle. Therapeutic interventions like low-dose rapamycin aim to address these underlying inflammatory processes, offering a novel approach to improve oral health and potentially mitigate the systemic effects associated with periodontal disease.
The theoretical basis that has led researchers to explore rapamycin for periodontal disease lies in its dual action. By inhibiting mTORC1, rapamycin reduces the production of pro-inflammatory cytokines, effectively calming the chronic inflammatory response in the gums. Additionally, by reactivating autophagy, rapamycin helps cells clear away bacterial debris and damaged tissue, further reducing inflammation and promoting tissue repair. This combination of reduced cytokine-driven inflammation and enhanced cellular cleanup suggests a potential pathway to improve periodontal health and preserve gum and bone structures.
Bone Remodeling and Inflammation: Rapamycin has been shown to reduce the activity of osteoclasts, which are responsible for bone degradation, by lowering oxidative stress and the production of inflammatory markers. This action helps restore the balance between bone resorption and formation, which is crucial for maintaining bone health, especially in conditions like periodontal disease.
Rejuvenation of Oral Health: Short-term treatment with rapamycin in aging mice resulted in significant improvements in oral health, including periodontal bone regeneration, reduced inflammation, and a shift toward a healthier oral microbiome. These findings indicate that rapamycin not only mitigates the progression of periodontal disease but also has the potential to reverse age-related degeneration in oral tissues, offering systemic health benefits.
Systemic Inflammation and Health Risks: Poor oral health, particularly periodontal disease, can lead to systemic inflammation by releasing pro-inflammatory cytokines into the bloodstream. This systemic spread can exacerbate chronic inflammatory conditions, such as cardiovascular disease and diabetes, by promoting processes like atherosclerosis and increasing insulin resistance, highlighting the interconnectedness between oral health and overall systemic health.
Link Between Oral Health and Cardiovascular Disease: There is a well-established connection between periodontal disease and cardiovascular health, as chronic gum inflammation can allow bacteria to enter the bloodstream, contributing to arterial plaque formation. Studies have shown that individuals with severe periodontal disease are at a higher risk for cardiovascular conditions, with various oral bacteria identified in arterial blockages, emphasizing the importance of maintaining oral health to mitigate the risk of heart disease and related systemic issues.
Impact on the Oral Microbiome: The oral microbiome is a complex ecosystem crucial for both oral and systemic health, where the overgrowth of pathogenic bacteria like Porphyromonas gingivalis can lead to chronic inflammation and periodontal disease. Effective management of bacterial growth and biofilm formation is essential for maintaining oral health, as biofilms protect harmful bacteria from the immune system and treatments.
Rapamycin and Oral Microbiome: Emerging research suggests that low-dose rapamycin may significantly reduce microbial growth and biofilm formation, including its effectiveness against resistant pathogens like Candida haemulonii. By targeting inflammatory pathways and inhibiting mTORC1 activity, rapamycin not only helps prevent periodontitis but also holds promise for modulating the oral microbiome. Its favorable safety profile positions it as a potential therapeutic option for long-term management of periodontal disease.
Rapamycin, by modulating the mTOR pathway, controls immune response and inflammation, promotes autophagy to clear cellular debris, and enhances the survival and regenerative capacity of human periodontal ligament stem cells, particularly in the presence of cytotoxic agents like sodium hypochlorite. This suggests that rapamycin may significantly improve outcomes in regenerative endodontic procedures, supporting effective tissue regeneration and the long-term success of dental implants.
Introduction
Inflammation is the body's natural defense mechanism against infection and injury. It helps to eliminate harmful stimuli such as pathogens and damaged cells, initiating tissue repair. However, chronic and uncontrolled inflammation can lead to persistent tissue damage rather than healing. This unchecked inflammation is a crucial contributor to the progression of many age-related conditions, including periodontal disease.
Periodontal disease is a common inflammatory condition that begins with bacterial overgrowth in the mouth. While bacteria trigger the initial infection, the body's excessive immune response causes gum tissue and bone breakdown, accelerating the disease. This chronic inflammation, if left untreated, can result in significant tissue destruction, tooth loss, and a heightened risk of systemic issues such as cardiovascular disease.
Emerging research indicates that low-dose rapamycin may offer a novel approach to managing periodontal disease by modulating mTORC1 activity, the pathway responsible for regulating inflammation. By controlling this pathway, rapamycin could reduce the harmful effects of chronic inflammation, preserve gum and bone health, and prevent the further progression of the disease.
In this article, we will examine the current research on using low-dose rapamycin in treating periodontal disease, exploring how it modulates inflammation and discussing its broader implications for oral and systemic health.
Rapamycin's Role in Inflammation and mTOR Overactivation
Rapamycin is a well-known inhibitor of the mammalian target of rapamycin (mTOR), a protein complex that plays a key role in regulating cell growth, metabolism, and aging. Think of mTOR as the cell's "command center," directing processes that determine how cells function and age. There are two distinct mTOR complexes—mTORC1 and mTORC2— with rapamycin primarily targeting mTORC1. [1]
mTORC1 serves as a central signaling hub, responding to environmental cues like nutrients, growth factors, and stress. When nutrient levels are high, mTORC1 is activated to promote cell growth and division. However, when nutrient intake is chronically high mTORC1 remains persistently active. This can occur when nutrient sensors in the body, such as insulin and amino acids like leucine, constantly signal mTORC1 to prioritize growth. Over time, this chronic activation can lead to mTORC1 becoming overactive, shifting cellular focus away from repair and maintenance, and instead promoting growth and inflammation. [2]
This overactivation of mTORC1 is linked to several age-related diseases, including obesity, type 2 diabetes, and chronic inflammation. By inhibiting mTORC1, rapamycin helps interrupt this cycle, pushing cells to focus more on stress resistance, repair, and reducing inflammation. This modulation of mTORC1 is why rapamycin is considered a promising therapeutic for extending healthspan and managing age-related diseases.
One of mTORC1's key functions is regulating inflammation and immune activity. Inflammation is the body's natural defense mechanism, activated in response to injury or infection. During this process, immune cells release cytokines—small signaling proteins that help coordinate the body's immune response. You can think of cytokines like the body’s "dispatchers" in an emergency response system. When an injury or infection occurs, these dispatchers send urgent messages to immune cells, guiding them to the affected area and instructing them on how to respond.
Pro-inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), are essential for fighting infections and triggering inflammation, much like dispatchers calling in more resources for a severe crisis. However, when mTORC1 is chronically activated, it can lead to an overproduction of these cytokines, causing the immune system to remain on high alert. This constant "emergency signal" leads to chronic inflammation, which can contribute to age-related diseases like cardiovascular disease and diabetes. By inhibiting mTORC1, rapamycin helps dial down this chronic response, reducing unnecessary inflammation while still allowing the immune system to function properly when needed. [2]
However, while mTORC1 supports the immune system’s energy demands during active immune responses, it simultaneously inhibits autophagy—a critical cellular process responsible for clearing away damaged proteins, organelles, and other malfunctioning components. Autophagy acts as the cell’s internal housekeeping system, maintaining cellular health by recycling and eliminating defective structures. When autophagy is suppressed due to prolonged mTORC1 activity, damaged and dysfunctional cell components, such as misfolded proteins and defective mitochondria, start to accumulate within cells.
This accumulation triggers cellular stress responses, releasing damage-associated molecular patterns (DAMPs), which are signals that alert the immune system to perceived damage. DAMPs can activate immune cells and promote the release of pro-inflammatory cytokines, thereby intensifying inflammation. As the cycle progresses, the immune system stays in a heightened state of alert, responding to cellular debris and stress signals that should have been removed through autophagy. This chronic, low-grade inflammation, known as "inflammaging," is a hallmark of aging and is implicated in many age-related diseases.
Moreover, this persistent inflammation can contribute to the onset and exacerbation of autoimmune conditions, such as rheumatoid arthritis and lupus, where the immune system mistakenly targets healthy tissues. In these conditions, overactive mTORC1 signaling drives both the metabolic demands of the immune system and the inhibition of autophagy, creating a perfect storm where inflammation becomes self-sustaining and damaging. [2]
This is where rapamycin becomes significant. By selectively inhibiting mTORC1, rapamycin can help dampen excessive inflammation and interrupt the cycle of chronic inflammation driven by overactive mTORC1 signaling. When mTORC1 activity is reduced, rapamycin allows autophagy to resume, helping cells clear away damaged components like misfolded proteins and dysfunctional mitochondria. This cellular “cleanup” reduces the release of DAMPS, which in turn lowers the immune system’s inflammatory response. The reduction in pro-inflammatory cytokine production further mitigates chronic inflammation, potentially slowing the progression of age-related inflammatory diseases and autoimmune conditions. [2]
Low-dose rapamycin is often preferred because it balances mTORC1 inhibition without significantly suppressing immune function. Higher doses of rapamycin, while effective at blocking mTORC1, can also inhibit mTORC2, which plays a vital role in maintaining immune cell survival and function. Excessive inhibition of mTORC2 can lead to immunosuppression, impairing the body’s ability to fight infections and maintain a balanced immune response. By using low doses, rapamycin selectively targets mTORC1, reducing inflammation while minimizing the risk of compromising immune defenses. This precision makes rapamycin a promising tool for treating chronic inflammatory and autoimmune conditions while preserving overall immune health.
The Role of Inflammation in Oral Health and Periodontal Disease
Periodontal disease, a condition largely driven by chronic inflammation triggered by bacterial overgrowth, has drawn increasing interest from researchers exploring therapeutic interventions like low-dose rapamycin. By targeting the underlying inflammatory processes, rapamycin offers a novel approach to improving oral health.
Think of your gums as a protective barrier around your teeth, much like a castle wall defending against invaders. When bacteria accumulate in the mouth—particularly in the spaces between the teeth and gums—this invasion triggers the body’s inflammatory response, similar to sounding an alarm when the castle is under attack. In a healthy scenario, inflammation is a controlled response aimed at eliminating harmful bacteria and repairing tissue. However, in periodontal disease, this defense system becomes dysregulated. The immune response not only targets harmful bacteria but also begins attacking beneficial bacteria and the surrounding gum tissue, causing more harm than good.
Chronic inflammation in the gums is like a fire that never fully extinguishes. Initially, it aims to fend off bacterial invaders, but if left unchecked, it begins to damage the very structures it's meant to protect—specifically, the gum tissue and the underlying bone that supports the teeth. This destructive cycle is primarily driven by pro-inflammatory cytokines such as IL-6 and TNF-α, which act as molecular messengers that amplify the inflammatory response. [3]
Unchecked chronic inflammation can lead to significant damage, contributing to two major conditions associated with periodontal disease: gingivitis and periodontitis.
- Gingivitis: This is the early stage of periodontal disease, marked by gum inflammation (gingiva). Symptoms include redness, swelling, and bleeding during brushing or flossing. Gingivitis is usually caused by plaque buildup, a sticky film of bacteria on the teeth. Fortunately, gingivitis is typically reversible with good oral hygiene.
- Periodontitis: If gingivitis is not treated, it can progress to periodontitis, a more severe disease that destroys the structures supporting your teeth, including the bone. Symptoms may include persistent bad breath, gum recession, pockets forming between the gums and teeth, and, in advanced cases, tooth mobility or loss. Periodontitis can also have systemic effects, contributing to other health issues such as heart disease and diabetes.
The theoretical basis that has led researchers to explore rapamycin for periodontal disease lies in its dual action. By inhibiting mTORC1, rapamycin reduces the production of pro-inflammatory cytokines, effectively calming the chronic inflammatory response in the gums. Additionally, by reactivating autophagy, rapamycin helps cells clear away bacterial debris and damaged tissue, further reducing inflammation and promoting tissue repair. This combination of reduced cytokine-driven inflammation and enhanced cellular cleanup suggests a potential pathway to improve periodontal health and preserve gum and bone structures.
New research has begun to test this hypothesis. In a 2023 study by Feng et al., titled "Rapamycin Inhibits Osteoclastogenesis and Prevents LPS-Induced Alveolar Bone Loss by Oxidative Stress Suppression," rapamycin was shown to reduce the bone-resorbing activity of osteoclasts—cells primarily responsible for breaking down bone. To understand this process more fully, it helps to look at the dynamic relationship between three key cell types involved in bone metabolism: osteoblasts, osteoclasts, and osteocytes.
Osteoblasts are cells that build and repair bone tissue by secreting the proteins that form the bone matrix, which eventually mineralizes to become hard bone. Osteoclasts, on the other hand, are cells that break down bone in a process called bone resorption. They dissolve the mineral components and degrade the bone matrix, which is crucial for reshaping bone during growth and healing or for freeing up minerals like calcium for use elsewhere in the body. Osteocytes are former osteoblasts embedded in the bone matrix, and they play a role in maintaining the bone by regulating both osteoblast and osteoclast activity. Together, these cells create a balance between bone formation and degradation—known as bone remodeling—that is essential for bone health.
However, this balance can become disrupted. In certain conditions, such as periodontal disease, osteoclasts become overactive and degrade bone tissue at a rate that exceeds the capacity of osteoblasts to rebuild it. Oxidative stress—a condition where harmful reactive oxygen species (ROS) accumulate in cells—can exacerbate this imbalance. When osteoclasts experience oxidative stress, they become hyperactive, leading to excessive bone degradation. This not only weakens the bone but also intensifies inflammation by increasing the production of RANKL (receptor activator of nuclear factor kappa-Β ligand), a protein that promotes osteoclast formation and activity.
Feng’s study provides important insights into how rapamycin can help restore this balance. By activating the Nrf2/GCLC signaling pathway, rapamycin reduces oxidative stress, calming the hyperactivity of osteoclasts and lowering the production of RANKL. This results in less bone resorption and reduced inflammation in rats with periodontitis. Additionally, the study found that rapamycin enhances autophagy in osteoclasts—a cellular process that removes damaged components—which further helps to reduce inflammation and limit bone degradation.
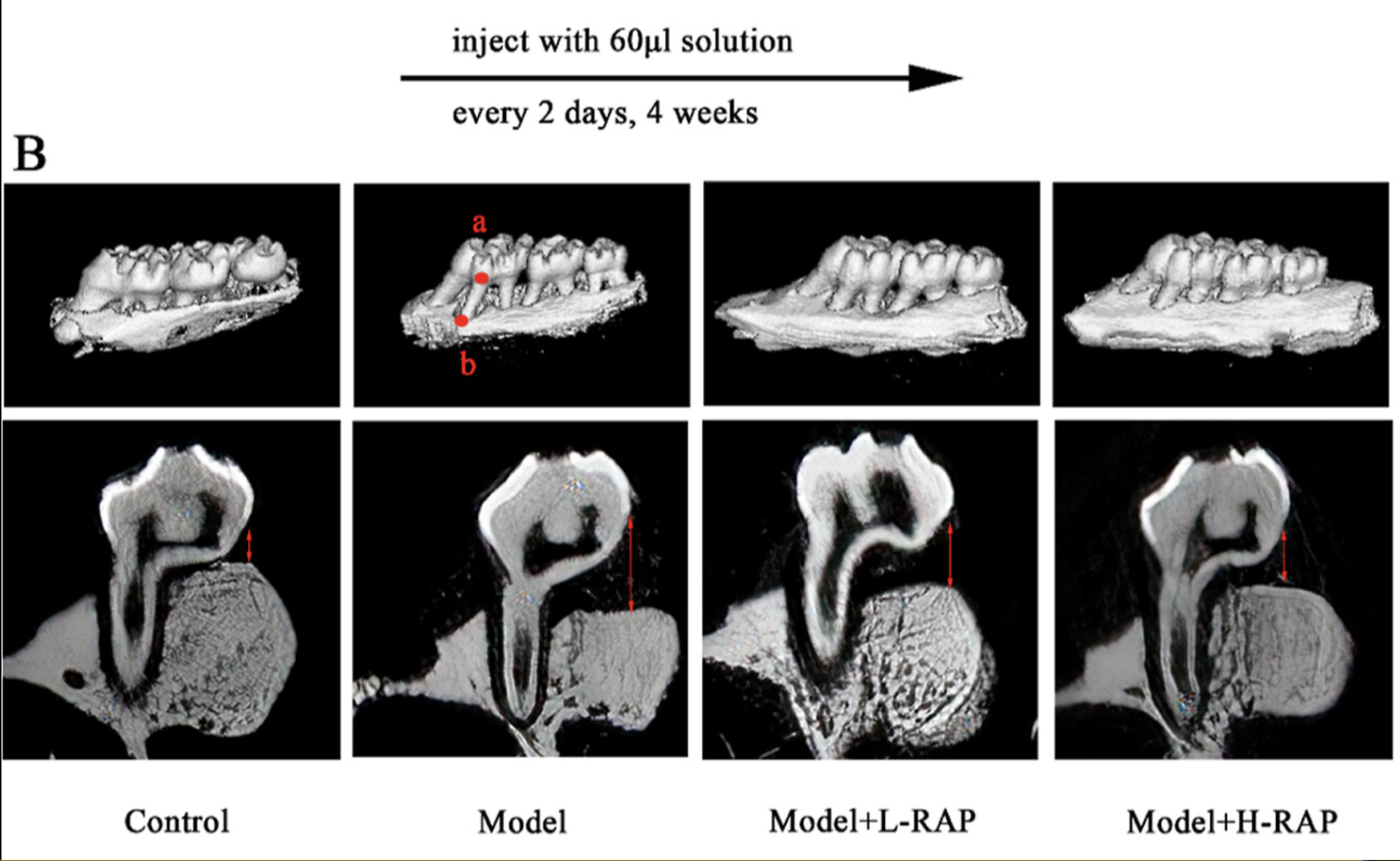
In the provided CT scans from the Feng et al. study, a model of periodontitis induced by lipopolysaccharide (LPS) in rats is depicted. The images compare the effects of different treatments on bone loss associated with this inflammatory condition.
- Control Group (Left):This image shows healthy bone structure, with no signs of bone erosion or loss.
- Model Group (Second from Left): This image indicates significant bone loss due to induced periodontitis. The bone density is reduced, and the structural integrity has deteriorated, as shown by the increased distance between the cemento-enamel junction (CEJ) and the alveolar bone crest (ABC).
- Model + L-RAP (Low-Dose Rapamycin, Third from Left): This image demonstrates partial rescue of bone structure with low-dose rapamycin treatment. The bone density improves, and the distance from the CEJ to the ABC decreases, indicating that bone loss is being mitigated.
- Model + H-RAP (High-Dose Rapamycin, Right): This image shows the most significant improvement in bone preservation. High-dose rapamycin not only improves bone density but also restores a nearly normal CEJ-to-ABC distance, suggesting that higher doses of rapamycin provide superior therapeutic effects.
These findings provide compelling evidence that rapamycin can help protect against bone loss in periodontal disease by inhibiting excessive osteoclast activity and promoting a more balanced bone remodeling process. By reducing oxidative stress and enhancing autophagy, rapamycin addresses the root causes of both inflammation and bone resorption, offering a potential therapeutic pathway to improve periodontal health.
Further supporting these findings, a 2020 study led by An et al., titled "Rapamycin Rejuvenates Oral Health in Aging Mice," and led by renowned rapamycin expert Matt Kaeberlein, explored the effects of short-term rapamycin treatment on aging mice with periodontal disease. Periodontal disease tends to worsen with age, often leading to increased bone loss, inflammation, and changes in the oral microbiome. The researchers sought to determine whether rapamycin could reverse some of these age-related effects and rejuvenate the oral health of middle-aged mice. [5]
Over an eight-week period, the study examined three key aspects of oral health: periodontal bone loss, tissue inflammation, and microbiome composition. Remarkably, the short-term rapamycin treatment demonstrated significant rejuvenating effects.
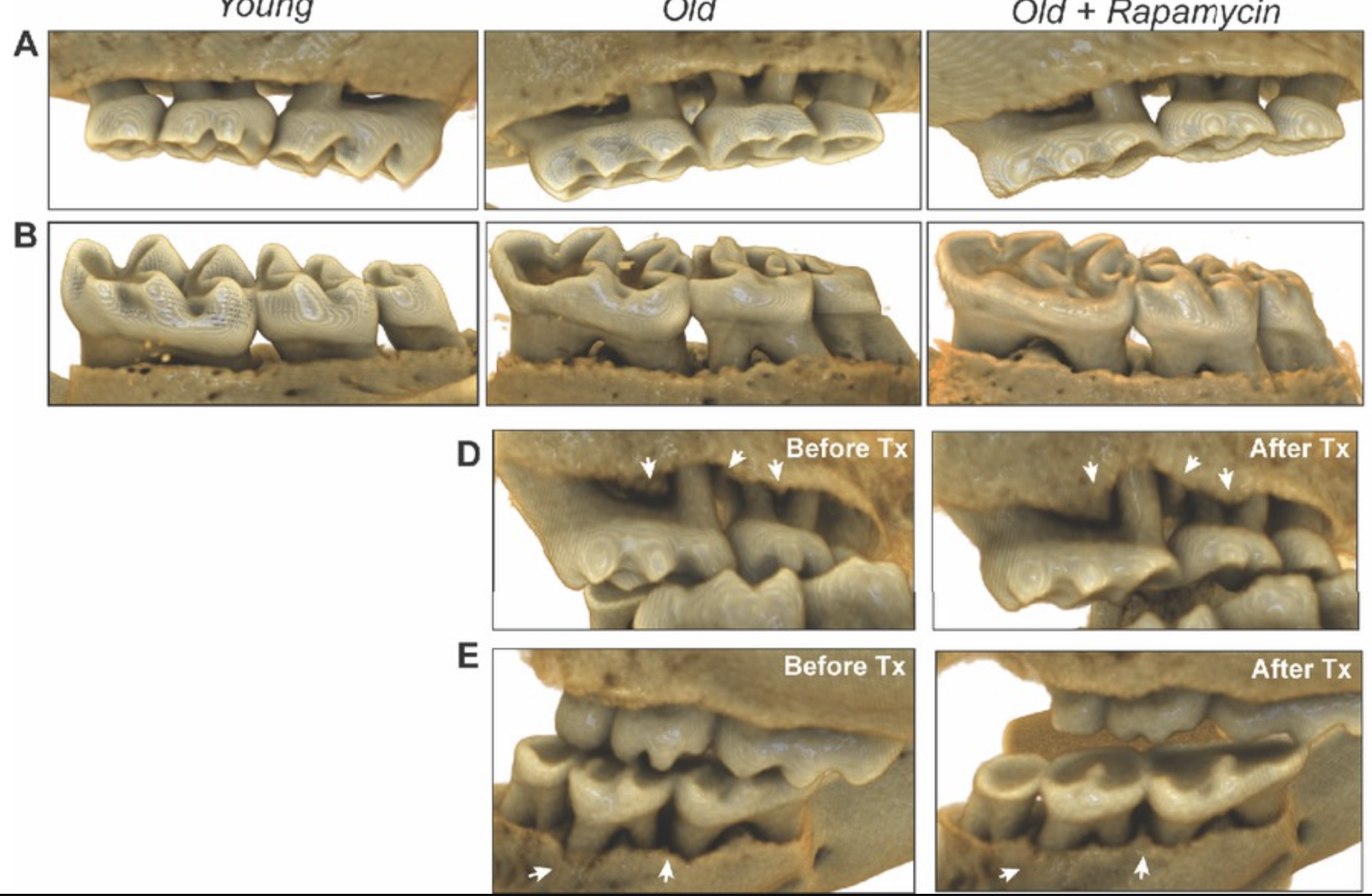
The aged mice experienced periodontal bone regeneration, a reduction in both gingival and periodontal inflammation, and a shift in the oral microbiome towards a more youthful composition. These findings suggest that rapamycin not only mitigates the progression of periodontal disease but also promotes a reversal of age-related oral degeneration, highlighting its potential as a therapeutic intervention to maintain oral health during aging. By effectively managing inflammation, rapamycin could slow the progression of periodontal disease and offer systemic health benefits beyond the mouth. [5]
Oral Health and Its Impact on Systemic Diseases
The mouth acts as a gateway to the rest of the body, and poor oral health can contribute to systemic health issues through various interconnected mechanisms. One of the primary pathways is via systemic inflammation. As previously discussed, periodontal disease triggers localized inflammation in the oral cavity, leading to the release of pro-inflammatory cytokines into the bloodstream. Once these cytokines circulate throughout the body, they can exacerbate inflammatory processes in distant organs and tissues.
This systemic spread of inflammation can worsen conditions that are already characterized by chronic inflammation, such as cardiovascular disease (CVD) and diabetes. For instance, in CVD, the excess cytokines can contribute to the formation of atherosclerotic plaques in arteries, while in diabetes, they may increase insulin resistance, further complicating glucose regulation. In both cases, poor oral health can serve as a constant source of inflammatory mediators, amplifying disease progression and highlighting the critical connection between oral health and overall systemic health. [6]
One of the most well-established connections between oral and systemic health is the link between periodontal disease and cardiovascular disease. Chronic inflammation of the gums can provide a pathway for bacteria from the mouth to enter the bloodstream. Once in circulation, these bacteria can contribute to the formation of arterial plaques, which narrow blood vessels and increase the risk of heart attacks and strokes. In addition, the systemic inflammation triggered by periodontal disease can exacerbate atherosclerosis, a condition characterized by the buildup of plaques in the arteries, further heightening cardiovascular risk.
Numerous studies have demonstrated that individuals with severe periodontal disease are at a significantly higher risk for developing cardiovascular conditions. In a notable study by Ford et al. (2006), researchers found that certain oral bacteria can adhere to the endothelial cells lining blood vessels, promoting plaque formation. Their analysis of clogged arteries revealed the presence of a variety of oral bacteria, including Streptococcus mutans (found in 78% of samples) and Aggregatibacter actinomycetemcomitans (7%). Other species commonly associated with gum disease, such as Tannerella forsythia, Prevotella intermedia, and Porphyromonas gingivalis (P. gingivalis), were also identified in arterial blockages, suggesting a strong and direct link between oral bacterial infection and cardiovascular complications. These findings underscore the critical role of maintaining oral health to reduce the risk of heart disease and other systemic issues. [6]
However, the effects of periodontal disease extend beyond the heart. Other systemic conditions linked to poor oral health include:
Diabetes
There is a well-documented bidirectional relationship between periodontal disease and type 2 diabetes (T2D). T2D occurs when the body becomes resistant to insulin, the hormone responsible for helping tissues absorb glucose for energy. As insulin resistance worsens, blood glucose levels rise, leading to various health complications. Chronic inflammation from periodontal disease can exacerbate this issue by further impairing insulin sensitivity, making it more difficult to manage blood glucose levels effectively.
Conversely, poor glycemic control in individuals with diabetes weakens the immune system, increasing susceptibility to infections, including periodontal disease. Elevated blood sugar levels create an environment in which bacteria can thrive, worsening gum inflammation and accelerating the progression of periodontal disease. This vicious cycle—where gum disease worsens blood sugar control, and uncontrolled diabetes exacerbates periodontal issues—highlights the importance of maintaining good oral hygiene in managing diabetes and preventing further complications. Effective oral care and regular dental check-ups can play a critical role in breaking this cycle and improving overall health outcomes for people with diabetes. [7]
Respiratory Diseases
Oral bacteria can also have a significant impact on respiratory health. When bacteria from the mouth are inhaled into the lungs, they can contribute to respiratory infections, including pneumonia. This risk is particularly high for individuals with weakened immune systems, such as older adults or those with chronic lung conditions like chronic obstructive pulmonary disease (COPD) or asthma. In these vulnerable populations, inhaled oral bacteria can exacerbate existing respiratory issues or lead to new infections. Maintaining good oral hygiene can help reduce the risk of respiratory infections by limiting the bacterial load in the mouth and preventing harmful pathogens from being aspirated into the lungs.
Cognitive Decline and Alzheimer's Disease
Emerging research is beginning to reveal a potential link between oral bacteria, chronic inflammation, and neurodegenerative diseases such as Alzheimer’s disease. Several studies have found the presence of oral bacteria in the brains of individuals with Alzheimer’s, raising the possibility that these pathogens may contribute to cognitive decline [7, 8]. For example, a notable study by Riviere et al. (2002) examined post-mortem brain samples from individuals with Alzheimer’s and discovered elevated levels of Porphyromonas gingivalis (P. gingivalis)—a bacterium commonly associated with periodontal disease. This finding has led researchers to investigate whether oral pathogens could play a role in the onset or progression of neurodegenerative conditions. [8]
Once in the brain, oral bacteria such as P. gingivalis can release toxic enzymes known as gingipains. These enzymes have the ability to degrade nerve cells, particularly in areas critical to memory and cognitive function. The release of gingipains triggers an inflammatory response in the brain, potentially contributing to the development of neuroinflammation—a key factor in Alzheimer’s disease. Over time, this neuroinflammation may accelerate the destruction of neurons, exacerbating memory loss and cognitive decline.
This growing body of research highlights how oral health is not isolated to the mouth but may have far-reaching effects on other parts of the body. Conditions such as cardiovascular disease, diabetes, respiratory infections, and now neurodegenerative diseases like Alzheimer’s are increasingly being linked to oral health. These connections underscore the critical importance of maintaining good oral hygiene to support not only oral health but also overall systemic well-being.
The Role of Rapamycin in Oral and Systemic Health
The connection between oral health and systemic well-being is becoming increasingly clear, as research continues to reveal how imbalances in oral bacteria and chronic inflammation can have far-reaching consequences. Conditions such as cardiovascular disease, type 2 diabetes, respiratory infections, and neurodegenerative disorders are all linked to poor oral hygiene and periodontal disease. As we’ve seen, these imbalances can trigger systemic inflammation and contribute to the progression of multiple diseases. Low-dose rapamycin presents a promising potential therapeutic approach by targeting the underlying mechanisms of inflammation and cellular hyperactivity that become pathological as we age.
One of the primary ways rapamycin may improve oral and systemic health is by reducing oral inflammation and limiting bacterial growth. In addition to its potential role in managing periodontal disease, rapamycin has shown promise in combating oral cancer, a condition with significant systemic consequences. A study by Semlali et al. (2022) explored the effects of oral rapamycin in the context of oral cancer, a disease that can metastasize from the mouth to other parts of the body, leading to secondary cancers. Chronic inflammation, a hallmark of oral cancer, not only contributes to cancer progression but also increases the risk of systemic inflammatory conditions, such as cardiovascular disease and diabetes, by releasing pro-inflammatory molecules into the bloodstream. [9]
Current treatment options for oral cancer, such as surgery, radiation, and chemotherapy, often come with considerable side effects, underscoring the need for more effective and less harmful treatments. In their study, Semlali et al. investigated how rapamycin could mitigate the effects of oral cancer. They tested varying doses of rapamycin on human gum cancer cells to examine its impact on cell division, autophagy, and cancer-related gene expression. Their findings were promising: low doses of rapamycin slowed cancer cell growth, reduced the formation of new cancer cell colonies, and inhibited the migration of cancer cells. Furthermore, rapamycin promoted programmed cell death (apoptosis) by activating key enzymes like caspase-9 and caspase-3, which are essential for the controlled elimination of cancer cells.
In addition to these anti-proliferative effects, the study demonstrated that rapamycin blocked several key cancer-promoting inflammatory pathways, including MAPK, NF-κB, and Wnt/β-catenin signaling. These pathways are known to drive cancer progression and inflammation, suggesting that rapamycin’s inhibition of these signals could help prevent cancer from spreading and limit its systemic impact. The results of this study highlight rapamycin’s significant potential not only for managing oral cancer but also for reducing the associated systemic inflammation, which can lead to broader health complications.
Rapamycin's Effect on the Oral Microbiome and Bacterial Growth
The oral microbiome is a complex ecosystem made up of bacteria, fungi, viruses, and archaea that coexist in a delicate balance, playing a crucial role in both oral and systemic health. While many microorganisms are beneficial or harmless, certain bacteria can become pathogenic under favorable conditions. One of the most prominent pathogens in periodontal disease is Porphyromonas gingivalis (P. gingivalis). Along with other harmful bacteria such as Tannerella forsythia (T. forsythia) and Treponema denticola, P. gingivalis can trigger chronic inflammation, destroying gum tissue and tooth loss.
The overgrowth of these bacteria is often facilitated by forming biofilms—protective layers that bacteria create by adhering to tooth surfaces. These biofilms shield the bacteria from the immune system and antimicrobial agents, making them difficult to eliminate. Left unchecked, biofilms can lead to the progression of gingivitis and periodontitis, which makes controlling bacterial growth essential for maintaining oral health. Numerous studies have demonstrated the role of P. gingivalis and other pathogens in developing periodontal diseases. For example, a study by Homann et al. (2001) found that poor dental hygiene can increase the risk of oral cancer by promoting the production of acetaldehyde, a carcinogen produced by oral bacteria, especially in individuals who consume alcohol heavily. [10] Acetaldehyde encourages cancer cell growth in the periodontal regions, emphasizing the importance of maintaining a healthy oral microbial balance to prevent oral cancers.
In addition to proper dental hygiene, rapamycin may also significantly reduce microbial growth and biofilm formation. Alves et al. (2024) investigated the effects of low-dose rapamycin on biofilms formed by Candida haemulonii (C. haemulonii), a fungal species that can cause oral infections, particularly in immunocompromised individuals or those using dentures. C. haemulonii is known for its resistance to many common antifungal treatments, making it difficult to control. The study found that rapamycin effectively killed most fungal strains at certain concentrations. Even at lower doses, similar to those used in human treatments, rapamycin reduced the fungi's ability to form biofilms and decreased the production of harmful enzymes. The study also indicated that rapamycin might reduce the fungus's capacity to cause disease. [11]
While research into the effects of low-dose rapamycin on the modulation of the oral microbiome is still emerging, the focus has primarily been on rapamycin's ability to inhibit inflammatory processes and mTORC1 activity, thereby preventing periodontitis and related dental diseases. However, as demonstrated in the C. haemulonii study, rapamycin's potential to regulate microbial profiles is promising. Furthermore, rapamycin may indirectly influence microbial compositions by targeting inflammatory pathways linked to bacterial overgrowth. More research is needed to understand how rapamycin affects the microbiota in periodontal regions entirely.
Given the favorable safety profile of low-dose rapamycin for long-term administration, it presents a promising therapeutic option for the management of periodontal disease. In the following section, we will examine the critical role of anti-inflammatory therapies in periodontal disease management and discuss how low-dose rapamycin may be utilized as a chronic treatment to maintain and promote oral health.
Rapamycin for Long-Term Management of Periodontal Disease
Chronic periodontal disease is a leading cause of tooth loss worldwide, primarily driven by bacterial infections that lead to persistent inflammation. This inflammation damages the gums and underlying bone structure, making long-term management essential for preventing further tissue destruction and maintaining oral health. Standard treatments include professional cleanings, proper oral hygiene, and, in more severe cases, antibiotics or anti-inflammatory medications. However, due to the chronic nature of periodontal disease, short-term treatments are often insufficient.
As we have established, inflammation plays a central role in the progression of periodontal disease. In response to bacterial buildup, the immune system activates inflammatory pathways that, over time, can destroy healthy tissues. Long-term control of this inflammation is crucial, especially in high-risk populations, to prevent further damage and maintain oral health. Unfortunately, prolonged use of traditional anti-inflammatory drugs or antibiotics comes with risks, including side effects and the development of drug resistance. This highlights the need for new, safer treatment options with fewer side effects.
Certain populations, such as older adults, individuals with diabetes, and those with autoimmune diseases, are particularly susceptible to chronic periodontal disease. These groups often experience heightened inflammation and are at greater risk for oral infections. Rapamycin, with its ability to reduce chronic inflammation without the need for high doses, presents a promising option for managing periodontal disease in these vulnerable groups. Unlike traditional anti-inflammatory drugs that provide short-term relief, low-dose rapamycin could offer a sustainable, long-term approach to controlling inflammation in the gums.
Looking ahead, clinical trials are focusing on evaluating rapamycin's efficacy in managing periodontal disease over the long term. The U.S. Food and Drug Administration (FDA) has approved Dr. Jonathan An from the University of Washington's Department of Oral Health Sciences to lead the first-ever study evaluating rapamycin in older adults with periodontal disease. [12] The study will include adults aged 50 and above who will receive intermittent doses of rapamycin over an eight-week period. Researchers will monitor the compound's effects on oral health throughout the study to better understand its long-term impact.
With ongoing research, the potential of low-dose rapamycin as a treatment for chronic oral diseases is becoming more apparent. In the next section, we will explore how rapamycin compares to traditional therapies in managing periodontal disease, highlighting its potential benefits for long-term oral health management.
Rapamycin Compared to Traditional Therapies
One key advantage of low-dose rapamycin over traditional therapies, such as antibiotics or nonsteroidal anti-inflammatory drugs (NSAIDs), is its ability to minimize side effects. Chronic use of antibiotics can disrupt the body's natural bacterial balance, contributing to antibiotic resistance and gut dysbiosis. Similarly, long-term NSAID use is associated with gastrointestinal issues and kidney damage. In contrast, low-dose rapamycin has been shown to have a relatively mild side effect profile, making it more suitable for long-term use.
Another benefit of rapamycin is its mechanism of action, which reduces the likelihood of drug resistance—a growing concern in infection management. Unlike antibiotics that target bacteria directly, rapamycin modulates the body's inflammatory response, reducing tissue damage without directly attacking bacteria. This approach decreases the chance of bacteria developing resistance, offering a significant advantage for long-term therapy.
Bacteria can become resistant to antibiotics in several ways, including invading and colonizing inside host cells, protecting them from both the immune system and antibiotics. Fortunately, studies show that low-dose rapamycin can overcome these defense mechanisms and kill bacteria before they cause long-term damage. Qiu et al. (2019) developed a biomolecule combining streptomycin (an antibiotic) with decylamine (derived from alcohol) and hyaluronan (a natural substance in the body). This biomolecule was designed to encapsulate rapamycin and enter host cells, where streptomycin is released in acidic environments to kill bacteria, and rapamycin enhances autophagy to fight infections. [13] This novel drug delivery system demonstrated how rapamycin could enhance the effects of streptomycin, potentially reducing bacterial resistance.
Additionally, rapamycin may provide benefits beyond its anti-inflammatory properties. Research suggests that rapamycin can inhibit biofilm formation. Biofilms create a protective barrier for bacteria, making it difficult for antibiotics to penetrate and eradicate the infection. Rapamycin could help reduce the bacterial load in the mouth by preventing biofilm formation, providing extra protection against periodontal disease.
Managing chronic periodontal disease requires long-term strategies that extend beyond traditional treatments like antibiotics or NSAIDs. For high-risk populations, such as older adults and individuals with diabetes, chronic inflammation in the gums is a persistent threat to oral health. Low-dose rapamycin offers a promising alternative for these individuals, providing a means to control inflammation over the long term while minimizing the side effects and resistance risks associated with conventional therapies.
Low-Dose Rapamycin in Dental Surgeries and Implants
Inflammation is a natural response to tissue injury during surgery, playing a crucial role in healing by recruiting immune cells to clear debris and promote tissue regeneration. However, excessive or prolonged inflammation can delay healing, cause pain, and lead to complications like peri-implantitis—a destructive inflammatory process affecting the tissues surrounding a dental implant. Peri-implantitis is often triggered by bacterial infections, common in the oral cavity due to the high bacterial load. Postoperative bacterial colonization of the implant site can lead to conditions like peri-implant mucositis and, in severe cases, bone loss around the implant. This compromises osseointegration, the process by which the implant fuses with the bone, which is essential for long-term implant success. Therefore, preventing and controlling both inflammation and bacterial infections is critical to ensuring the success of dental implants.
Rapamycin, through its modulation of the mTOR pathway, can regulate immune cell activation and cytokine production, helping to control inflammation at the surgical site. Its anti-inflammatory effects can prevent chronic inflammation, contribute to peri-implantitis, improve healing, and reduce postoperative complications. Additionally, rapamycin promotes autophagy—a process that clears damaged cell components—supporting tissue regeneration by removing cellular debris and aiding the healing of soft tissues and bone around the implant.
Research is increasingly investigating the potential of rapamycin to enhance dental treatments, particularly in the realm of regenerative therapies. A study by Elashiry et al. (2023) examined the effects of rapamycin on the survival and bone-forming capacity of human periodontal ligament stem cells (hPDLSCs) in the presence of sodium hypochlorite (NaOCl), a chemical commonly used to disinfect root canals during endodontic procedures. To fully appreciate the significance of this research, it's important to understand the foundational principles of regenerative dental treatments. [14]
Regenerative endodontic procedures (REPs) represent a significant advancement in dental medicine, offering the ability to regenerate tissues inside a tooth—especially in cases where root development has been halted due to infection or trauma. These procedures are designed to eliminate infection and create an environment that fosters continued root growth. The success of REPs depends on three key components: stem cells, growth factors, and a scaffold to support the formation of new tissue.
Stem cells play a central role in this process, as they have the unique ability to differentiate into various tissue types, including bone, ligament, and dental pulp. These cells can either originate from areas surrounding the tooth, such as the periodontal ligament, or be introduced from external sources. However, a significant challenge in REPs is maintaining a microenvironment conducive to stem cell survival and proliferation—particularly when disinfectants like NaOCl are used. While NaOCl is highly effective at eliminating bacteria, its cytotoxic effects can also damage stem cells, compromising the tissue regeneration process.
In the study by Elashiry et al., the researchers focused on human periodontal ligament stem cells (hPDLSCs), which are crucial for regenerative endodontic procedures due to their ability to differentiate into the cell types necessary for periodontal tissue repair. A critical component of REPs is ensuring that the infected tooth is cleared of harmful bacteria, but this process often involves the use of NaOCl, which, while effective as a disinfectant, is also known to be cytotoxic. This cytotoxicity poses a challenge by damaging hPDLSCs, thereby limiting their ability to support tissue regeneration.
The objective of the study was to determine whether rapamycin could protect these valuable stem cells and enhance their regenerative capacity, even in the presence of the cytotoxic NaOCl. The results were promising: stem cells treated with rapamycin showed significantly improved survival rates and remained healthier despite exposure to NaOCl. Additionally, these cells demonstrated enhanced regenerative capabilities, as indicated by their ability to deposit calcium—an important marker of bone formation. Moreover, the rapamycin-treated cells exhibited increased alkaline phosphatase activity, an enzyme essential for bone growth, and upregulated expression of genes involved in bone development. [14]
These findings suggest that rapamycin not only protects hPDLSCs from the cytotoxic effects of NaOCl but also enhances their capacity for tissue regeneration. By promoting stem cell survival and differentiation into bone-forming cells, rapamycin may play a pivotal role in improving the success of regenerative endodontic procedures, supporting the potential for full tissue regeneration even in challenging microenvironments.
Safety and Side Effects of Rapamycin in Dental Applications
Low-dose rapamycin has shown significant potential for dental applications due to its ability to control inflammation and support tissue regeneration. Importantly, at lower doses, rapamycin avoids the more severe side effects typically seen with higher doses, making it suitable for long-term use in managing conditions like periodontal disease. However, like any treatment, some considerations need to be carefully evaluated. In some individuals, low-dose rapamycin can still lead to mild side effects, such as oral ulcers or mucositis, though these instances tend to be uncommon and less severe compared to higher doses.
One concern in oral health is the possibility of developing conditions like oral mucositis, which involves inflammation and ulceration of the mucous membranes. While this condition is rare in low-dose regimens, monitoring signs of discomfort is essential. Additionally, careful management is needed in immunocompromised individuals, as rapamycin's immune-modulating effects could potentially increase susceptibility to oral infections like candidiasis.
A study by Hudson et al. (2024) evaluated the effects of off-label low-dose rapamycin use on oral health in 333 adults. The findings were reassuring, with only about 26% of participants reporting mild changes in their oral health, such as occasional mouth sores. Importantly, the study found no direct link between the dosage or duration of rapamycin use and the development of these sores, and more serious side effects were infrequent [15].
One of the current challenges in using rapamycin for dental applications is that no specific formulation is designed for oral use. This makes precise dosing difficult and increases the potential for systemic exposure, even with low doses. To address this, localized delivery methods—such as topical gels or slow-release dental coatings—are being explored. These methods would allow for targeted application of rapamycin directly to the oral tissues, reducing systemic exposure and minimizing potential side effects.
Early research supports the effectiveness of localized rapamycin delivery. For example, Sutter et al. (2019) studied the use of an in situ forming implant (ISFI) to deliver low-dose rapamycin at the site of Vascularized Composite Allotransplantation (VCA), a procedure often performed after extensive oral surgeries. The implant released rapamycin steadily at the transplant site, allowing for high drug concentration locally while keeping systemic levels low. This approach improved tissue acceptance and minimized the need for more potent immunosuppressive drugs, which often cause more severe side effects. [16]
As research continues, localized delivery methods may help optimize Rapamycin's use, making it a valuable option for managing conditions like periodontitis while maintaining oral and systemic health.
Future Outlook of Rapamycin in Dental and Systemic Health
Although targeted dental formulations of rapamycin, such as topical gels or slow-release coatings, have yet to be fully developed, oral administration of low-dose rapamycin remains a promising therapeutic approach. Oral administration at low doses has effectively reduced chronic inflammation, modulated immune responses, and inhibited harmful bacterial growth. This makes it a viable option for managing conditions like periodontal disease driven by inflammation.
Unlike higher doses, low-dose rapamycin avoids many severe side effects, such as immunosuppression and delayed wound healing, making it safer for long-term use. By targeting critical inflammatory pathways like mTORC1, low-dose rapamycin helps regulate excessive inflammation without directly attacking bacteria, reducing the risk of drug resistance—a common issue with antibiotics.
While localized delivery methods like topical gels or slow-release coatings are ideal for minimizing systemic exposure, oral dosing can still provide substantial benefits. It allows rapamycin to impact the oral cavity and systemic conditions linked to inflammation, such as cardiovascular disease and diabetes. Until more targeted formulations are developed, low-dose oral rapamycin offers a practical, effective way to harness its therapeutic potential for oral and overall health.
- Bakhshi, S. (2023, October 22). Healthspan Research Review: Rapamycin Research and clinical trials: A synthesis of recent scientific findings. Healthspan. https://gethealthspan.com/science/article/rapamycin-research-synthesis-recent-scientific-findings
- Konopka, A.R., Lamming, D.W., RAP PAC Investigators. et al. Blazing a trail for the clinical use of rapamycin as a geroprotecTOR. GeroScience (2023).
- Loos, B. G., & Van Dyke, T. E. (2020). The role of inflammation and genetics in periodontal disease. Periodontology 2000, 83(1), 26–39.
- Feng, C., Liu, Y., Zhang, B. Y., Zhang, H., Shan, F. Y., Li, T. Q., Zhao, Z. N., Wang, X. X., & Zhang, X. Y. (2023). Rapamycin Inhibits Osteoclastogenesis and Prevents LPS-Induced Alveolar Bone Loss by Oxidative Stress Suppression. ACS omega, 8(23), 20739–20754.
- An, J. Y., Kerns, K. A., Ouellette, A., Robinson, L., Morris, H. D., Kaczorowski, C., Park, S. I., Mekvanich, T., Kang, A., McLean, J. S., Cox, T. C., & Kaeberlein, M. (2020). Rapamycin rejuvenates oral health in aging mice. eLife, 9, e54318.
- Ford PJ, Gemmell E, Chan A et al. Inflammation, heat shock proteins and periodontal pathogens in atherosclerosis: an immunohistologic study. Oral Microbiol Immunol. 2006;21:206–211. doi: 10.1111/j.1399-302X.2006.00276.x
- Kamer, A. R., Craig, R. G., Niederman, R., Fortea, J., & de Leon, M. J. (2020). Periodontal disease is a possible cause of Alzheimer's disease. Periodontology 2000, 83(1), 242–271.
- Riviere G.R., Riviere K.H., Smith K.S. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer's disease. Oral Microbiol. Immunol. 2002;17:113–118. doi: 10.1046/j.0902-0055.2001.00100.x.
- Semlali, A., Papadakos, S., Contant, C., Zouaoui, I., & Rouabhia, M. (2022). Rapamycin inhibits oral cancer cell growth by promoting oxidative stress and suppressing ERK1/2, NF-κB, and beta-catenin pathways. Frontiers in oncology, 12, 873447.
- Homann, N., Tillonen, J., Rintamäki, H., Salaspuro, M., Lindqvist, C., & Meurman, J. H. (2001). Poor dental status increases acetaldehyde production from ethanol in saliva: a possible link to increased oral cancer risk among heavy drinkers. Oral oncology, 37(2), 153–158.
- Alves, V., de Andrade, I. B., Corrêa-Junior, D., Avellar-Moura, I., Passos, K., Soares, J., Pontes, B., Almeida, M. A., Almeida-Paes, R., & Frases, S. (2024). Revealing the impact of Rapamycin on the virulence factors of the Candida haemulonii complex. Current research in microbial sciences, 7, 100247.
- LevySmith, A. (2024b, January 29). UW periodontal study receives FDA approval for anti-aging drug use. UW School of Dentistry.
- Qiu, Y., Lu, C., Chen, P., Sun, F., Wang, D., Wang, Z., Hou, C., Mu, H., & Duan, J. (2019). Synergistic clearance of intracellular pathogens by hyaluronan-streptomycin micelles encapsulated with rapamycin. Carbohydrate polymers, 210, 364–371.
- Elashiry, M. M., Raafat, S. N., Tay, F. R., & Saber, S. M. (2023). Effect of rapamycin on human periodontal ligament stem cells that have been exposed to sodium hypochlorite. Life sciences, 329, 121989.
- Hudson J, Kaeberlein T, Mahal A, Wong N, Ghorbanifarajzadeh M, Radella F, Isman A, Nyquist A, Zalzala S, Haddad G, Kaeberlein M, An JY. Evaluation of off-label rapamycin use on oral health. Geroscience. 2024 Oct;46(5):4135-4146. doi: 10.1007/s11357-024-01221-0. Epub 2024 Jun 6. PMID: 38839644; PMCID: PMC11335702.
- Sutter, D., Dzhonova, D. V., Prost, J. C., Bovet, C., Banz, Y., Rahnfeld, L., Leroux, J. C., Rieben, R., Vögelin, E., Plock, J. A., Luciani, P., Taddeo, A., & Schnider, J. T. (2019). Delivery of Rapamycin Using In Situ Forming Implants Promotes Immunoregulation and Vascularized Composite Allograft Survival. Scientific reports, 9(1), 9269.