Rapamycin Added to Diet in Late Mid-Life Delays Age-Related Hearing Loss in UMHET4 Mice
Richard A. Altschuler 1,2,3 *, Lisa Kabara1 , Catherine Martin1 , Ariane Kanicki 1 , Courtney E. Stewart 1,2, David C. Kohrman1 and David F. Dolan1 1 Kresge Hearing Research Institute, Department of Otolaryngology, Head and Neck Surgery, University of Michigan, Ann Arbor, MI, United States, 2 VA Ann Arbor Health Care System, Ann Arbor, MI, United States, 3 Department of Cell and Developmental Biology, University of Michigan, Ann Arbor, MI, United States
Published: 07 April 2021.
This is a continuation of a first study which demonstrated rapamycin added to diet at 4 months of age had significantly less age-related outer hair cell loss in the basal half of the cochlea at 22 months of age compared to mice without rapamycin. The present study tested adding rapamycin to diet later in life, at 14 months of age, and added a longitudinal assessment of auditory brainstem response (ABR).
The present study used UMHET4 mice, a 4 way cross in which all grandparental strains lack the Cdh23753A allele that predisposes to early onset, progressive hearing loss. UMHET4 mice typically have normal hearing until 16–17 months, then exhibit threshold shifts at low frequencies/apical cochlea and later in more basal high frequency regions.
The mice ABR thresholds at 4, 12, 24, and 48 kHz were assessed and tested at 12, 18, and 24 months of age and compared to baseline ABR thresholds acquired at 5 months of age to determine threshold shifts (TS).
Threshold shifts at 12 months of age — No threshold shift from baseline at any frequency
Threshold shifts at 18 months of age — Mice with rapamycin added to diet had a significantly lower mean TS at 4 and 12 kHz compared to mice on the control diet. There was no significant difference in TS at 24 and 48 kHz.
Threshold shifts at 24 months of age — The mean 4 kHz TS in the rapamycin diet group was no longer significantly lower than the control diet group, while the 12 kHz mean remained significantly lower. Mean TS at 24 and 48 kHz in the rapamycin diet group became significantly lower than in the control diet group at 24 months.
The results show that a later life addition of rapamycin can decrease age-related hearing loss in the mouse model, however, it also suggests that this decrease is a delay/deceleration rather than a complete prevention.
Introduction:
Age-related hearing loss (ARHL) occurs in approximately one-third of people in the United States over the age of 65 increasing to approximately half of those over the age of 75 (e.g., Gates, 2006; Gates et al., 2010). ARHL can reduce ability to communicate, quality of life and social integration and has been identified as a major risk factor for depression and dementia (e.g., Gates et al., 2010; Lin et al., 2011; Davis and Smith, 2013).
One major cause of ARHL is an age-related loss of sensory hair cells, predominantly outer hair cells, and an accompanying decrease in auditory sensitivity as measured by threshold shifts (TS) in auditory brain stem response (ABR) in animal models and audiometric thresholds in people. The underlying mechanisms responsible for age-related hair cell loss remain unknown and there are no treatments currently being clinically applied to prevent or reduce this pathology.
The National Institute on Aging Intervention Testing Program (NIA-ITP) tests for treatments that can be added to diet to increase lifespan, using UMHET3 mice, a four-way cross, as a model. Four-way cross mice (from four different grandparent strains) provide for genetic heterogeneity and reduce strain specific effects. Among several effective treatments identified through NIA-ITP, addition of rapamycin to diet at 9 months of age was found to increase life span by 26% in male mice and 23% in female mice (Miller et al., 2014). We hypothesized that ARHL might share underlying mechanisms, such that treatments that enhance life span could also reduce and/or delay ARHL. This is consistent with studies that demonstrate the positive effects of rapamycin on age-related disorders in animal models, including decreases in cardiac pathology (Dai et al., 2014); muscle weakness (Bitto et al., 2016), cancer incidence (Anisimov et al., 2011), and cognitive decline (Halloran Hussong et al., 2012; Majumder et al., 2012).
In a previous study (Altschuler et al., 2018) we evaluated cochleae from 22 months old UMHET3 mice that had rapamycin added to their diet at 4 months of age as well as from control littermates with normal diet. The 22 months old rapamycin-treated mice had significantly less loss of outer hair cells in the basal half of the cochlea compared to the untreated controls. This sparing of hair cell loss was restricted to the basal half of the cochleae, while the apical half of the cochleae exhibited equivalently large losses of outer hair cells in both rapamycin-fed and normal diet controls (Altschuler et al., 2018).
These results showing rapamycin could reduce or delay age-related hair cell loss in the basal half of the cochlea at 22 months of age raised two pertinent issues. First, the apparent limitation of the treatment effect of rapamycin to the basal half of the cochlea could reflect differences in mechanisms underlying hair cell loss along the cochlear spiral. Alternatively, the effect of rapamycin treatment could be due to delaying rather than preventing age-related hair cell loss. Hair cell loss occurs earlier in apical vs. basal cochlea in most mouse strains (for reviews; Gratton and Vazquez, 2003; Ohlemiller, 2006). If the effect of rapamycin is to “delay” rather than prevent age-related hair cell loss, a treatment induced difference in apical cochleae might also have been present at an earlier time, but by 22 months of age the delay was over and the hair cell loss had equilibrated. The current study was designed to address these points by generating a longitudinal assessment of auditory brain stem response (ABR) thresholds at 4, 12, 24, and 48 kHz in individual mice at 5, 12, 18, and 24 months of age. The current study also addressed a second question of whether beginning rapamycin treatment at a more clinically relevant later age would still be effective in reducing or delaying ARHL. Recent studies have shown that rapamycin can extend life span in mice even when added to diet at 19–20 months of age (Harrison et al., 2009; Zhang et al., 2014) and can also reduce age-related pathologies such as cancer incidence and decreased muscle (Zhang et al., 2014) and cardiac function (Quarles and Rabinovitch, 2020) with late life application. We therefore chose to add rapamycin at a later time but prior to the first appearance of ARHL. Three of the four “grandparent strains” of the UMHET3 mice carry homozygous ahl alleles (Cdh23753A) that predispose to early onset, hair cell loss and progressive deafness in mice, thus restricting the progeny that can be used in auditory aging studies and decreasing the utility of this four way cross for auditory aging studies (Noben-Trauth et al., 2003; Mianné et al., 2016). For this reason, earlier studies from our group (Schacht et al., 2012; Altschuler et al., 2015) developed a different four-way cross, UMHET4, in which the grandparent strains lack the ahl predisposing Cdh23753A alleles. We returned to use of UMHET4 mice in the current study so that all of the progeny could be used. Our previous studies using UMHET4 mice (Schacht et al., 2012; Altschuler et al., 2015) and pilot animals in the current study showed that UMHET4 mice generally have a later appearing ARHL than UMHET3 mice and that ABR TS do not commonly initiate until around 18 months of age. The current study therefore tested the influence of adding rapamycin to mouse diet at 14 months of age.
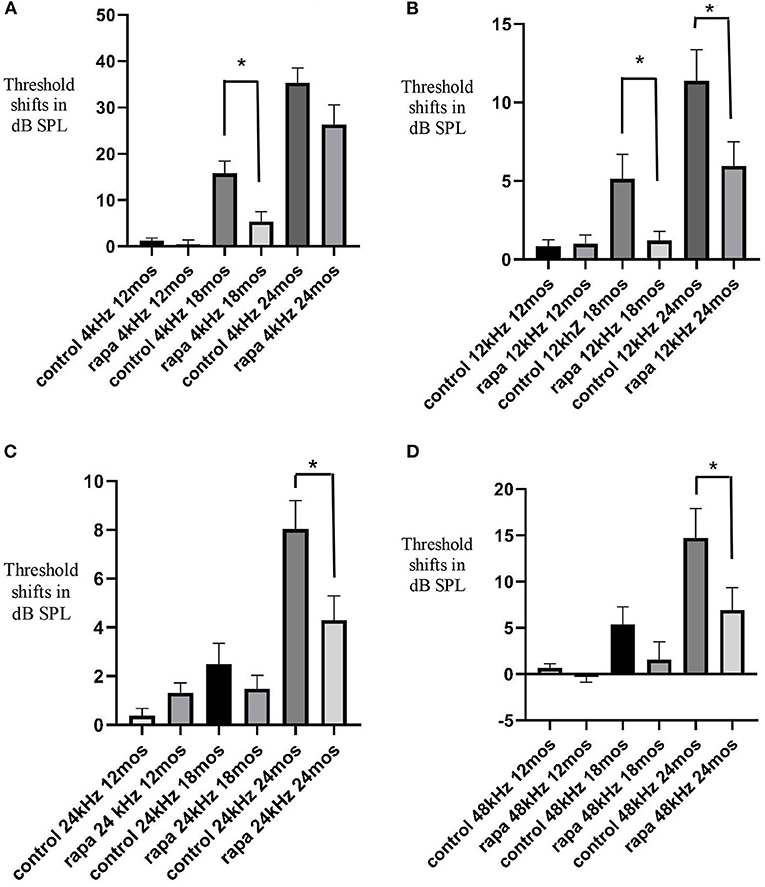
FIGURE 1. Comparison of mean auditory brain stem response threshold shifts (compared to 5 months of age) in the group with rapamycin added to diet at 14 months of age (rapa) vs. the group on control diet without rapamycin (control) at 12, 18, and 24 months of age at 4 kHz (A), 12 kHz (B), 24 kHz (C) and 48 kHz (D). Asterisks indicate significant differences. Please note differences between A, B, C, and D in scale bars for dB SPL on the “y” axis.
Discussion:
The results of the present study extend results of our previous study (Altschuler et al., 2018) that found rapamycin added to diet at 4 months of age reduced outer hair cell loss in the basal half but not apical half of the cochleae of 22 months old UMHET3 mice. The present study used a longitudinal measure of ABR to show rapamycin added to diet reduced mean threshold shifts at 4 and 12 kHz in 18 months old UMHET4 mice, largely by reducing the percent of mice showing TS. It is not well understood why hair cell loss occurs first in apical regions, then in basal regions in most mouse models and it has been suggested that different mechanisms could be influencing basal vs. apical age-related hearing loss (e.g., Schulte and Schmiedt, 1992; Dubno et al., 2013; Wu et al., 2020).
Since 4 kHz is processed in the apical half of the mouse cochlea, ~1.25 mm from apex (Viberg and Canlon, 2004), the results of the present study show that the influence of rapamycin is not restricted to the basal half of the cochlea. This provides indirect evidence that at least some components underlying age-related hearing loss (those that can be influenced by rapamycin) are present in both basal and apical cochleae.
Rapamycin acts on mammalian-target-of-rapamycin (mTOR) pathways (both mTORC1 and mTORC2). These pathways are multifaceted and in turn act on other functional signaling pathways including those associated with metabolism, proliferation, immune response and cell survival (Inoki et al., 2005a,b; Perl, 2015 for reviews; Wataya-Kaneda, 2015). The mTORC1 pathway can influence endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) (e.g., Ye et al., 2015). ER stress-related factors have been shown to increase in the cochleae of aged mice (Wang et al., 2015) and ER stress-mediated apoptosis has been associated with noise-induced, ototoxic drug-induced and age-related hearing losses (Oishi et al., 2015; Wang et al., 2015; Hu et al., 2016; Mahdi et al., 2016). ER stress pathways could therefore be a target of the rapamycin effect on ARHL. Rapamycin could also stimulate the survival pathway of p-Akt (S473) via mTOR2 signaling, including reducing mitochondrial stress. Rapamycin could also act through its influence on the inflammatory response or through inhibition of oxidative stress pathways previously implicated in hair cell pathology (Yamasoba et al., 2013, for review). Future studies will be necessary to identify the specific target or targets and pathways underlying the treatment effect seen in the current study.
Another important result is that the rapamycin treatment-related sparing of 4 kHz hearing loss at 18 months is no longer present at 24 months of age and there is an associated large hair cell loss in the apical cochlea of most rapamycin diet and control diet mice at 24 months of age, with no difference between the groups. This suggests that rapamycin treatment delays but does not prevent hearing loss. It would be valuable to identify the specific mechanisms by which rapamycin delays hair cell loss, not only to increase understanding of general mechanisms underlying ARHL but to determine if the delay could be extended and even turned into prevention. The timing of the last ABR and terminal euthanasia in the present study was before large TS generally occurs at higher frequencies in the UMHET4 mouse model. A greater rapamycin induced sparing of hair cell loss and TS than observed in the current study might therefore be found at a later age when greater hair cell loss is occurring, as observed in the previous study in UMHET3 mice where ARHL occurs more rapidly. The lack of correlation between ABR TS and OHC loss in the more basal cochlea at 24 months of age is consistent with reports of age-related reduction or loss in OHC function with reduced distortion product otoacoustic emissions (DPOAE) appearing before age-related OHC loss (e.g., Syka, 2010, for review). One explanation is an age-related disruption of prestin in morphologically intact OHC (Chen et al., 2009; Syka, 2010). It would be valuable to examine the influence of rapamycin treatment on age-related decrements in DPOAE. The variability in the progression and extent of ARHL seen in the control diet UMHET4 mice may reflect their genetic diversity and we have previously shown this variability can be correlated with polymorphisms in specific genetic loci (Schacht et al., 2012). The variability seen in the treatment effect of rapamycin in the rapamycin diet group might also reflect UMHET4 genetic diversity and it would be interesting to identify such differences in future studies.
The present study also addressed the question of whether beginning rapamycin treatment later in life than the 4 months of age used in Altschuler et al. (2018) would be effective. The results show beginning treatment later in life, at 14 months of age is still effective. This is consistent with studies showing late life rapamycin delivery also enhances life span and delays/reduces age-related declines in cardiac, muscle and cognitive functions (Quarles and Rabinovitch, 2020, for review). The literature also suggests that late life intermittent administration of rapamycin and rapamycin-like compounds (“rapalogs”) with less side-effects in people, can also increase life span and decrease age-related declines (Anisimov et al., 2011; Arriola Apelo et al., 2016; Shavlakadze et al., 2018; Quarles and Rabinovitch, 2020). It would therefore be valuable to test late-life intermittent treatment effects of rapamycin and rapalogs on ARHL. Thus, with this data we can conclude that rapamycin delays age-related hearing loss within mice with further lateral considerations for the same effect in humans.
- Altschuler, R. A., Kanicki, A., Martin, C., Kohrman, D., and Miller, R. A. (2018). Rapamycin but not acarbose decreases age-related loss of outer hair cells in the mouse Cochlea, Hearing Reserarch 370, 11–15. doi: 10.1016/j.heares.2018.09.003 PubMed Abstract | CrossRef Full Text | Google Scholar
- Altschuler, R. A., Zabezhinski, M. A., Popovich, I. G., Piskunova, T. S, Semenchenko, A. V., Tyndyk, M. L., et al. (2015). Age-related changes in auditory nerve—inner hair cell connections, hair cell numbers, auditory brain stem response and gap detection in UM-HET4 mice, Neuroscience 292, 22–33. doi: 10.1016/j.neuroscience.2015.01.068 PubMed Abstract | CrossRef Full Text | Google Scholar
- Anisimov, V. N., Zabezhinski, M. A., Popovich, I. G., Piskunova, T. S., Semenchenko, A. V., Tyndyk, M. L., et al. (2011). Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle 10, 4230–4236. doi: 10.4161/cc.10.24.18486 PubMed Abstract | CrossRef Full Text | Google Scholar
- Arriola Apelo, S. I., Pumper, C. P., Baar, E. L., Cummings, N. E., and Lamming, D. W. (2016). Intermittent administration of rapamycin extends the life span of female C57BL/6J Mice. J Gerontol A Biol Sci Med Sci. 71, 876–881. doi: 10.1093/gerona/glw064 PubMed Abstract | CrossRef Full Text | Google Scholar
- Bitto, A., Ito, T. K., Pineda, V. V., LeTexier, N. J., Huang, H. Z., Sutlief, E., Tung, H., et al. (2016). Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. elife 5:e16351. doi: 10.7554/eLife.16351 PubMed Abstract | CrossRef Full Text | Google Scholar
- Chen, G.-D., Li, M., Tanaka, C., Bielefeld, E. C., Hu, B. H., Kermany, M. H., et al. (2009). Aging outer hair cells (OHC) in the Fischer 344 rat cochlea: function and morphology. Hear. Res. 248, 39–47. doi: 10.1016/j.heares.2008.11.010 PubMed Abstract | CrossRef Full Text | Google Scholar
- Dai, D. F., Karunadharma, P. P., Chiao, Y. A., Basisty, N., Crispin, D., and Hsieh, E. J. (2014). Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13, 529–539. doi: 10.1111/acel.12203 PubMed Abstract | CrossRef Full Text | Google Scholar
- Davis, A., and Smith, P. (2013). Adult hearing screening: health policy issues-what happens next. Am. J. Audiol. 22, 167–170. doi: 10.1044/1059-0889(2013/12-0062) PubMed Abstract | CrossRef Full Text | Google Scholar
- Dubno, J. R., Eckert, M. A., Lee, F.-S., Matthews, L. J., and Schmiedt, R. A. (2013). Classifying human audiometric phenotypes of age-related hearing loss from animal models. J. Assoc. Res. Otolaryngol. 14, 687–701. doi: 10.1007/s10162-013-0396-x PubMed Abstract | CrossRef Full Text | Google Scholar
- Gates, G. A. (2006). The effect of noise on cochlear aging. Ear Hear. 27:91. doi: 10.1097/01.aud.0000194512.51849.ab PubMed Abstract | CrossRef Full Text | Google Scholar
- Gates, G. A., Gibbons, L. E., McCurry, S. M., Crane, P. K., Feeney, M. P., and Larson, E. B. (2010). Executive dysfunction and presbycusis in older persons with and without memory loss and dementia. Cogn. Behav. Neurol. 23, 218–223. doi: 10.1097/WNN.0b013e3181d748d7 PubMed Abstract | CrossRef Full Text | Google Scholar
- Gratton, M. A., and Vazquez, A. E. (2003). Age-related hearing loss: current research. Curr. Opin. Otolaryngol. Head Neck Surg. 11, 367–371. doi: 10.1097/00020840-200310000-00010 PubMed Abstract | CrossRef Full Text | Google Scholar
- Halloran Hussong, S. A., Burbank, R., Podlutskaya, N., Fischer, K. E., and Sloane, L. B. (2012). Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience 223:102–13. doi: 10.1016/j.neuroscience.2012.06.054 PubMed Abstract | CrossRef Full Text | Google Scholar
- Harrison, D. E., Strong, R., Sharp, Z. D., James, F., and Nelson, C. M. Kevin, A. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395. doi: 10.1038/nature08221 PubMed Abstract | CrossRef Full Text | Google Scholar
- Hu, J., Li, B., Apisa, L., Yu, H., Entenman, S., Xu, M., Stepanyan, R., et al. (2016). ER stress inhibitor attenuates hearing loss and hair cell death inCdh23erl/erlmutant mice. Cell Death Dis. 7:e2485doi: 10.1038/cddis.2016.386 PubMed Abstract | CrossRef Full Text | Google Scholar
- Inoki, K., Corradetti, M. N., and Guan, K. L. (2005b). Dysregulation of the TSC-mTOR pathway in human disease. Nat. Genet. 37, 19–24. doi: 10.1038/ng1494 PubMed Abstract | CrossRef Full Text | Google Scholar
- Inoki, K., Ouyang, H., Li, Y., and Guan, K. L. (2005a). Signaling by target of rapamycin proteins in cell growth control. Microbiol. Mol. Bio.l Rev. 69, 79–100. doi: 10.1128/MMBR.69.1.79-100.2005 PubMed Abstract | CrossRef Full Text | Google Scholar
- Johnson, K. R., Zheng, Q. Y., and Noben-Trauth, K. (2006). Strain background effects and genetic modifiers of hearing in mice. Brain Res.1091, 79–88. doi: 10.1016/j.brainres.2006.02.021 PubMed Abstract | CrossRef Full Text | Google Scholar
- Lin, F. R., Ferrucci, L., Metter, E. J., An, Y., Zonderman, A. B., and Resnick, S. M. (2011). Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 25, 763–770. doi: 10.1037/a0024238 PubMed Abstract | CrossRef Full Text | Google Scholar
- Mahdi, A. A., Rizvi, S. H. M., and Parveen, A. (2016). Role of endoplasmic reticulum stress and unfolded protein responses in health and diseases. Ind. J. Clin.l Biochem. IJCB 31, 127. doi: 10.1007/s12291-015-0502-4 PubMed Abstract | CrossRef Full Text | Google Scholar
- Majumder, S., Caccamo, A., Medina, D. X., Benavides, A. D., Javors, M. A., Kraig, E, Strong, R., et al. (2012). Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell 11, 326–335. doi: 10.1111/j.1474-9726.2011.00791.x PubMed Abstract | CrossRef Full Text | Google Scholar
- Mianné, J., Chessum, L, Kumar, S., Aguilar, C., Codner, G., Hutchison, M., et al. (2016). Correction of the auditory phenotype in C57BL/6N mice via CRISPR/Cas9-mediated homology directed repair Genome Medicine 8:16. doi: 10.1186/s13073-016-0273-4 PubMed Abstract | CrossRef Full Text
- Miller, R. A., Harrison, D. E., Astle, C. M., Fernandez, E., Flurkey, K., Han, M., et al. (2014). Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 13, 468–477. doi: 10.1111/acel.12194 PubMed Abstract | CrossRef Full Text | Google Scholar
- Noben-Trauth, K., Zheng, Q. Y., and Johnson, K. R. (2003). Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat. Genet. 345, 21–23. doi: 10.1038/ng1226 PubMed Abstract | CrossRef Full Text | Google Scholar
- Ohlemiller, K. K. (2006). Contributions of mouse models to understanding age- and noise-related hearing loss. Brain Res. 109, 189–102. doi: 10.1016/j.brainres.2006.03.017 PubMed Abstract | CrossRef Full Text | Google Scholar
- Oishi, N., Duscha, S., Boukari, H., Meyer, M., Xie, J., Wei, G., et al. (2015). XBP1 mitigates aminoglycoside-induced endoplasmic reticulum stress and neuronal cell death. Cell Death Dis. 6:e1763. doi: 10.1038/cddis.2015.108 PubMed Abstract | CrossRef Full Text | Google Scholar
- Perl, A. (2015). mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging. Ann N Y Acad Sci. 1346, 33–44. doi: 10.1111/nyas.12756 PubMed Abstract | CrossRef Full Text | Google Scholar
- Quarles, E., and Rabinovitch, P. S. (2020). Transient and later-life rapamycin for healthspan extension, Aging 12, 4050–4051. doi: 10.18632/aging.102947 PubMed Abstract | CrossRef Full Text | Google Scholar
- Schacht, J., Altschuler, R. A., Burke, D. T., Chen, S., Dolan, D., Galecki, A. T., et al. (2012). Alleles that modulate late life hearing in genetically heterogeneous mice. Neurobiol. Aging, 1842. 15–29. doi: 10.1016/j.neurobiolaging.2011.12.034 PubMed Abstract | CrossRef Full Text | Google Scholar
- Schulte, B. A., and Schmiedt, R. A. (1992). Lateral wall Na,K-ATPase and endocochlear potentials decline with age in quiet-reared gerbils. Hear Res. 61, 35–46. doi: 10.1016/0378-5955(92)90034-K PubMed Abstract | CrossRef Full Text | Google Scholar
- Shavlakadze, T., Zhu, J., Wang, S., Zhou, W., Morin, B., Egerman, M. A., et al. (2018). Short-term low-dose mTORC1 inhibition in aged rats counter-regulates age-related gene changes and blocks age-related kidney pathology. J Gerontol A Biol Sci Med Sci. 73, 845–852. doi: 10.1093/gerona/glx249 PubMed Abstract | CrossRef Full Text | Google Scholar
- Syka, J. (2010). The Fischer 344 rat as a model of presbycusis. Hear. Res. 264, 70–78. doi: 10.1016/j.heares.2009.11.003 PubMed Abstract | CrossRef Full Text | Google Scholar
- Viberg, A., and Canlon, B. (2004). The guide to plotting a cytocochleogram, Hear Res. 197, 1–10. doi: 10.1016/j.heares.2004.04.016 PubMed Abstract | CrossRef Full Text | Google Scholar
- Wang, W., Sun, Y., Chen, S., Zhou, X., Wu, X., Kong, W., and Kong, W. (2015). Impaired unfolded protein response in the degeneration of cochlea cells in a mouse model of age-related hearing loss. Exp. Gerontol, 70, 61–70. doi: 10.1016/j.exger.2015.07.003 PubMed Abstract | CrossRef Full Text | Google Scholar
- Wataya-Kaneda, M. (2015). Mammalian target of rapamycin and tuberous sclerosis complex. J. Dermatol. Sci. 79, 93–100. doi: 10.1016/j.jdermsci.2015.04.005 PubMed Abstract | CrossRef Full Text | Google Scholar
- Wu, P. Z., O’Malley, J. T., de Gruttola, V., and Liberman, M. C. (2020). Age-related hearing loss is dominated by damage to inner ear sensory cells, not the cellular battery that powers them. J. Neurosci. 40, 6357–6366. doi: 10.1523/JNEUROSCI.0937-20.2020 PubMed Abstract | CrossRef Full Text | Google Scholar
- Yamasoba, T., Lin, F. R., Someya, S., Kashio, A., Sakamoto, T., and Kondo, K. (2013). Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hear. Res, 303, 30–38. doi: 10.1016/j.heares.2013.01.021 PubMed Abstract | CrossRef Full Text | Google Scholar
- Ye, X., Luo, H., Chen, Y., Wu, Q., Xiong, Y., Zhu, J., et al. (2015). MicroRNAs 99b-5p/100-5p regulated by endoplasmic reticulum stress are involved in abeta-induced pathologies. Front. Aging Neurosci. 7:210. doi: 10.3389/fnagi.2015.00210 PubMed Abstract | CrossRef Full Text | Google Scholar
- Zhang, Y., Bokov, A., Gelfond, J., Soto, V., Ikeno, Y., and Hubbard, G. (2014). Rapamycin extends life and health in C57BL/6 mice. J. Gerontol. A Biol. Sci. Med. Sci. 69, 119–130. doi: 10.1093/gerona/glt056 PubMed Abstract | CrossRef Full Text | Google Scholar