Integrating Oxytocin and Rapamycin: A Synergistic Strategy for Enhancing Longevity and Combating Age-Related Diseases
Aging and age-related diseases present significant challenges to human health, driving the search for effective therapies that can slow or even reverse these processes. Among the most promising agents in this field are oxytocin and rapamycin, each offering powerful mechanisms to combat aging. Oxytocin is known for its role in social bonding and emotional well-being, but it also significantly influences stress regulation, cardiovascular health, and metabolism. Rapamycin, on the other hand, is a well-established mTOR inhibitor that promotes autophagy and mitigates cellular senescence, addressing key drivers of aging at the cellular level.
These two compounds are typically considered distinct, standalone therapies offering unique benefits. However, a deeper exploration of their mechanisms and effects reveals a compelling case for their combined use. This synergy arises from their ability to target different aspects of aging, potentially filling the gaps in each other's therapeutic profiles and enhancing overall efficacy.
This review will delve into the synergistic potential of Rapamycin and Oxytocin, exploring how their combined use could more effectively regulate inflammation, support cardiovascular and metabolic health, and manage stress—key factors in promoting longevity and reducing the burden of age-related diseases. This exploration aims to reveal the potential of combining oxytocin and rapamycin as a more powerful, integrated approach to longevity.
Rapamycin and Longevity
Rapamycin functions as an mTOR inhibitor, targeting the mammalian target of rapamycin (mTOR) complex, which plays a crucial role in regulating cell growth and function. Essentially, mTOR acts as the "command center" for cellular processes that influence the rate of aging.
mTOR is a nutrient-sensing enzyme. It acts as a molecular switch, responding to the abundance or scarcity of nutrients to then turn on or off the cell’s growth machinery. When nutrients are abundant mTOR shifts cellular focus toward growth and proliferation by stimulating the synthesis of proteins, lipids, and nucleic acids, which are essential building blocks for cell growth. This nutrient-driven activation of mTORC1 also suppresses autophagy, the process of cellular self-cleaning, to prioritize growth and biosynthesis over the recycling of cellular components. Conversely, when nutrients are scarce the cell is directed into a conservation mode and does not utilize cellular energy for growth. [1, 2, 4]
As we age, mTOR activity becomes overactive and stimulates excessive cellular growth. While mTOR functions optimally during youth to promote cell growth, protein synthesis, and overall vitality, this finely tuned regulation tends to degrade with age. Overactive mTOR signaling in older adults can contribute to a variety of aging-related issues, including cellular senescence, chronic inflammation, and metabolic dysfunction. This excessive activity is associated with the development of age-related diseases such as cancer, type 2 diabetes, and neurodegenerative conditions, as the balance between growth and maintenance becomes skewed in favor of unchecked cellular processes. [3]
Let’s explore how this elevated mTOR activity influences one of the key hallmarks of aging: cellular senescence.
The Role of mTOR in Cellular Senescence
One significant consequence of mTOR overactivity is the accumulation of senescent cells—cells that have ceased dividing but persist in the body, releasing harmful pro-inflammatory molecules. As we age, senescent cells become increasingly prevalent in our bodies. Cellular senescence is a natural process when cells enter a state of permanent growth arrest, meaning they stop dividing and replicating.
Senescence is triggered when a cell becomes damaged. When cells experience excessive stress or damage, they can become senescent, which prevents further proliferation of the damage and helps reduce the risk of tumorigenesis. We need senescence as a cellular program to stop the spread of cell damage when a cell replicates.
Although these cells have permanently exited the cell cycle and cannot replicate, they remain highly metabolically active, releasing pro-inflammatory and growth-promoting molecules that can contribute to developing various age-related diseases.
The fact that senescent cells cannot replicate impacts their size, activity, and pathology.
When a healthy cell is exposed to growth stimuli, it can balance out its growth by dividing. Senescent cells cannot share the burden of growth with other cells by simply dividing. Instead, when exposed to growth signals, they become very large and hyper-mitogenic. They release molecules that stimulate the proliferation of adjacent cells and resist programmed cell death.
Senescent cells also become highly inflammatory—a feature of their hyperfunctionality. They excrete a witch's brew of inflammatory molecules and growth factors known as the senescence-associated secretory phenotype (SASP). The SASP is a complex set of signaling molecules released by senescent cells, which can damage and stimulate nearby cells to divide and grow.
As a result, senescent cells are effectively "stuck" in a state of high activity, with both the mTOR gas pedal and the brakes pressed to the floor. The only way for senescent cells to be cleared from the body is through the action of nearby immune cells, which can recognize and remove them. As we age, however, the proportion of senescent cells increases as they become harder to remove—this has tremendous implications for the acceleration of aging.
Viewing cell senescence as a state of continuous growth stimulation in the absence of cell division helps to connect the process of cellular aging to the broader phenomenon of organismal aging.
Traditionally, we have believed that aging results from wear and tear and the gradual loss of cellular function. This understanding has been rooted in the idea that living organisms have a limited ability to repair themselves, leading to the accumulation of damage over time.
However, recent research has proposed an entirely different perspective, challenging the notion that aging results from the loss of cellular function. According to this counterintuitive theory, known as cellular hyperfunctionality, age-related diseases are caused by excessive cellular activity, not by a loss of function or damage to tissues. At the center of this hyperfunctionality is the excessive activity of mTOR driving excessive cellular growth and activity that causes the growth of unhealthy tissue. This shift in understanding has significant implications for how we approach the aging process and has captured the attention of longevity scientists.
The theory of hyperfunction in aging is a phenomenon popularized by Dr. Mikhail Blagosklonny; it aims to explain much of the aging process and the success of drugs like rapamycin through the lens of cellular overactivity.
Types of Cellular Hyperfunctions
Generally speaking, diseases of aging are characterized by cellular 'hyper-functions,' not the loss of cellular function or 'wear and tear' of tissues.
Blagosklonny outlines three hyperfunctional features to describe the morphology and pathology of a dysfunctional cell. These include hyperplasia, hypertrophy, and hyperfunctionality:
- Hyperplasia, is a form of cellular proliferation that is characterized by an increase in the number of cells within an organ or tissue. Senescent cells excessively excrete mitogens, which stimulate cell replication of adjacent cells, which can be pathological, as in the case of cancer or benign tumors.
- Hypertrophy, on the other hand, is an increase in the size of individual cells, leading to an overall increase in the size of the organ or tissue. However, if it persists for an extended period or becomes excessive, it can result in cellular dysfunction, tissue damage, or disease.
- Hyperfunctionality refers to the increased activity of cells or organs beyond what is normal or necessary. Cellular hyperfunctionality can occur due to several factors, including excessive stimulation by hormones or growth factors or abnormal signaling pathways. Hyperfunctionality can result in cellular damage, dysfunction, or disease.
Cell hypertrophy, hyper-function, and hyperplasia drive the acceleration of tissue dysfunction and aging in humans. This counterintuitive understanding of aging as a phenomenon of cellular overactivity has profound implications for how we think about decelerating the aging process.
Blagosklonny postulates that the heightened mTOR activity required for cellular growth and proliferation, which benefits us in youth, becomes detrimental as we age. This overactivity leads to tissue decline through excessive growth of unhealthy cells, contributing to the acceleration of age-related diseases. The same processes that once enabled growth now contribute to cellular dysfunction, resulting in chronic conditions associated with aging through the growth of unhealthy cells, which begin to compromise tissue function.
mTOR's overactivity over time is a key contributor to the aging process, and rapamycin offers a potential solution by inhibiting this overactivity. Acting as a molecular "brake," rapamycin inhibits the mTORC1 complex, mimicking the effects of nutrient deprivation and lifting the suppression on autophagy.
When autophagy becomes impaired—as is often the case in age-related chronic diseases—damaged cellular components accumulate, leading to tissue deterioration and accelerating disease progression. For example, in Alzheimer’s disease, the accumulation of tau proteins—a form of cellular waste in neuronal cells—contributes to neurodegeneration. Tau proteins form tangles inside neurons, disrupting their function and leading to cell death. This accumulation is a result of impaired autophagy, where the cell’s ability to clear out these toxic proteins diminishes over time.
By enhancing autophagy, rapamycin helps to clear out cellular debris and dysfunctional cells, potentially mitigating both aging and the onset of age-related diseases. This restoration of autophagy is crucial in conditions such as neurodegenerative diseases, cardiovascular disease, and metabolic disorders, where the buildup of damaged cells and proteins further exacerbates disease progression and tissue deterioration. A pivotal study by Wu et al. (2013) reinforces the critical role of autophagy in maintaining healthy tissue function and highlights rapamycin's potential in managing age-related kidney disease. As we age, disruptions in autophagy contribute to the deterioration of various tissues, including the kidneys. The kidneys play a crucial role in filtering blood and maintaining homeostasis, a process dependent on podocytes—specialized cells that line the glomeruli, the kidney's filtration units. Podocytes rely on efficient autophagy to recycle cellular components and sustain their function. However, with aging, the mechanisms governing autophagy become less effective, leading to increased susceptibility to podocyte injury and kidney disease. [5]
In their study, Wu et al. used rat models to investigate the relationship between mTORC1 activity, autophagy, and kidney health. They found that in rats with podocyte injury, mTORC1 activity was elevated, while autophagy was diminished. This imbalance led to significant podocyte damage and impaired kidney function, illustrating how the decline in autophagy contributes to tissue aging. Crucially, when the rats were treated with rapamycin, mTORC1 activity was inhibited, autophagy was restored, and podocyte damage was significantly reduced. These findings demonstrate rapamycin's potential to stimulate autophagy, not just in the kidneys, but as a mechanism to maintain healthy tissue aging more broadly. This study underscores the potential of rapamycin as a therapeutic intervention across various tissues, at least partially by enhancing autophagy, which is fundamental to preserving tissue function and longevity. [5]
Beyond its role in enhancing autophagy, mTORC1 inhibition by rapamycin also plays a crucial role in mitigating the hyperfunctions of senescent cells that make them so pathological. When mTORC1 activity remains unchecked, it not only blocks autophagy but also drives the hyperactivity of senescent cells. These cells, while no longer capable of dividing, remain highly active and secrete harmful pro-inflammatory molecules, contributing to tissue damage and spreading senescence to neighboring cells.
By inhibiting mTORC1, rapamycin diminishes this pathological hyperactivity of senescent cells, reducing the release of these inflammatory factors and slowing the spread of senescence. In addition, rapamycin promotes the clearance of accumulated cellular debris by restoring autophagy, further helping to prevent the onset and progression of cellular dysfunction. This dual action makes rapamycin a powerful tool in combating the cellular processes that drive aging and age-related diseases.
A study by Hussain et al. (2018) explored rapamycin's impact on cellular senescence in the context of chronic obstructive pulmonary disease (COPD), a severe lung condition that worsens with age. Their research found that lung cells in COPD patients exhibited heightened mTOR activity, which was strongly associated with increased cellular senescence and the progression of lung pathology. In transgenic mice engineered to have elevated mTOR activity in lung cells, the researchers observed increased senescence, along with COPD-like alterations in lung structure and function. [6]
However, when these mice were treated with low-dose rapamycin, the drug effectively inhibited mTORC1 activity, reduced cellular senescence, and suppressed the senescence-associated secretory phenotype (SASP)—a key driver of inflammation and tissue damage. As a result, inflammation was diminished, and lung function showed marked improvement. These findings suggest that rapamycin's ability to reduce cellular senescence could be a valuable therapeutic strategy not only for managing COPD but also for treating other age-related diseases characterized by senescence and chronic inflammation. [6]
Rapamycin's benefits extend beyond individual studies, with multiple investigations highlighting its potential in treating neurodegenerative diseases. In the study titled "Inhibition of mTOR by Rapamycin Abolishes Cognitive Deficits and Reduces Amyloid-Beta Levels in a Mouse Model of Alzheimer's Disease," Spilman et al. (2010) explored rapamycin's effects on Alzheimer's disease (AD) in mouse models and found that long-term rapamycin treatment significantly reduced memory problems associated with AD. The treatment also lowered levels of amyloid-beta 42 (Aβ42), a toxic protein that accumulates in the brains of AD patients and contributes to neurodegeneration. Rapamycin achieved these effects by inhibiting the mTOR pathway, which enhanced autophagy and improved the clearance of Aβ42, ultimately leading to better memory function. [7]
Recent research has brought additional attention to the J20 AD mouse model, which is genetically engineered to exhibit AD-like symptoms and serves as a crucial tool in studying Alzheimer's progression. In groundbreaking experiments, J20 AD mice treated with rapamycin retained their cognitive abilities, in stark contrast to untreated mice, which showed significant cognitive decline. Notably, rapamycin treatment in these experiments was initiated after the onset of symptoms, suggesting that the drug could potentially halt or even reverse the progression of AD-like symptoms—a hopeful development in the fight against Alzheimer's.
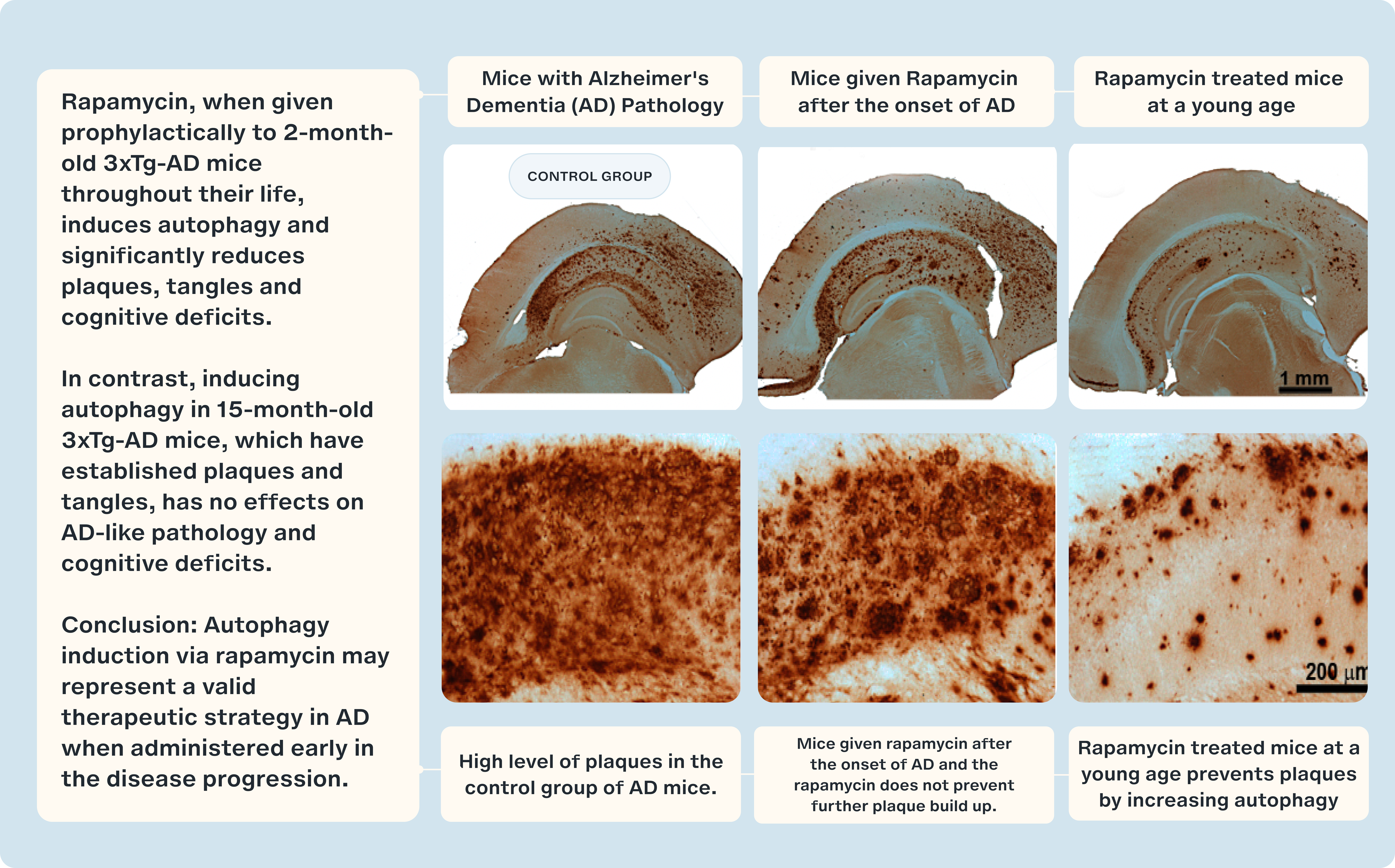
However, the relationship between mTOR inhibition and neurodegeneration is complex. Studies using the 3×Tg mouse model of Alzheimer's disease provide a more nuanced perspective. In the study titled "Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits," researchers showed that rapamycin, when administered prophylactically to 2-month-old 3xTg-AD mice throughout their lives, induced autophagy and significantly reduced the formation of amyloid plaques, tau tangles, and cognitive deficits. This finding supports the potential of rapamycin as an early intervention strategy to combat AD pathology. [25]
In contrast, when rapamycin treatment was initiated at 15 months of age, after plaques and tangles were already established, the therapeutic effects were far less pronounced. Inducing autophagy at this later stage had little to no effect on AD-like pathology or cognitive deficits, underscoring the importance of timing in therapeutic intervention. The study's conclusion emphasizes that autophagy induction via rapamycin may represent a valid therapeutic strategy in Alzheimer's disease, but its efficacy is heavily dependent on early administration during disease progression. [25]
These divergent results from different mouse models highlight two critical considerations: first, the necessity of maintaining balanced mTOR activity for optimal brain health; and second, the existence of a 'golden window' for therapeutic intervention, where early treatment may yield the most significant benefits. This underscores the importance of initiating therapies like rapamycin at the appropriate stage to maximize their impact in combating neurodegenerative diseases like Alzheimer's. [25]
While this article has highlighted research on the use of rapamycin for improving kidney function and as a prophylactic against Alzheimer's disease, the broader impact of rapamycin extends beyond these specific cases. By targeting two fundamental hallmarks of aging—senescence and autophagy—rapamycin has the potential to slow the aging process across a vast array of tissue types.
Rapamycin’s inhibition of mTORC1 offers a dual benefit: it promotes autophagy, enabling the clearance of damaged cellular components, and it prevents the onset of cellular senescence, thereby reducing the accumulation of dysfunctional cells. These combined effects make rapamycin a promising therapeutic for a wide range of age-related diseases, including but not limited to kidney disease and Alzheimer's. Its ability to modulate these critical aging processes positions rapamycin as a key candidate in the pursuit of healthier aging and extended lifespan across various tissues and organ systems.
Oxytocin and Longevity
Oxytocin is a hormone that plays a crucial role in social bonding, reproduction, and childbirth. Often referred to as the "love hormone" or "cuddle hormone," it is released in response to social bonding activities like hugging, kissing, or childbirth. However, its functions extend far beyond these. [8]
One of oxytocin's most critical roles is in the regulation of stress, where it serves as a counterbalance to the body's stress-response system. The hypothalamic-pituitary-adrenal (HPA) axis is a key system dedicated to managing stress. This axis involves intricate communication between the hypothalamus, the pituitary gland, and the adrenal glands. In response to stress, the hypothalamus initiates the process by releasing corticotropin-releasing hormone (CRH). CRH then stimulates the pituitary gland to release adrenocorticotropic hormone (ACTH) into the bloodstream, which in turn prompts the adrenal glands to release cortisol, the body's primary stress hormone. Cortisol helps the body manage stress by increasing energy availability and regulating various physiological processes.
However, oxytocin can modulate this stress response, exerting a calming effect on the HPA axis. In the study titled "The Yin and Yang of the Oxytocin and Stress Systems: Opposites, Yet Interdependent and Intertwined Determinants of Lifelong Health Trajectories," Moberg et al. (2024) explored how oxytocin influences this system and identified three key mechanisms through which it counterbalances the HPA axis. First, oxytocin reduces the secretion of CRH from the hypothalamus, thereby decreasing the activity of the stress-response cascade at its source. Additionally, oxytocin directly inhibits the release of ACTH from the pituitary gland and cortisol from the adrenal glands. These actions collectively help buffer the body's stress response, promoting a state of calm and reducing the long-term physiological impacts of stress. [9, 10, 11]
Oxytocin also plays a vital role in cardiovascular health by acting on specific receptors in heart cells and blood vessels to regulate blood pressure and heart rhythm. These receptors, proteins located on the surface or inside cells, recognize and bind to oxytocin, allowing the hormone to exert its physiological effects. Oxytocin receptors are present in both heart muscle cells and endothelial cells, which line blood vessels, making them key players in cardiovascular regulation.
In the study titled "Oxytocinergic Regulation of Cardiovascular Function: Studies in Oxytocin-Deficient Mice," Michelini et al. (2003) investigated how changes in oxytocin levels affect blood pressure, heart rate, and the body's ability to regulate these factors. The researchers used two groups of mice: an experimental group genetically modified to lack the oxytocin gene, resulting in undetectable levels of the hormone, and a control group with normal oxytocin levels. The study revealed that mice lacking oxytocin exhibited lower blood pressure, with an average of 102 mmHg compared to 110 mmHg in the control group, suggesting that oxytocin plays a role in stabilizing blood pressure.
Moreover, the research highlighted oxytocin's influence on the baroreflex—a critical mechanism that helps regulate cardiovascular function by adjusting heart rate and blood vessel constriction in response to changes in blood pressure. The study found that the baroreflex in oxytocin-deficient mice was less responsive and sensitive to a narrower range of blood pressure fluctuations compared to the control group. This reduced responsiveness indicates that oxytocin enhances the baroreflex, helping the body maintain cardiovascular stability during blood pressure changes [12].
The study concluded that oxytocin is essential for regulating blood pressure and supporting the body's ability to manage cardiovascular fluctuations, reinforcing its importance in overall cardiovascular health [12].
Oxytocin and Muscle Preservation
Beyond its roles in stress regulation and cardiovascular health, oxytocin has emerged as a key factor in muscle preservation and metabolic health. Seminal research by Irina and Michael Conboy from UC Berkeley’s Department of Bioengineering demonstrated that factors present in younger blood, including oxytocin, stimulate muscle stem cell (MuSC) activity to preserve muscle mass. Published in Nature, this groundbreaking study used a procedure called parabiosis, where two mice are surgically joined to share their circulatory systems. The results revealed that older mice paired with younger ones exhibited enhanced muscle repair compared to those paired with similarly aged mice. This enhanced repair capability was attributed to the younger blood environment, which is rich in factors that rejuvenate aging MuSCs, improving their regenerative potential and muscle repair capacity [26].
Conversely, when young muscle tissues were transplanted into older rats, these tissues experienced a decline in muscle mass and strength. This decline was further compounded by impaired angiogenesis—the formation of new blood vessels—within the transplanted muscle in the older host. Angiogenesis is critical for supplying oxygen and nutrients and removing waste products from tissues. However, in older hosts, this essential process was significantly hindered, limiting the growth and maintenance of grafted muscle tissue and contributing to the observed decline in muscle mass and function.
Building on this research, a follow-up experiment led by Dr. Elabd, Dr. Cousin, and Irina Conboy identified oxytocin as a key serum factor responsible for stimulating muscle tissue maintenance and repair. Published in Nature and titled "Oxytocin is an Age-Specific Circulating Hormone that is Necessary for Muscle Maintenance and Regeneration," this study investigated the effects of oxytocin administration on skeletal muscle tissue in elderly mice. The results were striking—oxytocin treatment significantly improved the function of aging muscle stem cells (MuSCs), enhancing muscle regeneration and overall muscle function.
Muscle stem cells possess specific receptors designed to recognize and bind to oxytocin. When oxytocin attaches to these receptors, it triggers a cascade of intracellular reactions that stimulate MuSC activity, promoting muscle growth and repair. This process is crucial for maintaining muscle mass, particularly as we age or recover from injury. Additionally, oxytocin exhibits anti-catabolic effects, preventing the breakdown of muscle proteins—a protective mechanism that is vital for preserving muscle mass, especially in aging individuals or those with conditions that accelerate muscle loss.
Recent studies suggest that the gradual decline in oxytocin levels with age may be a contributing factor to the weakening of muscle function, leading to conditions like sarcopenia. These findings highlight oxytocin's critical role in muscle strength and development, positioning it as a potential therapeutic target for mitigating age-related muscle decline.
Oxytocin also interacts with other hormones and growth factors involved in muscle maintenance. For instance, oxytocin helps protect muscle tissue from the catabolic effects of cortisol, the body's primary stress hormone. By regulating cortisol levels, oxytocin mitigates cortisol’s breakdown of muscle proteins, ensuring that muscle mass is preserved even during periods of stress. This interplay between oxytocin and other metabolic factors underscores its importance in maintaining muscle health and preventing the muscle deterioration that often accompanies aging or chronic illness.
Oxytocin's Role in Metabolism
Oxytocin's role in metabolism is equally important, as it influences energy expenditure and appetite regulation. Some studies suggest that oxytocin can help reduce food intake and increase energy expenditure, supporting overall metabolic health. In a study conducted by Professor Ernie Blevins from the University of Washington Diabetes Research Center, Blevins et al. (2015) examined the effects of oxytocin on body weight (BW) and appetite in Rhesus monkeys. The monkeys underwent a 12-hour fast and were provided with regular chow and a daily sweetened drink. After a week of receiving a vehicle (a substance with no active ingredients) to acclimate them to injections, they were administered oxytocin at doses of 0.2 mg/kg for two weeks, followed by 0.4 mg/kg for the next two weeks.
The researchers observed significant weight reduction in the oxytocin-treated monkeys, with BW decreasing by 3.3 ± 0.6 kg compared to the vehicle-treated group. The lower dose of oxytocin reduced 12-hour chow intake by 26%, while the higher dose further reduced 12-hour chow intake by 27% and 8-hour fructose drink intake by 18%. Additionally, oxytocin administration was associated with higher levels of free fatty acids and glycerol, indicating increased fat breakdown. These findings suggest that oxytocin aids in weight reduction by decreasing food intake and enhancing both energy expenditure and fat metabolism in Rhesus monkeys. [13]
Oxytocin Levels and Skin Senescence
The anti-aging potential of oxytocin has also been observed in human skin health. In a pilot clinical study by Hayre (2020), the effects of oxytocin on skin appearance were explored in women aged 48-61. This study was designed to investigate the correlation between oxytocin levels and skin appearance, focusing on oxytocin's role in inhibiting the senescence-associated secretory phenotype (SASP) in skin cells. When activated by oxytocin, receptors on human fibroblasts inhibited SASP, a pathway that leads to the release of proinflammatory cytokines contributing to skin aging. By blocking SASP activation, oxytocin potentially offers a protective mechanism against skin aging and inflammation.
The study methodology involved measuring oxytocin levels, capturing facial photographs, and assessing lifetime sun exposure in six female subjects. A skin age score (SAS) was calculated for each participant, which was then compared to the expected average SAS based on their age. A reduction in SAS indicated younger-looking skin compared to the average person of the same age. The results revealed that all subjects experienced a reduction in their SAS score, with an almost linear relationship between higher oxytocin levels and more youthful skin appearance. This finding suggests that higher oxytocin levels correlate with less visible skin aging, supporting its protective role against sun damage and age-related inflammation. [15]
Research has shown that oxytocin is a multifaceted hormone that plays a vital role in various physiological processes, including stress regulation, cardiovascular health, muscle preservation, and metabolism. As we age, oxytocin levels naturally decline, which may lead to several age-related diseases and conditions. This decline highlights the hormone's significance in maintaining overall health and well-being throughout the lifespan. The growing body of research underscores oxytocin's potential as a key factor in not only enhancing health but also combating the effects of aging, offering promising avenues for future therapeutic interventions to promote longevity and improve quality of life in older adults.
Oxytocin and Rapamycin: A Synergistic Approach
There are several parallels between oxytocin and rapamycin, as both compounds offer significant health benefits and share the potential to extend lifespan. However, the mechanisms through which they promote longevity differ. Rapamycin exerts its effects primarily by inhibiting mTOR complexes, particularly mTORC1. As we discussed in detail, this is crucial because, with age, mTORC1 can become hyperactive, leading to reduced autophagy, increased waste accumulation, cellular senescence, and uncontrolled cell division. By administering rapamycin, mTORC1 activity is modulated, allowing autophagy to resume normal function. This process aids in recycling and clearing damaged cellular components, preventing cells from progressing toward senescence.
Oxytocin enhances longevity through multiple mechanisms, and some research theorizes that it may also play a role in regulating telomere length. Telomeres are the protective caps at the ends of chromosomes that naturally shorten with age, leading to cellular senescence. There is growing interest in the possibility that oxytocin may help preserve telomere length, thereby delaying the onset of cellular aging. This potential protective effect is thought to be linked to oxytocin’s ability to reduce inflammation and oxidative stress—two major contributors to telomere shortening. By inhibiting the senescence-associated secretory phenotype (SASP), oxytocin helps minimize the release of inflammatory molecules that accelerate the aging process, supporting cellular health and longevity.
Beyond telomere maintenance, oxytocin also counteracts the effects of cortisol. It modulates the hypothalamic-pituitary-adrenal (HPA) axis by inhibiting the release of hypothalamic hormones (corticotropin-releasing hormone, CRH), anterior pituitary hormones (adrenocorticotropic hormone), and adrenal hormones (cortisol). By preventing the overactivation of this axis, oxytocin helps mitigate the negative health impacts of chronic stress, including accelerated aging.
Although few studies have explored the combined effects of oxytocin and rapamycin on aging and well-being, their combination is likely synergistic. This is because each compound targets distinct pathways. A combinatorial approach could activate multiple pathways simultaneously, potentially amplifying the benefits across various health domains. This includes effectively reducing inflammation, improving cardiovascular health, regulating metabolism, and managing stress.
Rapamycin, Oxytocin, and Inflammation
One of the primary driving forces of aging is chronic inflammation. Inflammation is the process by which our immune system becomes active, and it can be classified into two types: acute and chronic. Acute inflammation is a short-term response where the body's immune defenses are activated to clear foreign agents or injury. Chronic inflammation occurs when the immune system remains persistently active without a clear cause, leading to tissue damage. This chronic state is associated with various age-related diseases, including cardiovascular diseases, diabetes, and neurodegenerative disorders. As we age, the body's ability to regulate inflammation diminishes, resulting in a higher prevalence of chronic inflammatory conditions.
The combination of rapamycin and oxytocin offers a comprehensive approach to managing chronic inflammation, addressing both cellular and systemic levels. Rapamycin's primary mechanism of action involves reducing inflammation at the cellular level by promoting autophagy and inhibiting pro-inflammatory signals. Autophagy is crucial for cellular health as it allows for the degradation and recycling of damaged cellular components, preventing the accumulation of harmful debris that can trigger inflammation. By inhibiting the mTORC1 pathway, rapamycin effectively reduces the production of pro-inflammatory cytokines and other molecules that contribute to the chronic inflammatory state seen in aging and age-related diseases. [16]
Madrioli et al. (2023) published a pivotal study in the prestigious journal Nature, highlighting rapamycin's role as a powerful anti-inflammatory agent in the treatment of amyotrophic lateral sclerosis (ALS), a neurodegenerative disease characterized by the progressive death of motor neurons. The death of these neurons triggers a cascade of inflammatory responses, particularly the activation of microglia, the immune cells of the nervous system. This leads to the release of pro-inflammatory cytokines, such as IL-18, which exacerbate neuronal damage by promoting apoptosis and further microglial activation.
The study involved 63 ALS patients who were administered varying doses of rapamycin or a placebo over 18 weeks, followed by a 36-week observation period. The researchers found that rapamycin significantly reduced neuroinflammation by lowering levels of IL-18, which is known to drive further neuronal damage through the production of reactive oxygen species (ROS). Notably, the treatment was well-tolerated by patients, underscoring rapamycin's potential as a therapeutic agent for managing chronic inflammation in neurodegenerative and other age-related diseases.
This case study reinforces the broader role of rapamycin in decreasing systemic inflammation—a key driver of many age-related diseases—positioning it as a promising intervention to address the inflammatory components of aging. [16]
While rapamycin excels at targeting intracellular processes such as autophagy and cellular senescence, it may not fully address the broader, systemic inflammatory responses that can occur throughout the body. Oxytocin complements rapamycin by targeting the MAPK signaling pathway, offering a more global and independent mechanism for managing inflammation. The MAPK pathway plays a critical role in regulating various cellular activities, including the body's response to external stressors and inflammation. By modulating this pathway, oxytocin reduces systemic inflammation across multiple tissues and organs.
This systemic influence is particularly significant in conditions characterized by widespread inflammation, such as neurodegenerative diseases like Alzheimer's and Parkinson's, where inflammation extends beyond localized cellular damage. In these cases, oxytocin's ability to modulate systemic inflammatory responses could provide protection against the progression of disease. By working through a distinct pathway, oxytocin enhances the overall anti-inflammatory strategy, potentially offering therapeutic benefits in conditions where inflammation is both chronic and widespread. [17]
Rashed et al. (2011) demonstrated the anti-inflammatory properties of oxytocin in a study involving rats treated with cisplatin, a chemotherapeutic drug known for its nephrotoxic side effects. Cisplatin induces oxidative stress by activating NADPH oxidase, which leads to the production of reactive oxygen species (ROS) and subsequent activation of MAPK signaling, exacerbating cellular damage and inflammation. The study found that oxytocin administration significantly reduced levels of NADPH oxidase and MAPK, effectively mitigating cisplatin-induced inflammation. Additionally, oxytocin decreased the production of nitric oxide, a compound closely associated with inflammation, and provided protective effects on kidney tissues by reducing both damage and inflammation. [18]
By combining rapamycin’s ability to target intracellular processes with oxytocin’s systemic anti-inflammatory effects, these two compounds present a comprehensive approach to managing inflammation. Rapamycin addresses the root causes of cellular dysfunction, while oxytocin modulates the broader inflammatory responses that contribute to the progression of disease, offering a more holistic strategy for inflammation management. [18]
The combination of rapamycin and oxytocin offers a dual approach to managing the various triggers and perpetrators of inflammation. Inflammation is not only caused by cellular debris and stress responses but also by external factors such as infections, environmental toxins, and lifestyle-related issues like poor diet and lack of exercise. While rapamycin primarily addresses the internal cellular processes that lead to inflammation, oxytocin extends its benefits by counteracting the inflammatory effects of external stressors. For instance, oxytocin has been shown to improve social behaviors and reduce stress, which can directly impact lowering inflammation caused by psychological and social stressors. This dual approach addresses inflammation where it originates within the cells and in response to external factors, leading to a more comprehensive and sustained reduction in inflammatory processes. [16, 17]
Psychological stress significantly contributes to inflammation, and oxytocin's well-documented effects on reducing stress and promoting social bonding add another benefit. Chronic psychological stress exacerbates inflammation by triggering the release of stress hormones like cortisol, leading to prolonged inflammatory responses throughout the body. Elevated cortisol levels are associated with increased production of pro-inflammatory cytokines, contributing to a cycle of chronic inflammation that is difficult to break. While rapamycin's effects focus more on cellular pathways, it does not directly influence the psychological and social factors contributing to inflammation. Oxytocin, however, has been shown to reduce anxiety, enhance feelings of well-being, and promote social connections, which can indirectly lower stress-induced inflammation. These social and emotional benefits of oxytocin are particularly relevant in today's world, where chronic stress and social isolation are common issues. By improving mood, reducing anxiety, and fostering social bonds, oxytocin helps to create a more favorable psychological environment that is less conducive to the development and persistence of chronic inflammation. [14]
Furthermore, combining oxytocin and rapamycin can enhance the immune system's regulatory functions. While chronic inflammation is often harmful, the immune system must respond appropriately to threats such as infections and injuries. Using both rapamycin and oxytocin may fine-tune the immune response, ensuring that it is strong enough to defend against external threats while also being regulated enough to prevent excessive, chronic inflammation. Oxytocin's role in enhancing social behaviors and reducing stress could lead to a more balanced immune response, as chronic stress is known to dysregulate immune function. Meanwhile, rapamycin's ability to modulate autophagy and cytokine production ensures that the immune system does not overreact to internal cellular damage, thereby preventing the cascade of events that lead to chronic inflammation. [16, 17]
Rapamycin, Oxytocin, and Cardiovascular Health
As we age, our cardiovascular system experiences a gradual decline in functional capacity, increasing susceptibility to cardiovascular diseases. A primary contributor to this decline is damage to the blood vessels, particularly the endothelial cells lining them. These cells act as a barrier, keeping blood separate from surrounding tissues and organs. Over time, however, endothelial cells can become damaged or less effective, leading to endothelial dysfunction. This dysfunction is a key factor in the development of atherosclerosis, where fatty deposits accumulate in the arteries.
Age-related changes in diet and lifestyle exacerbate this process. As we grow older, we may consume more 'comfort foods' high in saturated fats, sugars, and processed ingredients, while engaging in less physical activity. These behaviors contribute to the buildup of fatty deposits in blood vessels. Atherosclerosis poses a significant health risk because these fatty deposits can clog arteries that supply the heart with oxygen and nutrients. When the heart muscle is deprived of these essential resources, it can result in a myocardial infarction, commonly known as a heart attack. According to the National Center for Health Statistics, heart attacks are one of the leading causes of death among the elderly in the U.S. [19]
Combining rapamycin and oxytocin could offer enhanced cardiovascular protection by targeting distinct mechanisms that, when combined, promote overall cardiovascular health.
Rapamycin improves endothelial function and reduces vascular inflammation by inhibiting mTOR signaling. This inhibition helps delay cellular senescence, promote autophagy, and suppress inflammation and fat buildup in blood vessels. In the study "Rapamycin-Loaded Biomimetic Nanoparticles Reverse Vascular Inflammation" published in Circulation Research, Boada et al. (2019) explored rapamycin's effects on vascular inflammation in mice with atherosclerosis. In this study, rapamycin was delivered to the blood vessel walls using biomimetic nanoparticles—tiny particles specifically designed to target particular areas within the body [20].
The results demonstrated that mice treated with these rapamycin-loaded nanoparticles had fewer proliferating immune cells in their arteries, lower levels of inflammatory markers, and reduced tissue breakdown compared to those receiving free rapamycin or no treatment. These findings suggest that when delivered effectively, rapamycin can stabilize advanced arterial plaques with minimal side effects, offering a targeted approach to managing vascular inflammation. However, its effects are primarily concentrated within the vasculature, indicating that additional strategies may be needed to address systemic inflammation more broadly. [20]
Oxytocin complements rapamycin by promoting heart health through its vasodilatory effects and hormonal regulation. Oxytocin binds to specific receptors in heart cells and blood vessels, where it plays a key role in regulating blood pressure and heart rhythm. One of its primary actions is inducing vasodilation by increasing the production of nitric oxide (NO). NO relaxes the smooth muscle cells that line blood vessels, reducing arterial wall stress and effectively lowering blood pressure. This vasodilation is crucial for reducing cardiovascular strain and improving circulation.
In addition to its effects on blood vessels, oxytocin stimulates the release of atrial natriuretic peptide (ANP), a hormone that helps prevent heart muscle hypertrophy (enlargement) and fibrosis (the formation of excess connective tissue). By promoting ANP release, oxytocin prevents the structural changes in the heart that impair function over time. This dual action—reducing blood pressure and preventing detrimental heart remodeling—highlights oxytocin’s critical role in maintaining overall heart health and function.
While rapamycin focuses on remodeling blood vessels, oxytocin is vital for maintaining the structural integrity of the heart muscle. By combining these two compounds, vascular and cardiac health can be addressed simultaneously, offering a more comprehensive therapeutic strategy for cardiovascular protection.
Rapamycin, Oxytocin, and Metabolic Regulation
Rapamycin and oxytocin are both potent metabolic regulators, each targeting distinct pathways. Combined, their effects complement one another, offering a more comprehensive approach to metabolic health.
Rapamycin partially regulates metabolic function, particularly by enhancing insulin sensitivity and reducing adiposity (fat accumulation), when taken at lower doses. Insulin sensitivity refers to how effectively the body's cells respond to insulin, the hormone responsible for regulating blood sugar levels. High insulin sensitivity allows cells to efficiently absorb glucose from the bloodstream, while low sensitivity—commonly known as insulin resistance—can lead to elevated blood sugar levels and increase the risk of type 2 diabetes (T2D). [21]
Rapamycin improves insulin sensitivity when taken at lower doses by inhibiting the mTORC1 pathway, which, when hyperactive, interferes with insulin signaling. This hyperactivity is often associated with age-related increases in insulin resistance and the development of T2D. By inhibiting mTORC1, rapamycin enhances insulin signaling, improving glucose uptake by cells and contributing to better overall metabolic function. Research by Zhou & Ye (2018) found that rapamycin improved insulin resistance and metabolic health in diabetic rats, primarily through its role in enhancing autophagy. However, the study also highlighted the importance of proper dosing. Chronic overuse of rapamycin can lead to metabolic side effects, such as dyslipidemia (abnormal cholesterol levels), which may increase cardiovascular risks. [21]
Oxytocin offers complementary benefits to rapamycin by improving insulin sensitivity and metabolic regulation through different mechanisms. Oxytocin activates AMP-activated protein kinase (AMPK), a critical enzyme that regulates energy balance. By activating AMPK, oxytocin enhances the body's response to insulin, improving glucose uptake and utilization. Additionally, oxytocin facilitates the translocation of glucose transporter type 4 (GLUT4) to the cell surface, which increases glucose absorption into cells, particularly in muscle and fat tissues. It also promotes the breakdown of fats for energy, aiding in managing body fat levels and contributing to overall metabolic health. [22]
In a notable clinical trial involving 21 older adults (average age 67.5 years) who were both obese and had slow gait speeds (an indicator of sarcopenia), researchers examined the effects of oxytocin supplementation. The participants were randomly assigned to receive either intranasal oxytocin or a placebo (an inactive substance) over eight weeks, in a double-masked, placebo-controlled setting [30]. The trial demonstrated that oxytocin administration was well-tolerated, with no significant side effects. Notably, those who received oxytocin experienced an increase in muscle mass, averaging a 2.25 kg gain, compared to the placebo group. There was also a tendency towards a decrease in fat mass.
The study also investigated the impact of oxytocin (OT) on low-density lipoprotein (LDL) levels in the blood. LDL, often termed "bad cholesterol," is a type of lipoprotein composed of fats and proteins, responsible for transporting cholesterol from the liver to other cells in the body. Elevated levels of LDL cholesterol are linked to an increased risk of atherosclerosis, a condition where arteries become narrowed and hardened due to cholesterol and other substance buildup. This can lead to serious cardiovascular diseases like heart attacks and strokes. Therefore, monitoring LDL levels is crucial for cardiovascular health. Participants who received oxytocin showed lower LDL levels compared to those in the placebo group. [30]
Combining rapamycin with oxytocin could offer a more balanced approach to metabolic health, potentially mitigating some of the side effects of rapamycin treatment. Oxytocin's ability to promote fat breakdown and suppress appetite can counterbalance the adverse metabolic effects of rapamycin, such as dyslipidemia. Moreover, the combination may allow for lower doses and shorter treatment durations, reducing the risk of side effects while maintaining therapeutic efficacy.
Furthermore, oxytocin directly enhances glucose metabolism by promoting the translocation of GLUT4 to the cell surface, increasing glucose uptake even in the presence of varying insulin levels. It also stimulates pancreatic beta cells to boost insulin secretion, further enhancing glucose regulation. This dual action—enhancing insulin sensitivity while also increasing insulin production—makes the combination of rapamycin and oxytocin a robust strategy for improving metabolic regulation. [22]
Rapamycin, Oxytocin, Stress and Cortisol
When we encounter stress, our body activates its stress response systems, releasing hormones such as cortisol and adrenaline. These hormones help the body adapt to stress by mobilizing energy and resources. However, when stress is chronic, the continuous activation of these systems leads to what is known as allostatic load. Allostatic load refers to the cumulative wear and tear on the body that results from repeated cycles of stress and the body's efforts to adapt. Initially, this load is a protective mechanism, helping the body maintain stability (homeostasis) and adapt to external stressors. But as we age and face more frequent or intense stressors, the burden can become overwhelming, leading to what is known as allostatic overload. This occurs when the body's ability to manage and recover from stress is exceeded, damaging various systems, including the cardiovascular, metabolic, and immune systems. This chronic strain can accelerate the aging process and increase vulnerability to stress-related diseases.
Rapamycin has shown some indirect effects in mitigating stress by targeting the mTOR pathway, which is linked to oxidative stress—a significant contributor to allostatic load. While rapamycin is not directly associated with managing psychological stress, its inhibition of mTOR activity can enhance the body's resilience to metabolic and oxidative stress, thereby improving cellular function and reducing damage. Moreover, since chronic stress often leads to increased inflammation, rapamycin's anti-inflammatory properties can help reduce the risk of stress-related diseases, providing further support for its therapeutic potential. [23]
Research supports rapamycin's role in managing stress-related conditions. For example, Zhang et al. (2023) investigated rapamycin's effects on mice subjected to chronic stress, a known risk factor for depressive disorders. In their study, mice exposed to chronic stress showed decreased myelination in the prefrontal cortex (PFC)—a brain region crucial for decision-making, emotional regulation, and social behavior—and increased mTOR activity. These changes are associated with depressive-like symptoms. Remarkably, chronic rapamycin treatment reversed these effects by increasing myelination and reducing mTOR signaling in the PFC. This led to a reduction in depressive symptoms, as measured by the sucrose preference test, which assesses anhedonia, a core symptom of depression. Mice treated with rapamycin showed a greater preference for sucrose, indicating improved mood and stress resilience. [23]
While rapamycin effectively targets physiological stress by reducing oxidative stress and protecting against cellular damage, it is not known to have any direct effects on psychological stress.
Oxytocin, however, addresses both physiological and psychological stress. It modulates the hypothalamic-pituitary-adrenal (HPA) axis to prevent the overproduction of cortisol, the stress hormone. Additionally, oxytocin influences key neurotransmitters such as dopamine and serotonin, which play crucial roles in mood regulation, pleasure, and anxiety reduction. By stabilizing these neurotransmitters, oxytocin helps alleviate psychological stress, reduce anxiety, and promote well-being. Furthermore, oxytocin directly impacts the amygdala, a brain region central to processing fear and anxiety, helping to mitigate emotional responses to stress. [14]
In a study by Guastella et al. (2009), oxytocin was shown to improve the outcomes of exposure therapy in individuals with social anxiety disorder (SAD). Participants who received oxytocin along with exposure therapy demonstrated improved self-evaluations of their appearance and speech performance, likely due to oxytocin's ability to reduce overactivity in the amygdala, which is typically heightened during stressful situations in individuals with SAD. [24]
The combination of rapamycin and oxytocin offers a superior approach to stress management compared to using either compound alone. While rapamycin effectively reduces physiological stress by targeting metabolic and oxidative pathways, oxytocin addresses physiological and psychological stress. Together, they provide a more holistic approach, supporting the body's physical responses and mental well-being. This combination reduces the wear and tear from allostatic load and enhances overall resilience to stress, making it a more powerful therapeutic strategy than relying on a single compound.
Practical Considerations for Combined Therapy
Combining rapamycin and oxytocin can produce synergistic effects across various health domains, including anti-inflammation, cardiovascular health, metabolic regulation, and stress response. Having established the benefits of this combinatorial approach, it is crucial to address the practical aspects of its implementation. Specifically, we must consider the optimal dosages, administration schedules, and safety monitoring strategies required to maximize the therapeutic benefits while minimizing potential side effects.
Optimizing Rapamycin Therapy: The Protective Role of Oxytocin in Mitigating mTORC2 Inhibition
Oxytocin may help mitigate the effects of mTORC2 inhibition, a concern observed in patients taking elevated doses or chronic daily dosing of rapamycin. The mTOR pathway, which plays a crucial role in cellular growth and metabolism, is divided into two main complexes: mTORC1 and mTORC2.
mTORC1: This complex, highly sensitive to rapamycin, governs processes such as protein synthesis, autophagy, and nutrient sensing. To conceptualize, mTORC1 functions like a cellular manager, assessing nutrient levels, coordinating intracellular machinery, and managing waste. Even at low doses, rapamycin profoundly affects mTORC1, which is often beneficial for promoting autophagy and reducing cellular senescence.
mTORC2: In contrast, mTORC2 is typically less affected by short-term rapamycin exposure. This complex regulates immune responses, cell survival, and lipid metabolism, acting as a stabilizer that maintains cellular structure and resilience under adverse conditions. At low doses, rapamycin predominantly targets mTORC1, but as the dosage increases, there’s potential for mTORC2 inhibition. This can negatively impact immune function and metabolic health, emphasizing the need for precise dosing to harness rapamycin's benefits while minimizing unintended effects on critical cellular processes.
Oxytocin may offer a complementary approach by stimulating the AMP-activated protein kinase (AMPK) pathway, which supports the maintenance of cellular structures. AMPK, in turn, can activate mTORC2. By increasing AMPK activity, oxytocin could theoretically reduce the adverse side effects associated with mTORC2 inhibition, thereby helping to balance rapamycin's effects on cellular health.
Safety Monitoring
Safety monitoring is paramount when administering a combined therapy of rapamycin and oxytocin. Regular assessment of key health metrics, including heart rate variability, blood pressure, inflammatory markers, and metabolic health indicators, is essential to ensure the combination therapy is safe and effective. This proactive approach will help mitigate risks associated with regular use of these compounds, ensuring a more effective and safer therapeutic experience.
Conclusion
Oxytocin and rapamycin each offer distinct benefits in anti-aging, addressing different aspects of the aging process. Rapamycin primarily targets cellular pathways, inhibiting mTOR to promote autophagy, reduce cellular senescence, and mitigate inflammation. Oxytocin, on the other hand, excels in regulating stress, enhancing cardiovascular function, and improving metabolic health. However, each of these compounds, when used alone, has limitations. For example, while rapamycin is effective in cellular maintenance, it does not directly address the psychological and emotional factors that can exacerbate aging. Similarly, oxytocin's ability to reduce stress and improve heart health does not extend to the cellular level where mTOR inhibition can prevent senescence and promote longevity.
By combining these therapies, one's strengths can compensate for the limitations of the other. Rapamycin's ability to enhance cellular health is complemented by oxytocin's role in stress regulation and cardiovascular support, resulting in a more comprehensive approach to aging. This synergy addresses multiple aging pathways simultaneously, offering a more holistic solution to age-related decline.
While oxytocin and rapamycin are powerful as standalone treatments, their combined use presents a more robust strategy for extending health span and promoting longevity. By integrating their complementary effects, we can achieve more significant improvements in combating the complex aging processes, paving the way for more effective anti-aging interventions.
- Bakhshi , S. (2023, October 22). Healthspan Research Review: Rapamycin Research and clinical trials: A synthesis of recent scientific findings. Healthspan. https://gethealthspan.com/science/article/rapamycin-research-synthesis-recent-scientific-findings
- Konopka, A.R., Lamming, D.W., RAP PAC Investigators. et al. Blazing a trail for the clinical use of rapamycin as a geroprotecTOR. GeroScience (2023). https://doi.org/10.1007/s11357-023-00935-x
- Rabanal-Ruiz, Y., Otten, E. G., & Korolchuk, V. I. (2017). mTORC1 as the main gateway to autophagy. Essays in biochemistry, 61(6), 565–584. https://doi.org/10.1042/EBC20170027
- Oh, W. J., & Jacinto, E. (2011). mTOR complex 2 signaling and functions. Cell cycle (Georgetown, Tex.), 10(14), 2305–2316. https://doi.org/10.4161/cc.10.14.16586
- Wu, L., Feng, Z., Cui, S., Hou, K., Tang, L., Zhou, J., Cai, G., Xie, Y., Hong, Q., Fu, B., & Chen, X. (2013). Rapamycin upregulates autophagy by inhibiting the mTOR-ULK1 pathway, resulting in reduced podocyte injury. PloS one, 8(5), e63799. https://doi.org/10.1371/journal.pone.0063799
- Houssaini, A., Breau, M., Kebe, K., Abid, S., Marcos, E., Lipskaia, L., Rideau, D., Parpaleix, A., Huang, J., Amsellem, V., Vienney, N., Validire, P., Maitre, B., Attwe, A., Lukas, C., Vindrieux, D., Boczkowski, J., Derumeaux, G., Pende, M., Bernard, D., … Adnot, S. (2018). mTOR pathway activation drives lung cell senescence and emphysema. JCI insight, 3(3), e93203. https://doi.org/10.1172/jci.insight.93203
- Spilman, P., Podlutskaya, N., Hart, M. J., Debnath, J., Gorostiza, O., Bredesen, D., Richardson, A., Strong, R., & Galvan, V. (2010). Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PloS one, 5(4), e9979. https://doi.org/10.1371/journal.pone.0009979
- Harvard Health. (2023, June 13). Oxytocin: The love hormone. https://www.health.harvard.edu/mind-and-mood/oxytocin-the-love-hormone
- professional, C. C. medical. (2020, April 4). Pituitary gland: What it is, Function & Anatomy. Cleveland Clinic. https://my.clevelandclinic.org/health/body/21459-pituitary-gland
- Herman, J. P., McKlveen, J. M., Ghosal, S., Kopp, B., Wulsin, A., Makinson, R., Scheimann, J., & Myers, B. (2016). Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Comprehensive Physiology, 6(2), 603–621. https://doi.org/10.1002/cphy.c150015
- Uvnäs-Moberg, K., Gross, M. M., Calleja-Agius, J., & Turner, J. D. (2024). The Yin and Yang of the oxytocin and stress systems: opposites, yet interdependent and intertwined determinants of lifelong health trajectories. Frontiers in endocrinology, 15, 1272270. https://doi.org/10.3389/fendo.2024.1272270
- Michelini, L. C., Marcelo, M. C., Amico, J., & Morris, M. (2003). Oxytocinergic regulation of cardiovascular function: studies in oxytocin-deficient mice. American journal of physiology. Heart and circulatory physiology, 284(6), H2269–H2276. https://doi.org/10.1152/ajpheart.00774.2002
- Blevins, J. E., Graham, J. L., Morton, G. J., Bales, K. L., Schwartz, M. W., Baskin, D. G., & Havel, P. J. (2015). Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. American journal of physiology. Regulatory, integrative and comparative physiology, 308(5), R431–R438. https://doi.org/10.1152/ajpregu.00441.2014
- Stevenson JR, McMahon EK, Boner W, Haussmann MF (2019) Oxytocin administration prevents cellular aging caused by social isolation. Psychoneuroendocrinology 103:52-60.
- Hayre N. (2020). Oxytocin Levels Inversely Correlate With Skin Age Score and Solar Damage. Journal of drugs in dermatology : JDD, 19(12), 1146–1148. https://doi.org/10.36849/JDD.2020.5063
- Mandrioli, J., D’Amico, R., Zucchi, E. et al. Randomized, double-blind, placebo-controlled trial of rapamycin in amyotrophic lateral sclerosis. Nat Commun 14, 4970 (2023). https://doi.org/10.1038/s41467-023-40734-8
- ZHANG, W., LIU, H. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 12, 9–18 (2002). https://doi.org/10.1038/sj.cr.7290105
- Rashed, L. A., Hashem, R. M., & Soliman, H. M. (2011). Oxytocin inhibits NADPH oxidase and P38 MAPK in cisplatin-induced nephrotoxicity. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 65(7), 474–480. https://doi.org/10.1016/j.biopha.2011.07.001
- Centers for Disease Control and Prevention. (2024, May 2). FASTSTATS - older persons health. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/fastats/older-american-health.htm
- Boada, C., Zinger, A., Tsao, C., Zhao, P., Martinez, J. O., Hartman, K., Naoi, T., Sukhoveshin, R., Sushnitha, M., Molinaro, R., Trachtenberg, B., Cooke, J. P., & Tasciotti, E. (2020). Rapamycin-Loaded Biomimetic Nanoparticles Reverse Vascular Inflammation. Circulation research, 126(1), 25–37. https://doi.org/10.1161/CIRCRESAHA.119.315185
- Zhou, W., & Ye, S. (2018). Rapamycin improves insulin resistance and hepatic steatosis in type 2 diabetes rats through activation of autophagy. Cell biology international, 42(10), 1282–1291. https://doi.org/10.1002/cbin.11015
- Zhang, H., Wu, C., Chen, Q., Chen, X., Xu, Z., Wu, J., & Cai, D. (2013). Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PloS one, 8(5), e61477. https://doi.org/10.1371/journal.pone.0061477
- Zhang, J., Li, W., Yue, Q., Liu, L., Hou, S. T., & Ju, J. (2023). Rapamycin Exerts an Antidepressant Effect and Enhances Myelination in the Prefrontal Cortex of Chronic Restraint Stress Mice. Neuroscience, 535, 99–107. https://doi.org/10.1016/j.neuroscience.2023.10.025
- Adam J. Guastella, Alexandra L. Howard, Mark.R. Dadds, Philip Mitchell, Dean S. Carson, A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder, Psychoneuroendocrinology,Volume 34, Issue 6,2009, Pages 917-923, ISSN 0306-4530,
- Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One. 2011;6(9):e25416. doi: 10.1371/journal.pone.0025416. Epub 2011 Sep 28. PMID: 21980451; PMCID: PMC3182203.
- Conboy, I. M., Conboy, M. J., Wagers, A. J., Girma, E. R., Weissman, I. L., & Rando, T. A. (2005). Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature, 433(7027), 760–764. https://doi.org/10.1038/nature03260
- Elabd, C., Cousin, W., Upadhyayula, P., Chen, R. Y., Chooljian, M. S., Li, J., Kung, S., Jiang, K. P., & Conboy, I. M. (2014). oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nature communications, 5, 4082. https://doi.org/10.1038/ncomms5082
- Jamshid Faraji, Mitra Karimi, Nabiollah Soltanpour, Alireza Moharrerie, Zahra Rouhzadeh, Hamid lotfi, S Abedin Hosseini, S Yaghoob Jafari, Shabnam Roudaki, Reza Moeeini, Gerlinde AS Metz (2018) Oxytocin-mediated social enrichment promotes longer telomeres and novelty seeking eLife 7:e40262 https://doi.org/10.7554/eLife.40262
- Benameur T, Panaro MA, Porro C. The antiaging role of oxytocin. Neural Regen Res. 2021 Dec;16(12):2413-2414. doi: 10.4103/1673-5374.313030. PMID: 33907023; PMCID: PMC8374585.
- Wronski, M. L., Plessow, F., Kerem, L., Asanza, E., O'Donoghue, M. L., Stanford, F. C., Bredella, M. A., Torriani, M., Soukas, A. A., Kheterpal, A., Eddy, K. T., Holmes, T. M., Deckersbach, T., Vangel, M., Holsen, L. M., & Lawson, E. A. (2022). A randomized, double-blind, placebo-controlled clinical trial of 8-week intranasal oxytocin administration in adults with obesity: Rationale, study design, and methods. Contemporary clinical trials, 122, 106909. https://doi.org/10.1016/j.cct.2022.106909
Related studies