Optimizing Glucose Metabolism: The Role of Exercise Modalities and Dietary Interventions
Glucose Metabolism and Its Regulation: Glucose serves as the primary energy source for the body's cells, derived from carbohydrates consumed in the diet. Insulin, a hormone produced by the pancreas, plays a crucial role in regulating blood glucose levels by facilitating glucose uptake into cells. Stable glucose levels are vital for preventing conditions like hyperglycemia and hypoglycemia, which can lead to severe health issues such as diabetes and cardiovascular disease. The liver and muscles store excess glucose as glycogen, which can be released during fasting or physical activity to ensure a continuous energy supply.
Impact of Exercise Modalities on Glucose Metabolism: Different types of exercise, such as aerobic and anaerobic activities, engage specific metabolic pathways that influence how the body uses glucose. Aerobic exercise, which relies on oxidative phosphorylation in the mitochondria, enhances glucose uptake and improves insulin sensitivity, making it effective for managing blood sugar levels. Anaerobic exercise, which relies on glycolysis, utilizes stored glycogen in muscles for rapid energy production, making it important for high-intensity efforts. Both types of exercise play a critical role in preventing insulin resistance and supporting metabolic health.
Role of Mitochondria in Exercise and Metabolism: Mitochondria are essential for energy production, particularly during exercise. Aerobic training increases the number, size, and efficiency of mitochondria, enhancing the body's ability to oxidize glucose and fatty acids for energy. These mitochondrial adaptations improve metabolic flexibility, allowing the body to switch between fuel sources more effectively. This is crucial for sustaining physical performance and maintaining energy balance, which supports overall metabolic health.
Dietary Influence on Glucose Metabolism: Macronutrients like carbohydrates, fats, and proteins significantly impact glucose metabolism. Carbohydrates are the primary source of glucose, while fats and proteins modulate the glycemic response and influence how the body manages blood sugar levels. Fiber plays a critical role in slowing carbohydrate absorption, leading to more stable blood glucose levels. The Mediterranean Diet, rich in whole foods, healthy fats, and polyphenols, is particularly effective in enhancing glucose metabolism, improving insulin sensitivity, and reducing the risk of metabolic disorders.
Synergistic Effects of Diet and Exercise: Combining regular exercise with targeted dietary interventions creates a powerful synergy that optimizes glucose metabolism and enhances overall metabolic health. Exercise improves insulin sensitivity, while a balanced diet rich in fiber, healthy fats, and lean proteins supports this effect. The timing of nutrient intake relative to exercise can also influence blood glucose levels, with strategies like carbohydrate timing helping to maintain stable glucose levels. Integrating exercise with a healthy diet can improve glycemic control, support cardiovascular health, and prevent metabolic disorders.
Case Study of the Mediterranean Diet: The Mediterranean Diet exemplifies a balanced approach to nutrition that significantly impacts glucose metabolism and metabolic health. It emphasizes whole, unprocessed foods like fruits, vegetables, whole grains, nuts, seeds, olive oil, and lean proteins. This diet is rich in polyphenols, antioxidants, and healthy fats, which enhance insulin sensitivity, reduce inflammation, and promote better glucose control. The Mediterranean Diet offers a sustainable strategy for preventing and managing metabolic disorders, contrasting sharply with diets high in processed foods and unhealthy fats.
When it comes to health and longevity, few factors are as influential as glucose metabolism. Glucose metabolism is the process by which our bodies convert the carbohydrates we consume into glucose, a simple sugar that serves as the primary energy source for our cells. This process is crucial for fueling daily activities and maintaining overall well-being. However, disruptions in glucose regulation are alarmingly common and can lead to serious health issues such as diabetes, cardiovascular disease, and nerve damage. Stable blood glucose levels are essential for preventing these chronic conditions, maintaining everyday vitality, and avoiding complications like fatigue and irritability.
This narrative review delves into the intricate relationship between glucose metabolism and exercise, exploring how different exercise modalities target specific metabolic pathways to optimize glucose utilization and overall metabolic health. By examining both aerobic and anaerobic exercise, the review highlights how these activities engage distinct metabolic processes, from glycolysis to oxidative phosphorylation, and their corresponding effects on glucose uptake, insulin sensitivity, and energy expenditure.
The review also investigates the synergistic effects of combining exercise with dietary interventions, focusing on how different macronutrients interact with exercise to influence glucose metabolism. The Mediterranean Diet is presented as a case study, illustrating its potential to enhance metabolic health through its emphasis on whole foods, healthy fats, and antioxidants.
By providing a comprehensive analysis of how various forms of exercise—from endurance training to high-intensity interval training (HIIT) and resistance training—impact glucose metabolism, this review aims to offer practical insights into optimizing metabolic pathways for improved health outcomes. The discussion is tailored to understanding how these exercise modalities, when integrated with a balanced diet, can significantly enhance glucose regulation, prevent metabolic disorders, and promote long-term well-being.
Glucose Metabolism Fundamentals
Glucose is a simple sugar and a primary energy source for the body's cells. It is derived from the carbohydrates we consume and absorbed into the bloodstream, where it travels to cells for energy. Maintaining stable blood glucose levels is essential for overall health and energy balance because it ensures that the body's cells receive a consistent energy supply, preventing hyperglycemia (high blood sugar) and hypoglycemia (low blood sugar). Proper glucose regulation helps to avoid complications such as fatigue, irritability, dizziness, and more severe conditions like diabetes, cardiovascular disease, and nerve damage. Stable glucose levels support optimal physical and cognitive function, promoting overall well-being.
The hormone insulin, produced by the pancreas, influences the regulation of glucose levels. It is crucial in managing your body's glucose levels. Its primary function is to facilitate glucose uptake into cells, where it can be used for energy, particularly in working muscles.
When blood glucose levels rise, such as after a meal, the pancreas releases insulin into the bloodstream. Insulin acts like a key, unlocking cells throughout the body—particularly in the liver, muscle, and fat tissues—allowing glucose to enter these cells. This mechanism is vital for the body's ability to use glucose as an energy source and for storing excess glucose, thereby preventing hyperglycemia, a condition characterized by dangerously high blood sugar levels.
Once inside the cells, glucose undergoes cellular respiration, a complex biochemical process that breaks down glucose molecules to produce ATP (adenosine triphosphate), the energy currency of the cell. ATP powers various cellular activities, enabling cells to perform their functions efficiently. Any glucose not immediately needed for energy is converted into glycogen, a polysaccharide that serves as a storage form of glucose.
Glycogen is primarily stored in two locations: the liver and skeletal muscles. The liver acts as a central storage depot and plays a critical role in maintaining blood glucose levels. Between meals or during periods of fasting, the liver breaks down glycogen and releases glucose back into the bloodstream to ensure a steady supply of energy for the body, particularly the brain, which relies heavily on glucose. Meanwhile, muscle glycogen is reserved for local use and is primarily utilized during physical activity. During exercise, the muscles rapidly break down glycogen to fuel muscle contractions, providing the energy necessary for sustained physical performance.
Conversely, when blood glucose levels drop, such as between meals, during fasting, or after prolonged physical exertion, the body initiates mechanisms to prevent hypoglycemia, a condition characterized by abnormally low blood sugar levels. In response to declining glucose levels, the pancreas reduces the secretion of insulin. Insulin’s primary role is to facilitate glucose uptake into cells, so this decrease signals the body to slow down glucose absorption by cells. By doing so, it helps to conserve the remaining glucose in the bloodstream, ensuring that vital organs, particularly the brain, have access to a continuous supply of glucose, which is critical for their function.
During these low-glucose states, the liver becomes the central player in maintaining blood sugar levels. The liver stores glucose in the form of glycogen, a polysaccharide that can be readily converted back into glucose. When the pancreas senses low blood glucose and reduces insulin output, it also triggers the release of another hormone called glucagon. Glucagon signals the liver to initiate glycogenolysis, a metabolic process where glycogen is broken down into glucose molecules. The liver then releases this glucose into the bloodstream, ensuring that vital organs, especially the brain, which cannot store glucose and relies on a constant supply from the blood, have the energy necessary to function.
This coordinated response not only stabilizes blood glucose levels but also maintains overall energy balance and metabolic health. It highlights the body’s intricate hormonal regulation systems designed to ensure that energy demands are met in various physiological states, such as fasting or physical activity.
The liver can also produce glucose through a process called gluconeogenesis, which is activated when glycogen stores are running low. In this process, non-sugar molecules like glycerol (a type of alcohol) and amino acids (the building blocks of proteins) are chemically modified into glucose. This process is a safety net when your diet is inadequate or you overexert yourself. In these situations, the net amount of carbohydrates received may be scarce, forcing your body to turn to gluconeogenesis as it is desperate for glucose. It begins breaking down proteins from muscles to produce glucose. This can lead to significant muscle loss and wastage over time. [3]
Understanding these principles and processes of glucose metabolism is critical to fully grasp the importance of a balanced diet and appropriate exercise load in maintaining optimal glucose levels, which impact overall health and energy levels.
Glucose Metabolism During Exercise
Our muscles require a steady supply of glucose during intense exercise to function. When glucose enters the muscles, it is metabolized to release energy, crucial for sustaining physical activity. Insulin plays a vital role in this process by facilitating the movement of glucose from the bloodstream into the muscles.
Skeletal muscles, in particular, are significant consumers of glucose during exercise. These muscles help you move and perform activities like walking and lifting objects. Unlike smooth and cardiac muscles, which work involuntarily to regulate the activity of your stomach, blood vessels, and heart, skeletal muscles are under your voluntary control.
In their seminal paper titled "Exercise-stimulated glucose uptake - regulation and implications for glycaemic control," Sylow et al. (2017) explored the intricate mechanisms underlying glucose uptake by skeletal muscles during physical activity. Published in the prestigious journal Nature, their research demonstrated that glucose uptake in skeletal muscles can increase by up to 50-fold during exercise compared to resting conditions. This dramatic enhancement is driven by a series of complex molecular mechanisms that optimize the delivery and transport of glucose across muscle cell membranes, ensuring that muscles have the necessary fuel to sustain prolonged physical exertion [1].
During exercise, the sensitivity of muscle cells to insulin is significantly heightened, leading to an amplified effect of insulin on glucose uptake. Additionally, exercise stimulates the translocation of glucose transporter proteins, particularly GLUT4, to the surface of muscle cells, where they facilitate the entry of glucose into the cells. This ensures that the muscles receive a continuous supply of energy necessary for contraction and endurance.
Richter et al. (2001) provided further insights into the molecular mechanisms governing glucose uptake in muscle cells in their influential paper titled "Glucose, exercise, and insulin: emerging concepts." The study focuses on the critical role of the GLUT4 transporter, which acts like a "delivery truck" for glucose, transporting it from the bloodstream into muscle cells where it is used as a source of energy [2].
During exercise, the body's demand for glucose significantly increases, and this is where GLUT4 plays a pivotal role. In response to physical activity, insulin is released and binds to receptors on muscle cells, initiating a cascade of signaling events that activate GLUT4. Under normal resting conditions, GLUT4 is stored in vesicles within the cell, but upon activation by insulin, it translocates to the cell membrane. Once in the membrane, GLUT4 facilitates the entry of glucose from the blood into the cell, where it is metabolized to produce the energy required for muscle contraction and endurance during exercise [2].
During physical exercise, GLUT4 translocation can occur through an insulin-independent pathway. Exercise stimulates muscle contractions that trigger different signaling pathways, including the activation of AMP-activated protein kinase (AMPK) and calcium signaling. These pathways promote GLUT4 translocation to the cell membrane independent of insulin, allowing glucose uptake to increase significantly during physical activity, even when insulin levels might be low.
This insulin-independent activation of GLUT4 not only ensures that muscles can efficiently use glucose during exercise but also has important implications for insulin sensitivity. Regular physical activity enhances the responsiveness of muscle cells to insulin, making them more efficient at glucose uptake even when at rest. This improved insulin sensitivity is a key benefit of exercise, helping to lower blood glucose levels and reducing the risk of insulin resistance, a condition that can lead to type 2 diabetes. Thus, exercise not only facilitates immediate glucose utilization through insulin-independent pathways but also contributes to long-term metabolic health by improving the body’s ability to respond to insulin.
In summary, glucose uptake during exercise involves the concerted action of insulin and GLUT4 proteins. When you exercise, insulin is released into your bloodstream, facilitating the movement of GLUT4 proteins to the membrane of muscle cells. Glucose then binds to these proteins and is transported into the cells for energy production.
Glucose is crucial in our ability to exercise and use muscles, but this relationship is bidirectional. While glucose fuels our exercise, exercise, in turn, helps us regulate and balance glucose levels. Different types of exercise have varying implications for glucose metabolism, highlighting the complex interplay between physical activity and glucose regulation. In the proceeding sections, we will explore the various types of exercise and their specific impacts on glucose metabolism.
Aerobic Exercise and Glucose Metabolism
Aerobic exercise is a form of physical activity that has a profound impact on glucose metabolism, playing a crucial role in both short-term energy utilization and long-term metabolic health. Activities such as walking, cycling, or swimming—often characterized by prolonged, steady-state exertion—elevate your heart rate and breathing, challenging your cardiovascular and respiratory systems. As you engage in aerobic exercise, your muscles become more efficient at utilizing oxygen, which is essential for producing the energy required to sustain these activities. This increased efficiency in oxygen use not only supports prolonged physical exertion but also enhances overall cardiovascular health and boosts your metabolism.
A key indicator of aerobic fitness is VO2 max, which measures the maximum amount of oxygen your body can utilize during intense exercise. Higher VO2 max levels are associated with a greater capacity for oxygen utilization, which directly impacts glucose metabolism. As your body becomes more adept at using oxygen, it also becomes more efficient at oxidizing glucose, which is a primary fuel source during aerobic activities. This efficiency helps to regulate blood glucose levels and improve insulin sensitivity over time. Consequently, individuals with higher VO2 max levels tend to have better glycaemic control and a reduced risk of developing insulin resistance and type 2 diabetes.
One of the key adaptations to aerobic exercise is an increase in the number and size of mitochondria in muscle cells. Mitochondria are the powerhouses of the cell, where glucose and other substrates are oxidized to produce ATP, the energy currency of the cell. With higher VO2 max levels, your muscles have more mitochondria, which means they can process more glucose through oxidative phosphorylation. This increased capacity allows the body to generate more ATP from glucose, which is particularly important during sustained exercise.
Zone 2 Training and Mitochondrial Health
Training for 60-120 minutes in a comfortable zone 2 training intensity can have significant improvements in mitochondrial health, notably increasing mitochondrial content (i.e., the number or size of mitochondria), and the efficiency of mitochondrial energetics (i.e., greater ability to convert glucose and lipids into fuel).
Utilizing stable isotope tracer methods, early work from Professor Stu Phillips' Lab at McMaster University showed a >150% increase in mitochondrial protein synthesis following 45 minutes at an intensity of 75% VO2 peak [16].
Mitochondrial content & size is also improved following as little as 6 weeks of zone 2 training [17]. A 2018 paper published by the lab of Professor Carsten Lundby from the Zürich Center for Integrative Human Physiology at the University of Zürich in Switzerland showed 4 weekly sessions of 60 minutes in zone 2 increased mitochondrial size by 55% (assessed via high-resolution transmission electron microscopy), with a concurrent 44% increase in citrate synthase activity [17].
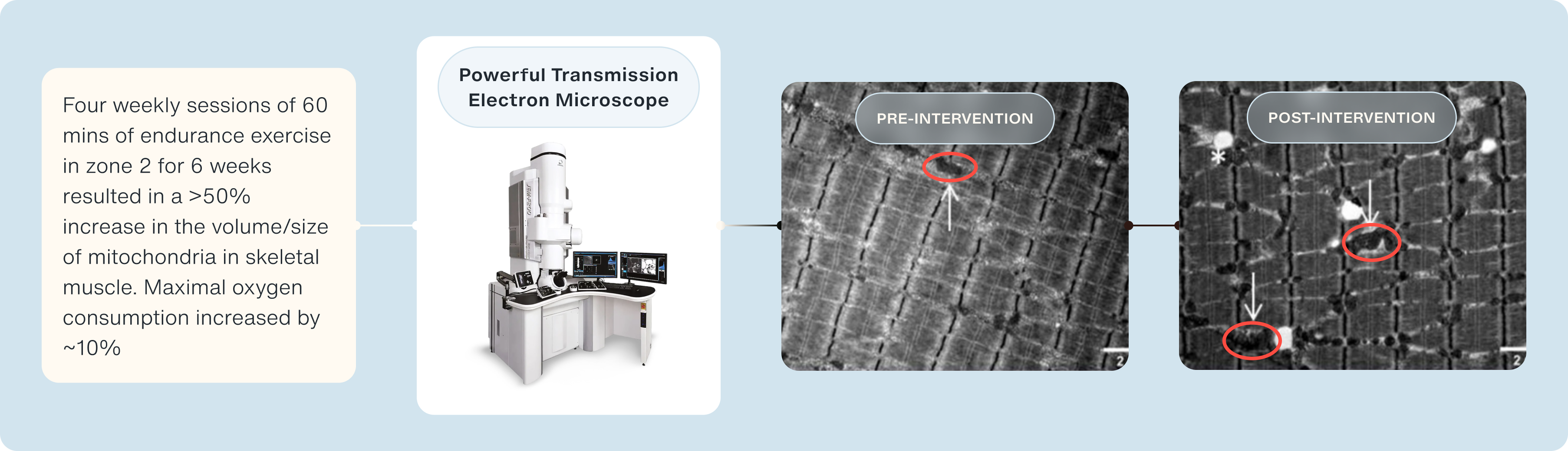
What is interesting is that contrary to previous hypotheses of an increase in mitochondrial biogenesis (i.e., the formation of new mitochondria), is that the surface area and density of mitochondria are improved following endurance exercise [17].
An important caveat when measuring mitochondrial content within skeletal muscle is the method used [18]. Transmission electron microscopy is the 'gold standard' and the best way to determine mitochondrial number, size, density, and location [18]. However, other methods can be used to determine the efficiency of these mitochondria by determining how much oxygen they consume in response to certain substrates.
A common technique is to use high-resolution respirometry. In a 2018 publication in Aging Cell, Dr Adam Konopka, PhD., presented the beneficial effects of aerobic endurance training in older adults, showing it's not too late to start exercising over the age of 60 [19].
Dr Konopka and his group showed 12 weeks of endurance training (3 sessions per week, 45-minute duration) increased VO2 max, which was underpinned by improvements in mitochondrial function [19]. Notably, following the 12 weeks of endurance exercise, skeletal muscle mitochondria developed better fatty acid oxidation and improved ADP sensitivity.
Endurance exercise training also increased the P/E ratio, which dictates an increased OXPHOS capacity (i.e., coupled respiration) and overall improved intrinsic mitochondrial health [19].
Enhanced Capillary Network
Regular aerobic exercise also promotes the growth of new blood vessels, a process known as angiogenesis. A denser capillary network improves oxygen delivery to muscle tissues, ensuring that more oxygen is available for glucose oxidation. One of the characteristics of aging is a reduction in capillary density within tissues, leading to reduced blood flow and, thereby, the delivery of oxygen and nutrients to support cellular and tissue health. With better oxygen supply, muscles can sustain higher rates of aerobic metabolism, allowing them to use glucose more efficiently as a fuel source.
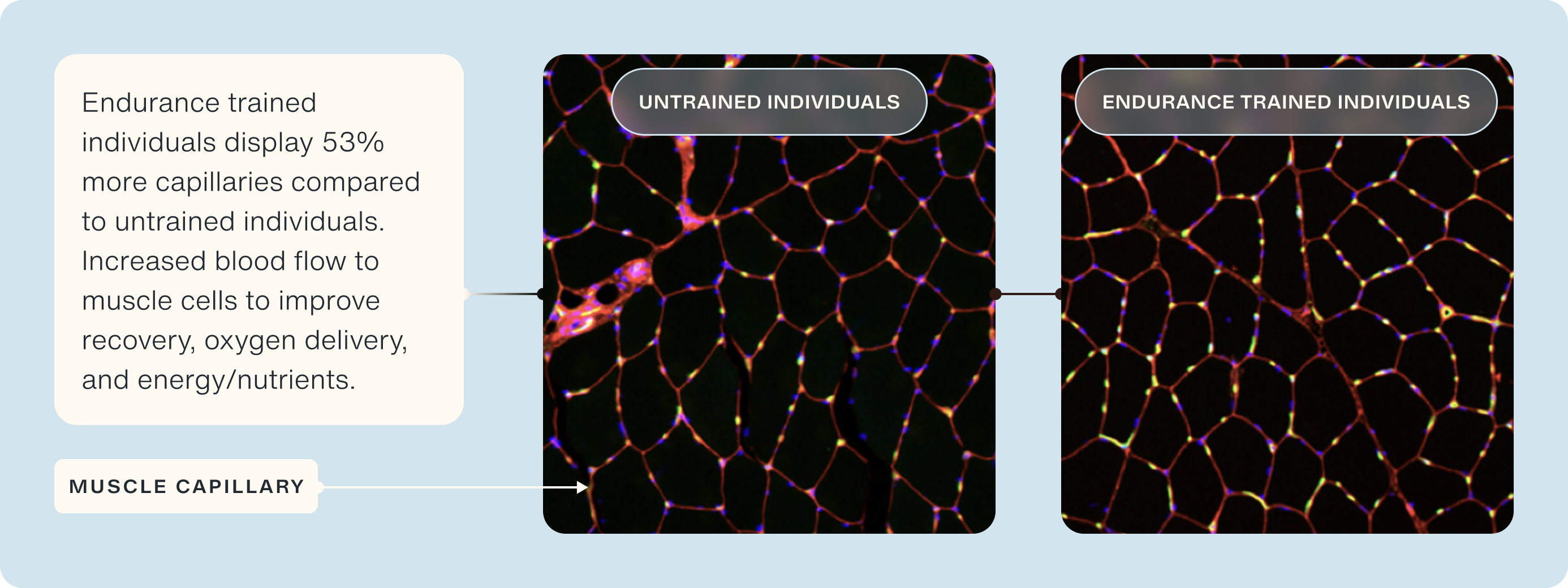
Notably, muscle capillary density is ~24% lower in older adults compared to younger adults [20], which has been correlated to reduced maximal oxygen consumption during exercise [21], post-meal insulin sensitivity [22], and blunted hypertrophic response to exercise [23]. Endurance-trained individuals display superior capillary density compared to age-matched untrained [24]. In particular, they display >50% more capillaries per muscle fiber, which were predominantly around type I muscle fibers (i.e., slow twitch/endurance-type muscle fibers) [24].
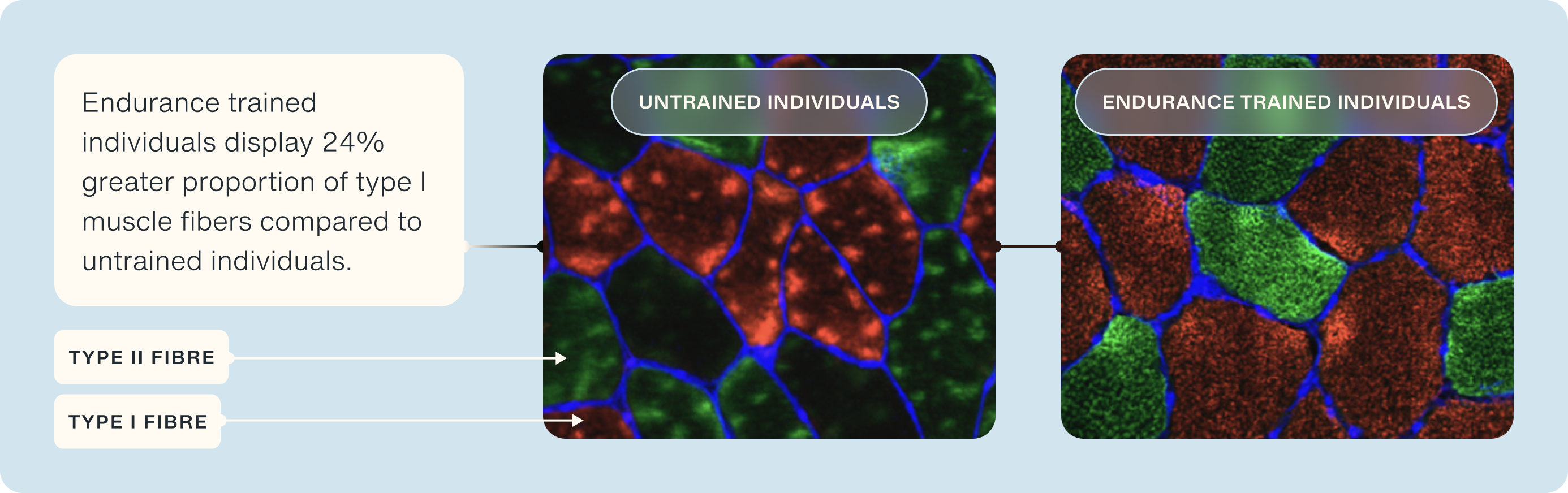
Improved Muscle Fiber Composition
Aerobic training can lead to significant changes in muscle fiber composition, specifically by increasing the proportion of slow-twitch (type I) muscle fibers. These fibers are distinct from fast-twitch (type II) fibers in both their structure and function. Slow-twitch fibers are characterized by their high oxidative capacity, meaning they are exceptionally efficient at using oxygen to produce energy. This efficiency is largely due to their rich mitochondrial content—mitochondria being the organelles where aerobic metabolism occurs [26].
Slow-twitch fibers are designed for endurance and are particularly well-suited for prolonged, steady-state activities such as long-distance running, cycling, or swimming. These activities demand a continuous supply of energy, which is met through the aerobic breakdown of glucose and fats within the mitochondria. The higher mitochondrial density in slow-twitch fibers allows for more effective oxidation of glucose, converting it into ATP, the energy currency of the cell [26]. This process supports sustained muscle contractions over extended periods, making slow-twitch fibers ideal for endurance sports and activities that require long-lasting energy output.
In contrast, fast-twitch (type II) fibers are more geared toward anaerobic metabolism, providing quick bursts of power and speed but fatiguing more rapidly. They rely more on glycolysis, a process that breaks down glucose in the absence of oxygen, leading to the production of lactate [27]. While fast-twitch fibers are crucial for high-intensity, short-duration activities like sprinting or heavy lifting, they are less efficient at using oxygen and glucose over extended periods.
The increase in slow-twitch fiber proportion through aerobic training not only enhances endurance performance but also improves your body’s ability to oxidize glucose during both exercise and rest. This shift in muscle fiber composition is beneficial for overall metabolic health, as it enhances glucose utilization and helps maintain stable blood sugar levels [28]. Additionally, the improved oxidative capacity of these fibers contributes to greater insulin sensitivity, reducing the risk of metabolic conditions such as type 2 diabetes.
Optimized Fat Utilization with Zone 2 Training
As aerobic fitness improves, your body undergoes several adaptations that enhance its ability to utilize fat as a primary fuel source during exercise. This shift toward greater fat oxidation is particularly evident during prolonged, moderate-intensity exercise, where the body relies more on fat and less on glucose as a source of energy. This adaptation occurs due to an increase in the enzymes involved in fat metabolism and an enhanced capacity of the mitochondria to oxidize fatty acids [30].
By becoming more efficient at using fat, the body is able to spare its limited glucose and glycogen stores. Glycogen, the stored form of glucose in muscles and the liver, is a crucial energy reserve, particularly during high-intensity exercise when the demand for quick energy is high. By conserving glycogen through increased fat utilization, the body ensures that glucose is available when it is most needed, such as during short bursts of intense activity or as exercise duration extends [31].
This ability to switch between fuel sources—fat and glucose—enhances overall metabolic flexibility, which is the capacity of the body to adapt fuel oxidation to changes in fuel availability and energy demand. Improved metabolic flexibility is associated with better glucose oxidation and overall metabolic health, reducing the risk of insulin resistance and metabolic disorders [32]. This shift not only supports endurance performance but also plays a critical role in maintaining stable blood sugar levels and optimizing energy utilization during various intensities of exercise [33].
Exercise Induced Increased Insulin Sensitivity
One of the significant benefits of aerobic exercise for glucose control is its ability to improve insulin sensitivity. Regular participation in aerobic activities makes your body better at responding to insulin. After an aerobic workout, you may notice lower blood glucose levels because your muscles become increasingly receptive to insulin. This increased sensitivity allows for more efficient absorption of glucose from the blood into the muscle cells, where it is used for energy. [4]
The mechanism behind this involves both insulin-dependent and insulin-independent pathways. During exercise, muscle contractions stimulate the translocation of GLUT4 transporters to the cell membrane independently of insulin. These transporters facilitate the uptake of glucose into muscle cells, thereby lowering blood glucose levels. Additionally, aerobic exercise increases the number and efficiency of mitochondria in muscle cells, enhancing their capacity to oxidize glucose for energy. Aerobic training also promotes the growth of blood vessels in skeletal muscles, allowing more blood to perfuse through the tissue and helping them absorb more glucose. [6]
Zone 2 exercise is particularly effective in improving glucose metabolism. It enhances the production of GLUT4 in muscle cells, increasing their capacity to take up glucose from the blood. This process allows the body to efficiently use both fat and glucose for energy, ultimately improving metabolic health and insulin sensitivity. [2]
Anaerobic Exercise and Glucose Metabolism
In addition to aerobic exercise, another type of exercise called anaerobic exercise has its unique effects on glucose metabolism. Anaerobic exercise involves short bursts of intense effort, such as weight lifting or sprinting. In Zone 5 exercise, which is typically characterized by very high-intensity efforts (90-100% of maximum heart rate), the primary fuel source is anaerobic energy production, predominantly relying on stored glycogen in the muscles. At this intensity, the body is unable to deliver oxygen quickly enough to meet the energy demands through aerobic metabolism, so it shifts to anaerobic glycolysis. This process breaks down glycogen to produce energy quickly, resulting in the production of lactic acid as a byproduct. This energy system is powerful but limited in duration, usually lasting for a few seconds to a couple of minutes at most, depending on the individual's conditioning.
While anaerobic exercise primarily focuses on short bursts of high-intensity activity, its long-term effects on glucose metabolism are profoundly beneficial. One of the key adaptations resulting from regular anaerobic exercise is an increase in muscle mass. Muscle tissue is metabolically active, meaning that more muscle mass translates to a greater capacity for glucose utilization. This is because skeletal muscle is a primary site for glucose uptake, especially during and after exercise, when muscles are in need of replenishing their energy stores [34].
As muscle mass increases, so does the number of glucose transporters, particularly GLUT4, within muscle cells. These transporters play a crucial role in facilitating the entry of glucose into the cells, where it can be used for energy production or stored as glycogen. Thus, the more muscle mass an individual has, the more glucose their body can handle effectively, reducing the likelihood of excess glucose remaining in the bloodstream and leading to hyperglycemia [35].
In addition to increasing muscle mass, anaerobic exercise significantly improves insulin sensitivity over time. Insulin sensitivity refers to how responsive your cells are to the effects of insulin, the hormone that regulates blood sugar levels. Regular engagement in anaerobic activities, such as resistance training, enhances the efficiency with which the body responds to insulin, making it more effective at lowering blood glucose levels [29]. This improvement in insulin sensitivity reduces the risk of developing insulin resistance, a precursor to type 2 diabetes, and contributes to better overall glucose control.
HIIT Training and Glucose Control
HIIT is an anaerobic exercise involving short bursts of intense exercise alternated with low-intensity recovery periods. This form of training is highly effective for improving glucose control and insulin sensitivity. Studies have shown that HIIT can significantly lower fasting glucose levels and enhance insulin sensitivity in various populations, including those with metabolic syndrome and type 2 diabetes (T2D). [1]
Metabolic syndrome is a cluster of conditions that increase the risk of heart disease, stroke, and T2D. It is characterized by abdominal obesity (excess fat around the waist), high blood pressure, hyperglycemia, and abnormally high cholesterol levels in the blood. Obesity is a crucial factor contributing to metabolic syndrome. HIIT, like aerobic exercise, promotes weight loss, helping to prevent the development of metabolic syndrome and T2D.
One of the significant benefits of HIIT is its ability to improve insulin sensitivity. This improvement occurs by increasing the GLUT4 transporters in muscle cells. When you engage in HIIT, your muscles increase their GLUT4 levels, making them more efficient at absorbing glucose from the bloodstream. [1]
Additionally, HIIT promotes the production of new mitochondria in muscle cells. By increasing the number of mitochondria, HIIT enhances the muscles' capacity to utilize glucose for energy, leading to better glucose control. [1]
Furthermore, the increased mitochondrial pool resulting from HIIT facilitates more efficient glucose uptake and breakdown. This process is crucial because, if not managed, high glucose levels can lead to the storage of glucose as fat tissue (adipose tissue), increasing the risk of obesity and metabolic syndrome. By promoting glucose utilization and preventing its excessive storage as fat, HIIT helps maintain healthy blood glucose levels and reduces the risk of metabolic disorders. It offers a powerful way to improve glucose metabolism and insulin sensitivity. [1]
Another form of anaerobic training is resistance training, which includes activities such as weight lifting, bodyweight exercises, and resistance bands. The mechanisms by which resistance training improves glucose metabolism are similar to those mediated by HIIT. Resistance training increases muscle mass, directly increasing the number of cells available to take up glucose from the bloodstream, providing a greater surface area for glucose absorption. It also stimulates the expression and activity of GLUT4 transporters in muscle cells. [7]
Additionally, resistance training enhances the number of insulin receptors on muscle cells, improving the cells' ability to respond to insulin and further aiding in glucose uptake. Once the muscles take up glucose, it is metabolized in the mitochondria to produce ATP, the cell's energy currency. Resistance training improves mitochondrial function and increases their number, enhancing the muscles' ability to convert glucose into energy. These mechanisms collectively promote glucose uptake by working muscles, where it is broken down by mitochondria into ATP, providing energy for muscular activity and improving overall glucose metabolism. [7]
Metabolic Adaptations to Zone 5 Training
Peter Attia recently recorded a podcast with Dr Iñigo San-Millán, PhD., an Assistant Professor at The University of Colorado's Medical School and the team performance director for the world-leading cycling team UAE Team Emirates. In this podcast, they went into detail regarding zone 2 training and touched upon the importance of zone 5 training and stressing the cardiovascular system once per week.
In this, Peter mentions his protocol of 4x4x4 minutes in zone 5, in which 4 minutes is completed in zone 5, followed by 4 minutes of rest, and repeated four times. As mentioned previously, HIIT has become ever-present in the fitness industry and is hailed as the ultimate training method to get fit, healthy, and burn fat. Although this is somewhat true, completing multiple HIIT sessions per week for a prolonged period of time can have detrimental effects to metabolic health [36].
In a somewhat provocative publication in Cell Metabolism, a team from The Swedish School of Sport and Health Sciences led by PhD graduate Dr. Mikael Flockhart, showed that 4-weeks of HIIT induced a 10% decrease in glycaemic control and a 40% reduction in dysfunction, which resulted in a decline in exercise performance [36].
Interestingly, not all research groups have observed this phenomenon. Research from Professor David Bishop's group at Victoria University, Melbourne, Australia, has observed the opposite and reported improvements in exercise performance & metabolic function [37]. In particular, Professor Bishop's group observed a 50% increase in mitochondrial content and a >40% increase in mitochondrial function [37].
An interesting discrepancy between the studies is the methods of analysis & normalization of the mitochondrial data. Notably, the publication from Mikael utilized isolated mitochondria from skeletal muscle biopsy samples [36], with Professor Bishop's group using permeabilized muscle fibers [37]. Therefore, when interpreting scientific research, it is important to evaluate the physiological relevance of the data and how this may impact your training.
What Are the Benefits of Lifelong Exercise on Metabolic Health?
Studying the effects of lifelong endurance exercise is an incredibly hard topic of research to undertake, primarily due to its niche population [38]. The pool of older adults who have maintained high levels of exercise and still train, race, and compete in competitive events is tiny!
However, a few research groups around the world have published evidence on the benefits of lifelong exercise on cardiovascular [39], musculoskeletal [40], and brain health [41]. Below we will briefly discuss how lifelong exercise can prevent traditional age-related decline and, in some cases, display similar physiological and metabolic characteristics to that of individuals <40 years younger, overall showing the importance of maintaining adequate amounts of endurance exercise across the lifespan.
Research from Professor Scott Trappe's research group from the Human Performance Laboratory at Ball State University has published several high-profile scientific articles characterizing the biological characteristics of highly active lifelong endurance athletes [39], [42–44].
Professor Trappe has consistently observed greater metabolic, cardiovascular, and musculoskeletal health compared to 'normal' older adults and, in some cases, display similar physiological profiles to that of younger individuals.
Notably, as leg muscle mass is a key predictor of sarcopenia, frailty, and quality of life, he showed in his 2020 publication in the Journal of Applied Physiology that individuals who have participated in lifelong exercise display minimal loss of quadriceps, and when compared to 'normal' healthy older adults, an attenuated decline of ~50% is observed [45].
Another key indicator of muscle health is the amount of fat that has infiltrated into skeletal muscle (termed 'intramuscular fat'). In the same study, Professor Trappe's group observed significantly less (~30%) intramuscular fat in lifelong exercisers compared to 'normal' older adults [45].
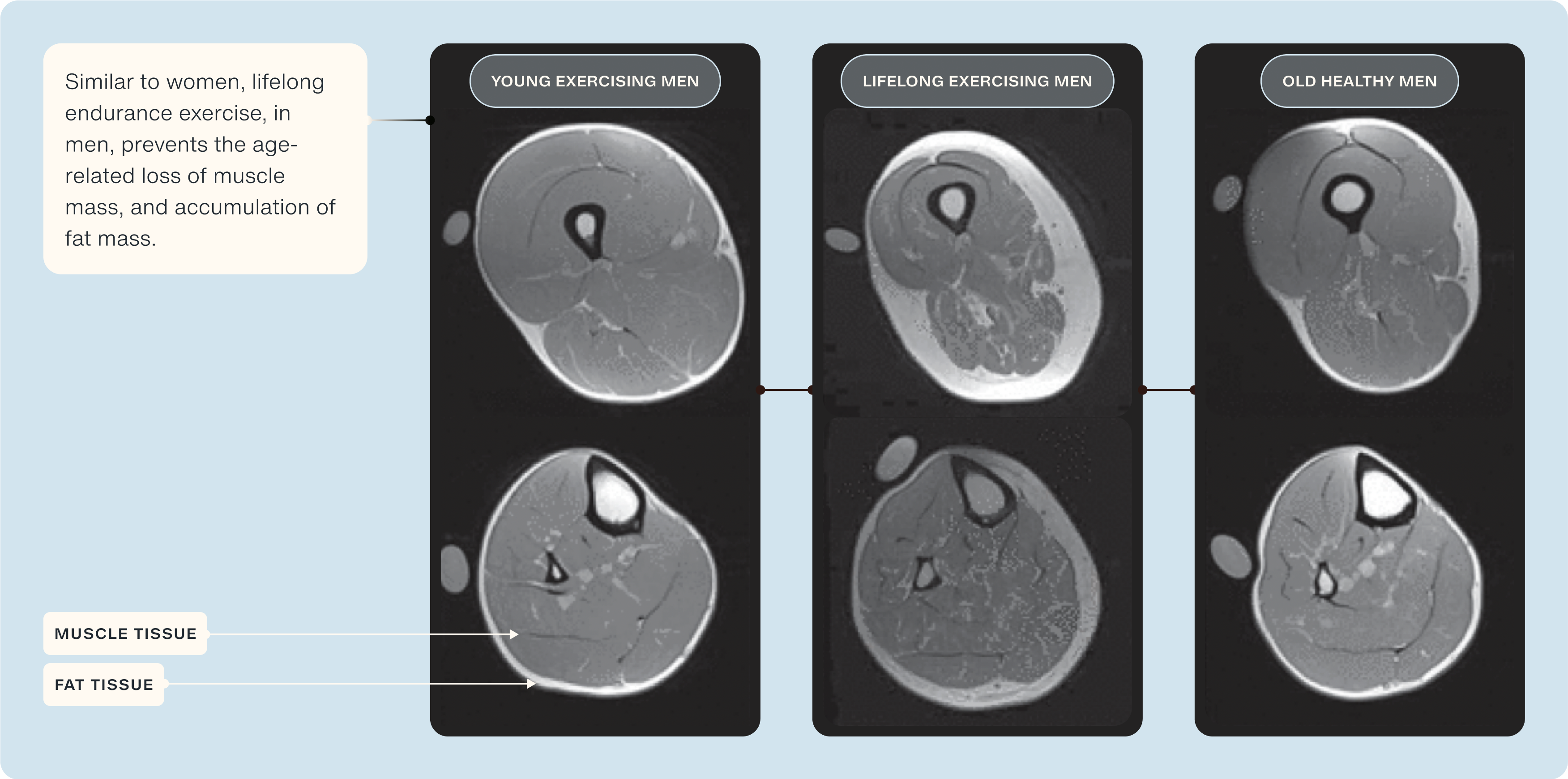
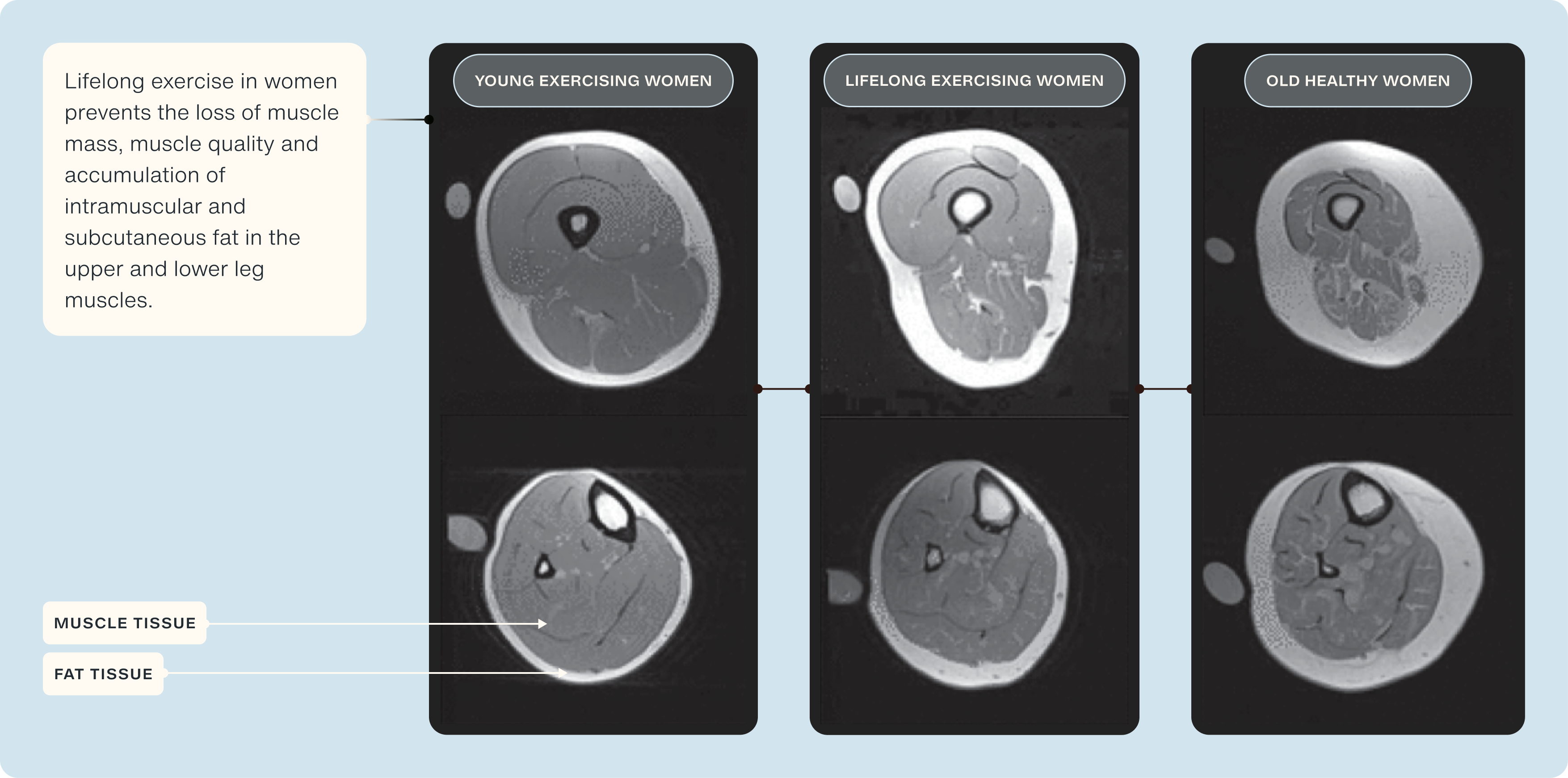
Maintaining lifelong endurance exercise has also been shown to maintain mitochondrial health into later life. Research from the University of Copenhagen's department for cell biology & physiology showed that highly trained 60-70 years old men had >100% more mitochondria, and their function were significantly better than age-matched untrained older adults and even better than younger adults [46].
More sophisticated proteomic investigations of 15 octogenarian world-class track and field MAs and 14 age-matched non-athlete's showed significantly greater VO2 max, as well as muscle strength & mass [47].
The research from Professor Russell Hepple's lab at the University of Florida showed 176 mitochondrial proteins were elevated in the >80 year old master athletes, owing to greater mitochondrial quality control (i.e., OPA1, MFN-2) [27]. As previously mentioned in the article, aging results in a decline in muscle capillary density. Research from Dr. James McKendry showed that individuals that had maintained lifelong endurance exercise had >35% more capillaries per fiber than both younger and older adults [48].
Diet and Glucose Metabolism
Now that we have a solid foundation on the intricate connection between exercise types and glucose metabolism let us look at another significant influence—our diet. Similar to exercise, our diet exerts a substantial effect on the way our body handles glucose. Various macronutrients, such as carbohydrates, fats, proteins, and fiber, affect our body's ability to process, utilize, and regulate glucose.
Carbohydrates
Carbohydrates are essential in influencing glucose metabolism. They are organic molecules that include both simple sugars like glucose and fructose and complex forms such as starch and cellulose. During digestion, carbohydrates break down into simpler sugars that can be absorbed into the bloodstream. This process begins in the mouth, where salivary amylases act on starches, and continues in the small intestine, where simple sugars are absorbed. These sugars, particularly glucose, are then available to produce ATP in the mitochondria. Hence, carbohydrates are the primary source of glucose in our body.
Carbohydrates are usually found in whole grains, fruits, vegetables, legumes, and dairy products. When consumed, they play a critical role in maintaining blood glucose levels, providing the primary fuel for cellular activities. The glucose derived from carbohydrates enters the bloodstream. It is transported to cells throughout the body, where it is either used immediately for energy or stored as glycogen in the liver and muscles for later use. [9]
The presence of adequate carbohydrates in the diet ensures that the body has a steady supply of glucose for ATP production, especially during periods of intense physical activity. During high-intensity or prolonged exercise, your body relies heavily on glycogen for energy. Glycogen stored in muscles and the liver is progressively depleted as you exercise. The depletion rate is influenced by exercise intensity; higher intensities lead to faster glycogen breakdown. This depletion plays a critical role in athletic performance, as running out of glycogen—commonly known as "bonking" in endurance sports—can cause significant fatigue. To delay depletion and prolong endurance, athletes might consume carbohydrates during exercise or engage in training that enhances glycogen storage capacity. [5]
Fats and Proteins
Fats and proteins also play significant roles in glucose metabolism, particularly in modifying the body's glycemic response to carbohydrates. The glycemic response refers to how blood glucose levels change in response to food intake, mainly carbohydrates.
When fats and proteins are consumed alongside carbohydrates, they can influence the glycemic response in complex ways. Fats, for instance, slow gastric emptying—the rate at which food leaves the stomach and enters the small intestine. This delay in digestion reduces the speed at which glucose is absorbed into the bloodstream, resulting in a lower initial spike in blood glucose levels after a meal [49]. However, this effect can be double-edged. Although dietary fat can reduce early postprandial (post-meal) glucose levels, it can also lead to a prolonged elevation in blood glucose levels several hours later. This extended glycemic response is due to the delayed absorption of carbohydrates, which eventually leads to a slower, more sustained release of glucose into the bloodstream [9].
Similarly, proteins have been shown to influence the glycemic response, albeit through different mechanisms. When consumed in significant amounts—typically 75 grams or more—proteins can cause a delayed and prolonged increase in postprandial glucose levels. This is partly due to the process of gluconeogenesis, where the liver converts certain amino acids from protein into glucose, contributing to a gradual rise in blood glucose levels. The combination of carbohydrates with high amounts of protein can, therefore, extend the glycemic response, which is particularly relevant for managing blood sugar levels in individuals with diabetes. [9]
Proteins stimulate the secretion of insulin and glucagon. Insulin helps lower blood glucose levels by facilitating glucose uptake into cells, while glucagon raises blood glucose levels by promoting glycogen breakdown in the liver. The net effect of protein intake on glucose metabolism depends on the balance between these hormones and the amount of protein consumed. Additionally, proteins are crucial for muscle repair and growth, indirectly supporting glucose metabolism by increasing muscle mass and enhancing glucose uptake. [9]
Understanding the roles of fats and proteins in glucose metabolism highlights the importance of a balanced diet. Consuming a combination of macronutrients in appropriate forms and amounts can help maintain stable blood glucose levels and support overall metabolic health. [9]
Fiber:
Fiber is a crucial dietary component, especially for those with metabolic dysregulation, as it helps regulate blood sugar levels and provides numerous other health benefits. Fiber, which includes varieties such as soluble and insoluble, does not contribute to glucose levels because it is not digested by the body.
Soluble fiber, found in foods like oatmeal, beans, and apples, helps manage glucose levels by slowing digestion and extending satiety, which can prevent overeating and help manage weight—a critical factor in controlling T2D. By slowing down the absorption of carbohydrates, soluble fiber prevents rapid spikes in blood glucose levels, contributing to more stable blood sugar control. [10]
Insoluble fiber in whole grains, vegetables, and wheat bran adds bulk to the stool and helps maintain regular bowel movements. While it does not directly influence glucose metabolism, it supports overall digestive health, indirectly benefiting glucose control by promoting a healthy gut microbiome and reducing inflammation.
The American Diabetes Association recommends consuming at least 14 grams of fiber per 1,000 calories. This recommendation underscores the importance of fiber in a balanced diet to manage blood glucose levels. Adequate fiber intake can improve glycemic control, reduce the risk of cardiovascular disease, and support weight management by promoting feelings of fullness and reducing overall calorie intake. [10]
Fiber also enhances insulin sensitivity. By slowing the release of glucose into the bloodstream, fiber helps the body manage blood sugar levels more effectively, reducing the demand for insulin. This can be particularly beneficial for individuals with insulin resistance, a common condition in those with T2D.
Now, let's explore how these macronutrients affect glucose metabolism through a case study of the Mediterranean Diet. We’ve chosen the Mediterranean Diet as a case study because of its significant distinction from the standard American diet, particularly in its emphasis on whole, unprocessed foods and its minimal reliance on processed products. This contrast allows us to better understand the impact of dietary patterns on metabolic health.
Case Study: The Mediterranean Diet
A great example of a dietary regimen that ensures an optimal balance of carbohydrates, proteins, fats, and dietary fiber is the Mediterranean Diet. This diet emphasizes the consumption of whole grains, fruits, vegetables, legumes, nuts, and seeds, providing a rich source of dietary fiber. It also includes healthy fats from olive oil and fish and moderate amounts of protein from lean meats and dairy. The Mediterranean Diet, rich in polyphenols and fibers, significantly impacts metabolic health by reducing risk factors associated with metabolic syndrome. [11]
Key components of the Mediterranean Diet, such as olive oil and red wine, contain polyphenols like resveratrol. Polyphenols are micronutrients with antioxidant properties found abundantly in natural plant food sources. Resveratrol is known for its anti-inflammatory and antioxidant effects, which help protect the body's cells from damage and reduce inflammation. These substances help regulate blood glucose levels by improving insulin sensitivity, meaning the body's cells can more effectively respond to insulin and absorb glucose from the bloodstream. [11]
One of the key ways polyphenols enhance metabolic health is through their impact on mitochondria, the energy powerhouses of the cell. Among the various polyphenols, resveratrol has garnered significant attention for its ability to modulate mitochondrial function. Resveratrol promotes the activity of Uncoupling Protein 1 (UCP1) within the mitochondria, particularly along the electron transport chain (ETC), which is a series of protein complexes responsible for producing ATP, the cell's main energy currency [50].
UCP1 plays a crucial role in thermogenesis, the process of heat production in the body. It acts as a "pressure release valve" by allowing protons to re-enter the mitochondrial matrix without passing through ATP synthase, the enzyme responsible for ATP production. This uncoupling mechanism causes a portion of the energy derived from food to be dissipated as heat rather than being stored as ATP or fat. By promoting this mild uncoupling, resveratrol helps to increase energy expenditure, thereby reducing the likelihood of excess energy being stored as fat [51].
This process not only prevents the buildup of excess fat but also supports mitochondrial health by reducing oxidative stress and enhancing mitochondrial biogenesis, the creation of new mitochondria. Improved mitochondrial function is linked to better metabolic health, as mitochondria are central to energy metabolism and play a key role in the regulation of glucose and lipid metabolism [52]. By enhancing UCP1 activity and promoting mitochondrial efficiency, resveratrol and other polyphenols contribute to improved metabolic flexibility and energy balance, which are critical factors in preventing metabolic disorders such as obesity and type 2 diabetes [11].
Additionally, the Mediterranean Diet's high content of monounsaturated and polyunsaturated fats, primarily from olive oil and fish, aids in improving fat profiles and enhancing overall cardiovascular health. Monounsaturated fats help reduce harmful atherogenic lipoproteins while maintaining good cholesterol levels (HDL), crucial for heart health. Polyunsaturated fats, including omega-3 fatty acids found in fish, have anti-inflammatory properties and further support heart health by reducing triglycerides and lowering blood pressure. [11]
The fiber in the Mediterranean Diet, found in whole grains, fruits, vegetables, legumes, nuts, and seeds, also plays a crucial role. Fiber slows the digestion and absorption of carbohydrates, leading to a more gradual rise in blood glucose levels and preventing sharp spikes. This moderation helps in maintaining stable blood glucose levels and improving overall glycemic control, which is especially beneficial for individuals with diabetes or those at risk.
In the DIRECT Trial titled "Weight Loss with a Low-Carbohydrate, Mediterranean, or Low-Fat Diet," published in the prestigious New England Journal of Medicine, Shai et al. (2008) investigated the effects of three different dietary regimens on weight loss and glucose metabolism in a cohort of obese patients. This comprehensive study, which spanned two years, involved 322 moderately obese individuals with an average age of 52 and a body mass index (BMI) of 31. Participants were randomly assigned to one of three diets: a low-fat, restricted-calorie diet; a Mediterranean, restricted-calorie diet; or a low-carbohydrate, non-restricted-calorie diet. [12]
The Mediterranean Diet group consumed the maximum dietary fiber among the three groups. In contrast, the low-carbohydrate group ate the least carbs and the most fats, proteins, and cholesterol. The average weight loss across the three groups was 2.9 kg for the low-fat group, 4.4 kilograms for the Mediterranean Diet group, and 4.7 kilograms for the low-carb group. While those in the low-carb diet demonstrated the maximum weight loss, they were also at increased risk of ketoacidosis—a severe condition involving elevated acidic content in the blood. Typically, the body strives to maintain an optimal blood pH of around 7.35-7.45. However, in ketoacidosis, levels can drop, leading to symptoms such as extreme thirst, frequent urination, nausea, vomiting, and severe abdominal pain. [12]
For the 36 participants with diabetes, those on the Mediterranean Diet had better blood sugar and insulin levels than those on the other regimens. This superior glucose control in the Mediterranean Diet group can be attributed to several factors:
- High Fiber Content: The Mediterranean Diet is rich in dietary fiber from whole grains, fruits, vegetables, legumes, nuts, and seeds. Fiber slows down the digestion and absorption of carbohydrates, leading to more stable blood glucose levels.
- Healthy Fats: The diet's high content of monounsaturated and polyunsaturated fats, primarily from olive oil and fish, helps improve insulin sensitivity and reduce inflammation, which is crucial for maintaining optimal blood glucose levels.
- Polyphenols and Antioxidants: Polyphenols like resveratrol in olive oil and red wine have anti-inflammatory and antioxidant effects that enhance insulin sensitivity and protect against oxidative stress, promoting better glucose metabolism.
- Moderate Protein Intake: The balanced protein intake from lean meats and dairy provides essential amino acids without overwhelming the body's ability to process them, unlike the high-protein intake in low-carb diets, which can lead to insulin resistance over time.
The Mediterranean Diet exemplifies a balanced approach to nutrition, focusing on whole, unprocessed foods such as fruits, vegetables, whole grains, nuts, seeds, olive oil, and lean proteins like fish. This contrasts sharply with the standard American diet, which is often laden with processed foods, refined sugars, and unhealthy fats, contributing to poor metabolic health.
Not only does the Mediterranean Diet support optimal blood glucose management, but it also promotes safe and sustainable weight loss. The study's findings suggest that while low-carb diets can lead to significant weight loss, they may carry potential health risks if not carefully managed. In contrast, the balanced, nutrient-dense Mediterranean Diet offers a safer, more practical option for long-term health management, providing the benefits of weight control and metabolic health without the associated risks of more restrictive diets.
Synergistic Effects of Combined Diet and Exercise
Now that we have covered the importance of diet and exercise let's explore how their combination ultimately affects how our bodies utilize glucose. Integrating nutrient intake timing with exercise routines can optimize metabolic responses and enhance overall health outcomes.
Consuming protein-rich foods and minimizing saturated fats, combined with well-timed exercise, enhances the secretion of hormones like Glucagon-Like Peptide-1 (GLP-1). GLP-1 regulates glucose metabolism and plays a significant role in managing metabolic syndrome, type 2 diabetes (T2D), and related health problems. GLP-1 stimulates insulin secretion and promotes glucose uptake from the blood into tissues, thereby helping regulate blood sugar levels. When you consume protein-rich meals, GLP-1 levels rise, which enhances insulin secretion and facilitates better glucose control. Exercise also boosts GLP-1 levels, making diet and exercise particularly effective for improving glucose metabolism. [13]
Combining a balanced diet and regular exercise can lead to several synergistic benefits. First, regular exercise improves the body's responsiveness to insulin, allowing cells to take up glucose more effectively. When combined with a diet rich in fiber, healthy fats, and lean proteins, the effect on insulin sensitivity is even more pronounced. Additionally, the timing of carbohydrate intake relative to exercise can influence blood glucose levels. Consuming carbohydrates before exercise can provide the necessary energy for physical activity, while consuming them after exercise can help replenish glycogen stores, helping to maintain stable blood glucose levels. [13]
Moreover, diets that include protein-rich foods and minimize saturated fats can stimulate the secretion of GLP-1. When combined with regular exercise, which also boosts GLP-1 levels, the overall effect is an enhanced regulation of glucose metabolism. This combination also supports weight management by promoting satiety and preventing overeating, as exercise helps burn calories and build muscle, increasing the resting metabolic rate.
Furthermore, both diet and exercise contribute to improved cardiovascular health. Exercise strengthens the heart and improves circulation, while a diet low in saturated fats and high in monounsaturated and polyunsaturated fats helps maintain healthy cholesterol levels, reducing the risk of cardiovascular diseases often associated with poor glucose metabolism. Finally, exercise induces the production of antioxidants and anti-inflammatory molecules. When paired with a diet rich in polyphenols and antioxidants, such as the Mediterranean Diet, the body is better equipped to combat inflammation and oxidative stress, which can impair glucose metabolism. [13]
Several studies underscore the importance of combining dietary interventions with exercise in glucose metabolism. Bianco et al. (2023) examined how a Mediterranean Diet and regular exercise (both aerobic and resistance) could improve the body's glucose handling over one year. Managing glucose levels within an appropriate range is crucial to avoiding metabolic disorders like type 2 diabetes (T2D) and Non-Alcoholic Fatty Liver Disease (NAFLD). In both conditions, glucose levels remain elevated due to insulin resistance. [13]
In the study, 58 individuals aged 18 to 65 with varying levels of NAFLD were tracked for 12 months. Participants followed a lifestyle regimen that included adhering to a Mediterranean diet and regularly engaging in resistance training and aerobic activity. To assess the effects of the regimen, the researchers tracked a key blood sugar marker called HbA1c, which indicates how well blood sugar levels are managed over time. [13]
The study reported a significant decrease in HbA1c levels over the year. HbA1c levels steadily decreased throughout the year for participants with moderate and severe NAFLD. For those with mild NAFLD, improvements began to show after nine months. The study demonstrated that a Mediterranean diet and regular exercise significantly improved blood sugar management, particularly HbA1c levels, in people with NAFLD. This suggests that lifestyle changes can be highly effective in improving glucose metabolism. [13]
This integrated approach to diet and exercise helps manage existing metabolic issues like T2D and NAFLD and plays a preventive role by enhancing overall health and mitigating the progression of these conditions and their related complications. [13]
Considerations:
Achieving an optimal combination of diet and exercise is a challenging feat. Balancing your diet and exercise regimen is crucial but can be complex depending on your circumstances. Key factors to consider include:
Glycemic Index
Understanding foods' glycemic index (GI) can help you maintain stable blood sugar levels and improve overall health. The glycemic index is a ranking system for carbohydrates based on their immediate impact on blood glucose levels, with pure glucose having a GI of 100. Foods with a high glycemic index, such as white bread and pretzels, cause rapid spikes in blood glucose. These quick increases can lead to energy crashes, increased hunger, and potential long-term health issues like insulin resistance. In contrast, low-GI foods like oatmeal and non-starchy vegetables gradually increase blood sugar, promoting sustained energy levels and reducing hunger. To effectively use the glycemic index, incorporate more low-GI foods into your meals to help maintain stable blood sugar levels, support metabolic health, and avoid the pitfalls of high-GI foods.
Continuous Glucose Monitoring
Continuous glucose monitoring (CGM) is a powerful tool for understanding how diet and exercise impact blood glucose levels. A CGM provides accurate, real-time data, allowing you to see how certain foods, activities, sleep, and stress affect your blood sugar. This information is invaluable for making informed lifestyle adjustments to maintain optimal blood glucose levels and improve overall metabolic health.
Using a CGM, you can monitor your blood glucose levels before meals or physical activities and then track how these levels change immediately after, one hour post, and two hours post-activity or meal. This helps you understand the glycemic impact of different foods and the physiological response to various exercises. For example, you might find that certain high-GI foods cause rapid spikes in blood sugar, while low-GI foods provide a more gradual increase, allowing for more sustained energy levels. [14]
Incorporating CGM data into your routine can help you optimize your diet by choosing foods that maintain stable blood sugar levels. Similarly, understanding how different types of exercise affect your glucose levels can guide you in tailoring your physical activity to support better metabolic control. For example, you may find that you need to eat closer to exercise or that you need a snack midway through your workout to maintain energy levels. Alternatively, you might discover that eating dinner earlier or later can help stabilize your blood sugar overnight. These insights gained from CGM can lead to more effective and personalized health strategies, enhancing your diet and exercise routines to support your overall well-being.
Adapting to Individual Needs:
Each individual's response to exercise and dietary changes can vary significantly based on factors like age, gender, diabetes status, type of diabetes (if applicable), and overall health. If you have diabetes, it is essential to tailor your management plan to fit your personal needs and lifestyle. For instance, if you take insulin, you may need to adjust your dosage based on the intensity of your exercise or the carbohydrate content of your meals. Coordinating with a healthcare professional is valuable to ensure that your meal plans and physical activities complement your diabetes management.
Similarly, gender plays a vital role in determining the type of diet and exercise regimen one should follow. Horton et al. (2006) investigated the effects of exercise on glucose metabolism in both men and women. Healthy men and women of average weight who were physically active and had similar fitness levels were selected for the study. Glucose levels were measured while resting (for 120 minutes), during exercise (90 minutes at moderate intensity), and after exercise (for 180 minutes). Before testing, the diet of all participants was matched for three days, and participants did not exercise before the test. [15]
The findings showed that women had lower rates of glucose appearance (how much glucose enters the blood) and disappearance (how much glucose is used by the body) than men. The maximum appearance and disappearance rates for women and men were 22.8 and 23.2 μmol/kg/min and 33.6 and 34.1 μmol/kg/min, respectively. After exercise, women's glucose appearance and disappearance rates were still lower than men's, but the difference diminished over time. The study demonstrated that women have lower glucose levels during and immediately after moderate exercise than men. This difference might be due to lower increases in certain hormones like glucagon and epinephrine during exercise. [15]
Such differences highlight the importance of adjusting diet and exercise to match gender-specific fluctuations in glucose metabolism. For example, women might benefit from consuming a balanced snack with a combination of carbohydrates and protein before moderate exercise to maintain stable blood sugar levels. Additionally, incorporating more frequent but less intense exercise sessions might be more effective for women in managing glucose levels than fewer, high-intensity workouts. These tailored adjustments can help optimize metabolic health and enhance the effectiveness of diet and exercise regimens for women.
Conclusion
Through this exploration, we have navigated the complex relationship between exercise, diet, and their combined effect on glucose metabolism. Like a well-orchestrated symphony, each component—from the intensity and type of physical activity to the quality and timing of nutrients—plays a crucial role in optimizing our body's ability to manage glucose. This equilibrium underscores the significance of a holistic approach to health, focusing on a balanced diet and regular exercise, and highlights the importance of personalized healthcare strategies to align with individual lifestyles and needs. The insights gleaned underscore the message that managing glucose metabolism is within our control through mindful lifestyle choices.
The dialog between diet and exercise offers a powerful testament to their synergistic potential in enhancing metabolic health. Imagine our bodies as a finely-tuned engine, where both fuel (diet) and maintenance (exercise) work hand in hand to ensure peak performance. By adopting practices such as the Mediterranean diet, engaging in aerobic and anaerobic training, adjusting our exercise and meal timing, and utilizing tools such as glycemic indexes and continuous glucose monitoring, we can adapt our practices to achieve optimal metabolic health.
This journey is challenging, yet achieving a balanced glucose metabolism is attainable. Making informed and conscious choices about our diet and exercise routines can significantly impact our overall health and well-being, paving the way for a healthier future.
- Sylow L, Kleinert M, Richter EA, Jensen TE. Exercise-stimulated glucose uptake - regulation and implications for glycaemic control. Nat Rev Endocrinol. 2017 Mar;13(3):133-148. doi: 10.1038/nrendo.2016.162. Epub 2016 Oct 14. PMID: 27739515.. https://doi.org/10.1038/nrendo.2016.162
- Richter, E. A., Derave, W., & Wojtaszewski, J. F. (2001). Glucose, exercise, and insulin: emerging concepts. The Journal of Physiology, 535(Pt 2), 313–322. https://doi.org/10.1111/j.1469-7793.2001.t01-2-00313.x
- The Liver & Blood Sugar. Diabetes Education Online. (2011, June 9). https://dtc.ucsf.edu/types-of-diabetes/type1/understanding-type-1-diabetes/how-the-body-processes-sugar/the-liver-blood-sugar/
- Blood glucose and exercise. Understanding Blood Glucose and Exercise | ADA. (n.d.). https://diabetes.org/health-wellness/fitness/blood-glucose-and-exercise
- Murray, B., & Rosenbloom, C. (2018). Fundamentals of glycogen metabolism for coaches and athletes. Nutrition Reviews, 76(4), 243–259. https://doi.org/10.1093/nutrit/nuy001
- Yaribeygi, H., Atkin, S. L., Simental-Mendía, L. E., & Sahebkar, A. (2019). Molecular mechanisms by which aerobic exercise induces insulin sensitivity. Journal of cellular physiology, 234(8), 12385–12392. https://doi.org/10.1002/jcp.28066
- Sahlin K. (1990). Muscle glucose metabolism during exercise. Annals of Medicine, 22(3), 85–89.
- Yaribeygi, H., Atkin, S. L., Simental-Mendía, L. E., & Sahebkar, A. (2019). Molecular mechanisms by which aerobic exercise induces insulin sensitivity. Journal of cellular physiology, 234(8), 12385–12392. https://doi.org/10.1002/jcp.28066
- Paterson, M., Bell, K. J., O'Connell, S. M., Smart, C. E., Shafat, A., & King, B. (2015). The Role of Dietary Protein and Fat in Glycaemic Control in Type 1 Diabetes: Implications for Intensive Diabetes Management. Current diabetes reports, 15(9), 61. https://doi.org/10.1007/s11892-015-0630-5
- The Liver & Blood Sugar. Diabetes Education Online. (2011, June 9). https://dtc.ucsf.edu/types-of-diabetes/type1/understanding-type-1-diabetes/how-the-body-processes-sugar/the-liver-blood-sugar/
- Kirwan, J. P., Sacks, J., & Nieuwoudt, S. (2017). The essential role of exercise in the management of type 2 diabetes. Cleveland Clinic journal of medicine, 84(7 Suppl 1), S15–S21. https://doi.org/10.3949/ccjm.84.s1.03
- Shai, I., Schwarzfuchs, D., Henkin, Y., Shahar, D. R., Witkow, S., Greenberg, I., Golan, R., Fraser, D., Bolotin, A., Vardi, H., Tangi-Rozental, O., Zuk-Ramot, R., Sarusi, B., Brickner, D., Schwartz, Z., Sheiner, E., Marko, R., Katorza, E., Thiery, J., Fiedler, G. M., … Dietary Intervention Randomized Controlled Trial (DIRECT) Group (2008). Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. The New England journal of medicine, 359(3), 229–241. https://doi.org/10.1056/NEJMoa0708681
- Bianco, A., Franco, I., Curci, R., Bonfiglio, C., Campanella, A., Mirizzi, A., Fucilli, F., Di Giovanni, G., Giampaolo, N., Pesole, P. L., & Osella, A. R. (2023). Diet and Exercise Exert a Differential Effect on Glucose Metabolism Markers According to the Degree of NAFLD Severity. Nutrients, 15(10), 2252. https://doi.org/10.3390/nu15102252
- Mayo Foundation for Medical Education and Research. (2024, January 9). Diabetes and exercise: When to monitor your blood sugar. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/diabetes/in-depth/diabetes-and-exercise/art-20045697
- Horton, T. J., Grunwald, G. K., Lavely, J., & Donahoo, W. T. (2006). Glucose kinetics differ between women and men, during and after exercise. Journal of Applied Physiology (Bethesda, Md.: 1985), 100(6), 1883–1894. https://doi.org/10.1152/japplphysiol.01431.2005
- Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA et al. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. Journal of Physiology 2008; 586: 3701–3717.https://doi.org/10.1113%2Fjphysiol.2008.153916
- Meinild Lundby AK, Jacobs RA, Gehrig S, de Leur J, Hauser M, Bonne TC et al. Exercise training increases skeletal muscle mitochondrial volume density by enlargement of existing mitochondria and not de novo biogenesis. Acta Physiologica 2018; 222: e12905. https://doi.org/10.1111/apha.12905
- Larsen S, Nielsen J, Hansen CN, Nielsen LB, Wibrand F, Stride N et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol 2012; 590: 3349–3360.
- Konopka AR, Laurin JL, Schoenberg HM, Reid JJ, Castor WM, Wolff CA et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell 2019; 18: 12880.
- Verdijk LB, Snijders T, Holloway TM, Van Kranenburg J, Van Loon LJC. Resistance training increases skeletal muscle capillarization in healthy older men. Med Sci Sports Exerc 2016; 48: 2157–2164.
- Hepple RT, Mackinnon SLM, Goodman JM, Thomas SG, Plyley MJ. Resistance and aerobic training in older men: effects on VO2 peak and the capillary supply to skeletal muscle. J Appl Physiol 1997; 82: 1305–1310.
- L Groen BB, Hamer HM, Snijders T, van Kranenburg J, Frijns D, Vink H et al. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol 2014; 116: 998–1005.
- Snijders T, Nederveen JP, Joanisse S, Leenders M, Verdijk LB, van Loon LJC et al. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J Cachexia Sarcopenia Muscle 2017; 8: 267–276.
- Shaw CS, Swinton C, Morales-Scholz MG, McRae N, Erftemeyer T, Aldous A et al. Impact of exercise training status on the fiber type-specific abundance of proteins regulating intramuscular lipid metabolism. J Appl Physiol 2020; 128: 379–389.
- Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273-91. doi: 10.1146/annurev.ph.38.030176.001421. PMID: 130825. https://doi.org/10.1146/annurev.ph.38.030176.001421
- Hawley JA. Adaptations of skeletal muscle to prolonged, intense endurance training. Clin Exp Pharmacol Physiol. 2002 Mar;29(3):218-22. doi: 10.1046/j.1440-1681.2002.03623.x. PMID: 11906487. https://doi.org/10.1046/j.1440-1681.2002.03623.x
- Spriet LL. Anaerobic metabolism in human skeletal muscle during short-term, intense activity. Can J Physiol Pharmacol. 1992 Jan;70(1):157-65. doi: 10.1139/y92-023. PMID: 1581850.
- Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001 Dec;86(12):5755-61. doi: 10.1210/jcem.86.12.8075. PMID: 11739435. https://doi.org/10.1210/jcem.86.12.8075
- Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol (Oxf). 2008 Jan;192(1):127-35. doi: 10.1111/j.1748-1716.2007.01783.x. PMID: 18171435. https://doi.org/10.1111/j.1748-1716.2007.01783.x
- Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol Respir Environ Exerc Physiol. 1984 Apr;56(4):831-8. doi: 10.1152/jappl.1984.56.4.831. PMID: 6373687. https://doi.org/10.1152/jappl.1984.56.4.831
- Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993 Sep;265(3 Pt 1):E380-91. doi: 10.1152/ajpendo.1993.265.3.E380. PMID: 8214047. https://doi.org/10.1152/ajpendo.1993.265.3.e380
- Goodpaster BH, Sparks LM. Metabolic Flexibility in Health and Disease. Cell Metab. 2017 May 2;25(5):1027-1036. doi: 10.1016/j.cmet.2017.04.015. PMID: 28467922; PMCID: PMC5513193. https://doi.org/10.1016/j.cmet.2017.04.015
- Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the "crossover" concept. J Appl Physiol (1985). 1994 Jun;76(6):2253-61. doi: 10.1152/jappl.1994.76.6.2253. PMID: 7928844. https://doi.org/10.1152/jappl.1994.76.6.2253
- Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006 Sep;84(3):475-82. doi: 10.1093/ajcn/84.3.475. PMID: 16960159. https://doi.org/10.1093/ajcn/84.3.475
- Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235-61. doi: 10.1146/annurev.med.49.1.235. PMID: 9509261. https://doi.org/10.1146/annurev.med.49.1.235
- Flockhart M, Nilsson LC, Tais S, Ekblom B, Apró W, Larsen FJ. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab 2021. doi:10.1016/j.cmet.2021.02.017.
- Granata C, Oliveira RSF, Little JP, Renner K, Bishop DJ. Mitochondrial adaptations to high-volume exercise training are rapidlyreversed after a reduction in training volume in human skeletal muscle. doi:10.1096/fj.201500100R.
- Lazarus NR, Harridge SDR. Inherent ageing in humans: the case for studying master athletes. Scand J Med Sci Sports 2007; 17: 461–463.
- Gries KJ, Raue U, Perkins RK, Lavin KM, Overstreet BS, D’acquisto LJ et al. Cardiovascular and skeletal muscle health with lifelong exercise. J Appl Physiol 2018; 125: 1636–1645.
- Wroblewski AP, Amati F, Smiley MA, Goodpaster B, Wright V. Chronic exercise preserves lean muscle mass in masters athletes. Phys Sportsmed 2011; 39: 172–178.
- Tseng BY, Uh J, Rossetti HC, Cullum CM, Diaz-Arrastia RF, Levine BD et al. Masters Athletes Exhibit Larger Regional Brain Volume and Better Cognitive Performance than Sedentary Older Adults. J Magn Reson Imaging 2013; 38: 1169–1176.
- Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation in women. J Appl Physiol 2020; 129: 1493–1504.
- Trappe S, Hayes E, Galpin A, Kaminsky L, Jemiolo B, Fink W et al. New records in aerobic power among octogenarian lifelong endurance athletes. J Appl Physiol 2013; 114: 3–10.
- Gries KJ, Minchev K, Raue U, Grosicki GJ, Begue G, Finch WH et al. Single-muscle fiber contractile properties in lifelong aerobic exercising women. J Appl Physiol 2019; 127: 1710–1719.
- Chambers TL, Burnett TR, Raue U, Lee GA, Finch WH, Graham BM et al. Skeletal muscle size, function, and adiposity with lifelong aerobic exercise. J Appl Physiol 2020; 128: 368–378
- Gudiksen A, Qoqaj A, Ringholm S, Wojtaszewski J, Plomgaard P, Pilegaard H. Ameliorating Effects of Lifelong Physical Activity on Healthy Aging and Mitochondrial Function in Human White Adipose Tissue. J Gerontol A Biol Sci Med Sci 2022; 77: 1101–1111
- Ubaida-Mohien C, Spendiff S, Lyashkov A, Moaddel R, Macmillan NJ, Filion ME et al. Unbiased Proteomics, Histochemistry, and Mitochondrial DNA Copy Number Reveal Better Mitochondrial Health in Muscle of High Functioning Octogenarians. Elife 2022; 11. doi:10.7554/ELIFE.74335.
- McKendry J, Joanisse S, Baig S, Liu B, Parise G, Greig CA et al. Superior Aerobic Capacity and Indices of Skeletal Muscle Morphology in Chronically Trained Master Endurance Athletes Compared With Untrained Older Adults. The Journals of Gerontology: Series A 2020; 75: 1079–1088.
- Collier, G., & O'Dea, K. (1983). The effect of co-ingestion of fat on the glucose, insulin, and gastric inhibitory polypeptide responses to carbohydrate and protein. The American Journal of Clinical Nutrition, 37(6), 941-944. https://doi.org/10.1093/ajcn/37.6.941
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006 Jun;5(6):493-506. doi: 10.1038/nrd2060. Epub 2006 May 26. PMID: 16732220. https://doi.org/10.1038/nrd2060
- Rasbach KA, Schnellmann RG. Isoflavones promote mitochondrial biogenesis. J Pharmacol Exp Ther. 2008 May;325(2):536-43. doi: 10.1124/jpet.107.134882. Epub 2008 Feb 11. PMID: 18267976. https://doi.org/10.1124/jpet.107.134882
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006 Dec 15;127(6):1109-22. doi: 10.1016/j.cell.2006.11.013. Epub 2006 Nov 16. PMID: 17112576. https://doi.org/10.1016/j.cell.2006.11.013