Connecting mTOR and Mitochondria: The Novel Synergy Between Rapamycin and Urolithin A
Introduction
Aging is a complex and multifaceted process marked by the gradual decline of cellular and bodily functions. At its core, aging is driven by a combination of interconnected biological mechanisms.
At its core, aging arises from a network of interconnected biological processes. One prominent factor is chronic, low-grade inflammation, often referred to as “inflammaging,” which exacerbates tissue damage and accelerates the progression of age-associated diseases. In parallel, the accumulation of damaged cellular components—including misfolded proteins, oxidized lipids, and dysfunctional organelles—places an increasing burden on the cell's natural repair and recycling systems. Compounding these challenges is a decline in the efficiency of cellular maintenance pathways, particularly those responsible for clearing damaged cellular components. These disruptions collectively impair cellular function, contributing to systemic dysfunction and the emergence of age-related pathology.
In response to these challenges, researchers have focused on therapies that target the fundamental drivers of aging rather than its symptoms. Two promising interventions have emerged: Rapamycin, a pharmacological agent that modulates the mTOR (mechanistic target of rapamycin) pathway, and Urolithin A, a dietary metabolite that improves mitochondrial health by activating mitophagy. Both compounds have garnered attention for their ability to influence cellular health at a deep, mechanistic level.
Although Rapamycin and Urolithin A act through distinct pathways, they converge on the goal of enhancing cellular quality control. Central to their effects are autophagy and mitophagy, two interconnected processes critical for maintaining cellular integrity. Autophagy serves as a broad recycling system, clearing damaged proteins and organelles to maintain overall cellular health. In contrast, mitophagy, a specialized form of autophagy, specifically targets dysfunctional mitochondria to preserve mitochondrial function. Together, these processes mitigate oxidative stress, sustain energy production, and support overall cellular function. However, the efficiency of both autophagy and mitophagy declines with age, leading to the accumulation of cellular damage and functional decline.
This review explores the complementary roles of Rapamycin and Urolithin A in addressing the cellular underpinnings of aging. By examining findings from clinical and preclinical studies, we highlight their distinct mechanisms of action and potential synergy. These insights suggest that integrating these compounds into therapeutic strategies could improve cellular health, combat age-related decline, and promote healthier aging.
Rapamycin and Autophagy
Known for its ability to target senescent cells, stimulate autophagy, and recalibrate cellular growth, rapamycin has emerged as one of the most intriguing molecules in aging research. To grasp how rapamycin promotes healthspan, it is important to understand its role in targeting the mTOR (mechanistic target of rapamycin) pathway.
mTOR acts as a molecular switchboard, integrating a range of signals—including nutrient availability, growth factors, and cellular energy levels—to dictate whether cells should prioritize growth or shift toward conservation and repair. When nutrients and energy are abundant, mTOR is activated, driving anabolic processes that enable cellular growth. These include protein synthesis (to build new cellular structures), lipid production (for membrane formation and energy storage), and nucleotide biogenesis (essential for DNA and RNA synthesis). mTOR is therefore essential for cellular growth. This activity is particularly vital during early life, supporting the rapid development of tissues and organs and ensuring successful growth and reproduction. Evolutionarily, this role is crucial, ensuring that cells capitalize on plentiful resources to promote growth and reproduction.
However, when nutrient resources are scarce, mTOR activity is downregulated, triggering catabolic pathways such as autophagy. From a logical perspective, when we are in a nutrient deficit we don’t have the energetic capacity to fuel cell growth. Instead, the cell has to breakdown cellular components to use for cellular energy in the absence of a fuel source. Often referred to as the cell's "recycling system," autophagy is the process by which damaged proteins, organelles, and other dysfunctional components are broken down and repurposed. This cleanup process serves dual purposes: it prevents the accumulation of cellular debris that can lead to dysfunction, and it generates substrates to meet energy demands during times of scarcity.
In a healthy state, mTOR must remain highly sensitive to nutrient signals and growth factors to toggle between cell growth and cell clean-up.
As we age, however, mTOR levels become persistently high. The continuous activation of mTOR shifts from being beneficial to counterproductive. Chronic mTOR activity locks cells into a pathological state of unchecked growth and overactivity—a term that the prominent mTOR researcher, Mikhail Blagosklonny, has termed hyperfunctionality. This persistent overactivity leads to cellular dysfunction, damaging the surrounding environment and tissue in which it resides, and contributes to the hallmark features of aging. There are three defining characteristics of cellular hyperfunctionality and chronic mTOR signaling.
Hyperplasia
Hyperplasia refers to an increase in the number of cells within a tissue or organ. While controlled cellular proliferation is essential for growth and repair, excessive and unregulated hyperplasia can become pathological. Senescent cells, which accumulate with age, secrete mitogens—molecules that stimulate cell division in neighboring cells. This can lead to aberrant cellular replication, contributing to conditions such as benign tumors and, in some cases, cancer. The overproduction of cells strains the surrounding tissue, creating structural and functional imbalances.
Hypertrophy
Hypertrophy is the enlargement of individual cells, resulting in an overall increase in tissue or organ size. While adaptive hypertrophy may occur in response to increased functional demands (e.g., muscle hypertrophy from exercise), chronic hypertrophy driven by mTOR overactivation can lead to detrimental outcomes. Enlarged cells may lose their ability to function efficiently, disrupt tissue architecture, and compromise organ performance. Prolonged hypertrophy has been implicated in conditions such as cardiac hypertrophy, where the heart's structure becomes maladaptive and leads to failure.
Hyperfunctionality
Hyperfunctionality describes cellular or organ activity that exceeds normal physiological requirements. Under the influence of excessive hormonal stimulation or dysregulated growth signaling pathways, cells may engage in overproduction of proteins, enzymes, or metabolic byproducts. While this hyperactivity might initially appear advantageous, it often results in metabolic strain, oxidative stress, and cellular damage. Over time, hyperfunctionality leads to dysfunction at the cellular and tissue levels, amplifying the risk of age-related diseases.
We typically think of aging as a loss of cellular function. However, this decline in function is often a downstream consequence of cellular hyperactivity. Excessive growth, proliferation, and metabolic activity impose stress on tissues, creating a cascade of dysfunction that ultimately impairs the system as a whole. Overactive cells consume resources inefficiently, produce harmful byproducts, and disrupt the intricate signaling networks required for a tissue to remain functional. This overactivity and "wear and tear" accelerate the decline of organ systems, underscoring the paradox of aging as a consequence of cellular hyperfunction—overactivity precedes the ultimate loss of function in a tissue.
One of the most striking examples of how hyperfunction contributes to loss of function is seen in heart attacks. While traditionally understood as a failure of the heart's ability to pump blood efficiently, this failure is often driven by the hypertrophy of smooth muscle cells in the heart. These cells enlarge, leading to a thickening of the heart walls—a condition known as pathological cardiac hypertrophy. This hyperfunction of smooth muscle cells disrupts the heart's architecture, impairing its ability to contract and pump blood effectively.
Beyond heart attacks, the hyperfunction, hyperplasia, and hypertrophy of smooth muscle cells are key drivers of other cardiovascular diseases. In atherosclerosis, smooth muscle cells proliferate excessively (hyperplasia) and migrate to arterial walls, contributing to plaque formation. Over time, this narrows the arteries, restricting blood flow and increasing the risk of heart attacks and strokes. Similarly, chronic hypertrophy of smooth muscle cells in the vasculature contributes to hypertension by stiffening arterial walls, further straining the cardiovascular system. These cellular changes illustrate how hyperfunction can culminate in systemic dysfunction, ultimately leading to life-threatening conditions.
Alzheimer's disease and other neurodegenerative disorders also exhibit classic features of cellular hyperfunction. In Alzheimer's, overactivation of the mTOR pathway leads to the excessive production and accumulation of Tau proteins. These proteins aggregate into neurofibrillary tangles, disrupting the structural integrity and function of neurons. As these aggregates build up, they impair synaptic communication, induce inflammation, and ultimately trigger widespread neurodegeneration. This process highlights how the hyperactivity of cellular pathways, while initially functional, becomes pathological over time, contributing to the progressive loss of cognitive and neurological function.
Chronic activation of mTOR has a dual detrimental effect: it drives excessive cellular growth while simultaneously suppressing autophagy. When autophagy is suppressed, it leads to the accumulation of damaged cellular parts, which impairs cellular function and increases inflammation. For instance, reduced autophagy has been strongly implicated in neurodegenerative disorders like Alzheimer’s disease, where the buildup of protein aggregates disrupts neuronal function, as well as in cardiovascular disease and cancer, where cellular waste and damaged structures contribute to tissue dysfunction and uncontrolled growth.
This is why researchers are so intensely focused on mTOR and rapamycin as a geroprotective intervention. Rapamycin serves as a molecular brake, curbing the chronic overactivation of mTOR and restoring its activity to more youthful levels. Simultaneously, it stimulates autophagy, allowing cells to restore their natural cleaning and recycling mechanisms. This stimulation of autophagy is pivotal to understanding rapamycin’s longevity benefits. [2, 3]
These effects are particularly important when it comes to senescent cells. Rapamycin exerts potent anti-inflammatory effects by targeting senescent cells—aged or damaged cells that have ceased dividing but remain metabolically active. These senescent cells secrete a range of pro-inflammatory molecules, collectively termed the senescence-associated secretory phenotype (SASP). SASP includes cytokines, chemokines, growth factors, and proteases, which create a pro-inflammatory microenvironment. Over time, the accumulation of SASP-expressing cells drives chronic, low-grade inflammation. This persistent inflammatory state accelerates tissue damage.
Rapamycin helps to mitigate inflammaging by reducing the activity of the mTOR pathway, which is closely linked to SASP production. SASP production is an example of a cellular hyperfunction—the overproduction of inflammatory factors. By curbing mTOR signaling, rapamycin limits the secretion of pro-inflammatory factors from senescent cells, thereby reducing their systemic impact. This ability to suppress SASP is not just a localized effect; it has far-reaching consequences for whole-body inflammation. Studies have shown that rapamycin can decrease levels of circulating inflammatory markers, highlighting its potential to address one of the key hallmarks of aging: chronic inflammation. [4, 5]
The reduction of SASP-driven inflammation is central to rapamycin’s longevity-enhancing effects. Chronic inflammation not only accelerates tissue degradation but also disrupts repair mechanisms, exacerbating tissue-wide functional decline. For example, in cardiovascular systems, rapamycin’s anti-inflammatory properties may help reduce arterial stiffness and prevent plaque instability, while in neurodegenerative conditions, they may protect neurons from inflammatory damage. This dual action—targeting both senescent cells and their inflammatory secretions—positions rapamycin as a cornerstone therapeutic for age-related inflammatory diseases. [4, 5]
While rapamycin's ability to stimulate autophagy and reduce inflammation underscores its broad therapeutic potential, its role in mitochondrial-specific autophagy—mitophagy—deserves closer examination. Mitochondria are central to cellular health, serving as the primary source of energy production and playing a key role in regulating metabolic and signaling pathways. However, as cells age, mitochondria accumulate damage, leading to reduced energy efficiency and the production of harmful reactive oxygen species (ROS). This decline in mitochondrial quality is a hallmark of aging and a driver of chronic diseases.
Given rapamycin’s well-established role in inducing autophagy through mTOR inhibition, one might expect it to have a robust effect on mitophagy as well. Interestingly, the evidence for rapamycin’s impact on mitophagy is less conclusive.
Rapamycin and Mitophagy: Mechanisms of Mitochondrial Quality Control in Aging and Disease
Rapamycin is well-established as a potent inducer of autophagy through its suppression of mTOR activity. Given this, it stands to reason that rapamycin might also significantly impact mitochondrial-specific autophagy, or mitophagy. However, evidence on rapamycin’s direct effects on mitophagy has been surprisingly limited. A recent 2022 study published in Cell Metabolism by Dr. Tom McWilliams offers critical insights into this relationship, particularly in the context of mitochondrial disease. This work utilized reporter models developed during Dr. McWilliams’ PhD research to investigate rapamycin’s role in mitophagy [6].
Reporter models are experimental tools that allow researchers to track and visualize specific cellular processes in real time. In the context of mitophagy, these models typically involve the use of fluorescent markers or genetically encoded biosensors that label mitochondria and their degradation pathways. For example, dual-fluorescent systems like MitoKeima and MitoQC are designed to distinguish between healthy mitochondria and those undergoing degradation. MitoKeima uses a pH-sensitive fluorescent protein targeted to mitochondria, which shifts its emission spectrum when mitochondria are sequestered in acidic lysosomes, indicating active mitophagy. Similarly, MitoQC employs a tandem fluorescent protein tag to differentiate mitochondria destined for autophagic degradation. These tools provide a dynamic and quantifiable means to study mitophagy under various experimental conditions.
Using such reporter models, the study examined the effects of rapamycin in a mitochondrial disease model that shares notable parallels with aging. The mitochondrial disease model used in the study shares notable parallels with aging, particularly in older adults (octogenarians and beyond), who frequently exhibit severe mitochondrial abnormalities [7]. In the disease model, key features include defects in the mitochondrial respiratory chain, accumulation of damaged mitochondria, and the presence of “ragged-red fibers” containing pathogenic mitochondrial DNA variants. These hallmarks closely mirror the mitochondrial dysfunction observed in aging [6].
The study revealed that administering rapamycin at a dose of 8 mg/kg/day over 70 days resulted in a 125% increase in mitophagy [6]. The researchers hypothesized that both mitochondrial disease and aging involve hyperactivation of autophagy-mediated degradation pathways. This is evidenced by the accumulation of P62 and phosphorylated ubiquitin in muscle fibers, markers indicative of impaired mitophagic clearance. In mitochondrial disease patient samples, levels of the autophagy adapter protein P62 were found to be more than 80-fold higher compared to healthy controls. Remarkably, rapamycin treatment reduced this hyper-autophagic state by approximately 90%, effectively restoring P62 levels to near-normal [6].
Further analysis in the supplementary data of the publication suggested that one of rapamycin’s mechanisms of action in enhancing mitophagy involves the downregulation of phosphorylated S6, a downstream marker of mTOR activity [6]. This finding highlights rapamycin’s ability to modulate mTOR signaling in a way that promotes mitochondrial quality control.
The study on rapamycin’s effects on mitophagy underscores the critical role of mitochondrial quality control in aging and disease. While rapamycin’s influence on mitophagy appears to be secondary to its broader autophagic activity, the importance of mitochondrial-specific autophagy cannot be overstated. Mitochondria are at the heart of cellular energy production and metabolic regulation, but their proper function is contingent upon their quality being rigorously maintained. This brings us to mitophagy, a specialized form of autophagy that directly addresses the unique challenges of mitochondrial health.
Mitophagy is a cornerstone mechanism for sustaining cellular function, particularly in aging tissues where mitochondrial dysfunction becomes a pervasive issue. To fully appreciate the significance of mitophagy, it’s essential to understand its role in maintaining mitochondrial efficiency, preventing oxidative stress, and supporting overall cellular health.
Mitophagy as a Mitochondrial Quality Mechanism
For good reason, we have focused extensively on general autophagy as a key mechanism for combating aging. When autophagy is impaired, a cascade of effects leads to the deterioration of tissue function and the onset of age-related chronic diseases. Just as important, but less highlighted, is mitochondrial-specific autophagy, or mitophagy. Mitophagy is a targeted form of autophagy that identifies and removes damaged, dysfunctional, or inefficient mitochondria.
We focus on mitochondrial health as a promoter of healthspan because compromised mitochondrial function affects nearly every hallmark of aging.
Mitochondria, often referred to as the "powerhouses" of the cell, are critical for cellular energy production. They convert nutrients into adenosine triphosphate (ATP), the molecule that powers nearly all biological functions, from muscle contractions to nerve impulses. However, like any machinery, mitochondria are susceptible to wear and tear. Stressors such as toxins, oxidative damage, and the natural demands of metabolism impair their function over time.
Mitophagy can be understood by likening mitochondria to the engines of a car. When operating efficiently, these engines burn fuel cleanly to produce energy. However, when they malfunction—whether due to poor maintenance or faulty components—they lose efficiency in producing energy (ATP) and emit more "exhaust fumes." These "fumes" represent reactive oxygen species (ROS), highly reactive molecules generated as byproducts of mitochondrial activity. A malfunctioning metabolic engine causes myriad harmful systemic effects.
While ROS at normal levels play important roles in cell signaling and immune responses, excessive "exhaust" becomes corrosive to the cellular environment. This overproduction leads to oxidative stress, a condition in which the balance between ROS and the body's ability to neutralize them is disrupted. Over time, this cumulative damage accelerates aging and contributes to various diseases, including neurodegenerative disorders like Alzheimer's and Parkinson's disease, cardiovascular ailments, and certain cancers.
This provides a framework for evaluating compounds that improve mitochondrial health. Specifically, such compounds should demonstrate the ability to increase ATP production while simultaneously reducing oxidative stress. Enhanced mitochondrial efficiency translates to improved energy output and minimizes the harmful byproducts that contribute to oxidative damage. This is what we mean by a healthy metabolic engine.
By identifying and selectively removing defective mitochondria, mitophagy preserves mitochondrial efficiency and reduces the production of excessive ROS. This process ensures that only healthy mitochondria remain, thereby supporting sustained energy production and minimizing the risks of oxidative damage.
Mitophagy and Aging
As you can imagine, mitophagy is crucial in tissues with high energy demands, such as the heart, brain, and muscles. In these tissues, cells depend on a constant supply of ATP to function correctly. If damaged mitochondria are not removed, they fail to produce sufficient energy and contribute to increased ROS levels, leading to the corrosion of the cell itself and ultimately tissue damage. [8]
Adding to the vulnerability, mitochondria contain their own DNA (mtDNA), which is separate from the cell’s nuclear DNA. Unlike nuclear DNA, mtDNA is not as well-protected and is more susceptible to damage from oxidative stress. When ROS accumulate, they can harm mtDNA, leading to mutations that impair mitochondrial function. Damaged mtDNA not only reduces ATP production but also impairs the mitochondria’s own mechanisms for self-repair and replication, creating a vicious cycle of dysfunction and mitochondrial decline.
Over time, this mtDNA damage can contribute to aging and the onset of age-related diseases, including neurodegenerative conditions like Alzheimer's and Parkinson’s, as well as cardiovascular diseases where the energy needs of tissues are high and must remain stable. [8]
At the same time, as we age, the efficiency of mitophagy declines. Cells become less adept at recognizing and removing dysfunctional mitochondria, allowing these damaged mitochondria to accumulate within tissues over time. Returning to the metabolic engine analogy, our cellular engines gradually become highly inefficient in generating energy. Just as a poorly maintained engine produces more exhaust, these malfunctioning mitochondria emit excessive "metabolic exhaust" in the form of ROS.
This excess ROS leads to chronic oxidative stress that damages critical cellular structures. Over time, this oxidative damage contributes to a progressive decline in cellular function and is implicated in a variety of age-related diseases, such as neurodegenerative disorders like Parkinson's and Alzheimer's, as well as cardiovascular conditions that depend on optimal energy metabolism. [8]
In neurodegenerative diseases, impaired mitophagy is closely linked to neuronal death. Neurons, unlike many other cell types, are largely non-replicative, meaning they cannot be readily replaced once lost. This makes them especially vulnerable to accumulated damage.
In Parkinson’s disease, for example, the PINK1/Parkin pathway—a key mechanism in mitophagy—frequently becomes defective. Mutations in the genes for PINK1 or Parkin disrupt the cell’s ability to clear damaged mitochondria, leading to a buildup of dysfunctional mitochondria that contribute to oxidative stress and, ultimately, the death of dopamine-producing neurons. This neuronal loss is what drives the hallmark motor symptoms of Parkinson’s disease, such as tremors, rigidity, and bradykinesia (slowness of movement). [8]
Can We Manipulate Mitophagy?
Emerging evidence suggests that enhancing mitophagy can positively impact longevity and lifespan. Several studies involving genetic mutation, dietary manipulations, or pharmacological interventions have demonstrated that promoting mitophagy extends the lifespan in various model organisms, including yeast [9], worms [10], flies, and mice [11].
Enhancing mitophagy through pharmacological interventions or lifestyle modifications holds promise for mitigating age-related diseases and promoting healthy aging. Here we will briefly discuss how geroprotective pharmacological interventions can improve mitophagic regulation, improve mitochondrial quality control as we age, and prevent the accumulation of damaged mitochondria.
Evaluating Urolithin A
Given the central role of mitophagy in aging and disease, researchers are exploring ways to enhance this process to support healthy aging. One promising compound is Urolithin A, a naturally occurring metabolite derived from ellagitannins—polyphenolic compounds found in foods such as pomegranates, berries, and nuts. After consumption, ellagitannins are metabolized by specific gut bacteria into ellagic acid, which is further converted into Urolithin A. However, the production of Urolithin A is highly dependent on an individual’s gut microbiota composition, making dietary habits and gut health critical factors in determining its availability and efficacy [12, 13].
The Challenge of Urolithin A Synthesis
The natural synthesis of Urolithin A is remarkably variable and occurs in only about 40% of the human population [14]. This variability arises from differences in gut microbiota composition, as specific bacterial species are required to convert dietary ellagitannins into active Urolithin A. Two key bacterial species—Gordonibacter urolithinfaciens and Ellagibacter isourolithinifaciens—play pivotal roles in this conversion process. Without these microbes, individuals are unable to produce sufficient levels of Urolithin A, limiting its potential benefits.
Given the variability in Urolithin A synthesis and the reliance on specific gut bacteria, supplementation has become a practical solution for elevating Urolithin A levels in the body [15]. By bypassing the need for microbial conversion, supplementation ensures consistent bioavailability, regardless of individual microbiome composition. This approach is particularly important for those who are "non-responders" due to the absence of the necessary gut bacteria, enabling a broader population to benefit from Urolithin A's mitophagy-enhancing effects.
Initial evidence supporting Urolithin A’s potential emerged in 2016 from research conducted in Professor Johan Auwerx’s lab. Their findings demonstrated that Urolithin A supplementation increased lifespan in C. elegans by an impressive 45%, primarily due to its effects on mitochondrial function [16]. Mechanistically, these benefits were attributed to Urolithin A’s ability to stimulate mitophagy, selectively removing defective mitochondria and preserving mitochondrial efficiency.
To explore this mechanism, researchers employed a sensitive reporter model of mitophagy called MitoKeima—a tool similar to the previously established MitoQC model. In this model, a 24-hour exposure of muscle cells to Urolithin A resulted in a ~104% increase in mitophagy compared to untreated controls [16]. This increase underscores Urolithin A’s capacity to promote mitochondrial-specific autophagy in cellular models, ensuring the selective degradation of dysfunctional mitochondria while allowing healthy mitochondria to thrive.
Beyond cellular models, Urolithin A has demonstrated promising effects in animal and human studies. In mice, middle-aged adults [17], and older adults [18], supplementation with Urolithin A was shown to increase the gene and protein expression of key markers associated with mitophagy. These findings highlight Urolithin A’s ability to support mitochondrial quality control across diverse biological systems and age groups.
Mechanistically, how does Urolithin A work?
Urolithin A exerts its effects on mitochondrial quality control at least partially by activating the PINK1/Parkin pathway, a critical signaling cascade involved in mitophagy. When mitochondria become dysfunctional, they lose their membrane potential, which functions much like a battery losing its charge. Just as a charged battery powers a device, a healthy mitochondrion depends on its membrane potential—a voltage difference across its membrane—to drive the production of ATP, the cell's energy currency.
When a mitochondrion’s "charge" diminishes, it can no longer produce energy efficiently, signaling to the cell that it is no longer functional. This loss of potential triggers the accumulation of PINK1 (PTEN-induced kinase 1) on the outer mitochondrial membrane. Acting like a quality control inspector, PINK1 recruits Parkin, an E3 ubiquitin ligase, which tags the damaged mitochondrion with ubiquitin molecules—essentially marking it for recycling. These ubiquitin "tags" instruct the cell to send the defective mitochondrion to the lysosome for degradation, ensuring it is replaced with a fully "charged" and functional mitochondrion. This highly selective process preserves mitochondrial efficiency and minimizes the accumulation of harmful byproducts like reactive oxygen species (ROS) [13, 19].
A growing body of evidence connects Urolithin A’s ability to stimulate mitophagy with potential therapeutic benefits. A study by Luan et al. (2021) explored Urolithin A as a potential treatment for Duchenne muscular dystrophy (DMD), a severe genetic disorder that primarily affects boys. DMD is characterized by progressive muscle weakness and degeneration caused by mutations in the dystrophin gene, which is essential for maintaining muscle cell structure and resilience. Dystrophin acts like a structural anchor, linking the muscle cell membrane to the extracellular matrix and enabling muscle cells to withstand repeated contractions. Without dystrophin, muscle cells become fragile, gradually weakening and deteriorating over time.
One of the critical challenges in DMD is mitochondrial dysfunction, which significantly accelerates disease progression. Mitochondria in DMD-affected muscle cells exhibit reduced energy production, impaired functionality, and heightened oxidative stress—akin to engines producing less power while emitting excessive exhaust. Compounding this issue, research has shown that genes involved in mitophagy are often underexpressed in DMD, further limiting the muscle cells’ ability to clear out damaged mitochondria effectively. Studies in DMD mouse models consistently reveal lower levels of mitophagy markers, highlighting a profound impairment in the mitochondrial recycling process in this disease context. [20]
The researchers in this study explored whether Urolithin A could activate the mitophagy process to address this issue. They tested the effects of Urolithin A on both mice and human muscle cells. In the mice, Urolithin A was added to their food at a dose of 50 mg per kg per day. In human trials, muscle cells were obtained from three healthy boys and three boys with DMD, all aged 4 to 7 years. These cells were treated with 25 μM of Urolithin A for varying durations (2, 6, and 24 hours). The study found that Urolithin A enhanced the clearance of defective mitochondria in both the mice and the DMD-affected human muscle cells. This improvement led to better mitochondrial function and increased the muscle's ability to use oxygen, suggesting that Urolithin A may reduce symptoms of DMD by promoting mitophagy. [20]
A recent study by Huang et al. (2023), titled Urolithin A Ameliorates Obesity-Induced Metabolic Cardiomyopathy in Mice via Mitophagy Activation, published in the prestigious journal Nature, explored Urolithin A’s role in managing metabolic cardiomyopathy (MC), a condition often associated with obesity. MC is characterized by the buildup of fat within heart cells, which disrupts normal cardiac function and leads to oxidative stress and mitochondrial dysfunction. This condition is particularly concerning because it impairs the heart's energy production, weakens contractility, and lacks effective treatment options.
In cases of obesity, cardiomyocytes (heart muscle cells) exhibit distinct mitochondrial abnormalities. Mitochondria in these cells often show impaired respiration, mislocalization within the cell, and disorganized mitophagy—a process critical for mitochondrial quality control. Mitochondria undergo constant dynamic cycles, including fission (division), fusion (joining), biogenesis (creation of new mitochondria), and mitophagy (selective degradation). Among these processes, mitophagy plays a key role in maintaining mitochondrial homeostasis by removing damaged or aged mitochondria, thus preventing the accumulation of dysfunctional organelles that could compromise cellular health.
In healthy cells, mitophagy is facilitated by pathways like the PINK1/Parkin or receptor-dependent pathways, both of which target damaged mitochondria for degradation and recycling. However, in the cardiomyocytes of MC mice, researchers observed enlarged mitochondria with collapsed membrane potentials, indicating severe dysfunction. These mitochondria, which are unable to maintain the necessary energy gradient, were not being cleared effectively, suggesting that impaired mitophagy was contributing to the condition. This buildup of damaged mitochondria likely exacerbates oxidative stress and further weakens mitochondrial function. [21]
In this study, the researchers sought to determine if poor mitophagy is a driving factor in MC and whether Urolithin A could mitigate these effects. Using an obesity-induced MC model in mice, they fed the mice a high-fat diet for 20 weeks to induce obesity and MC symptoms, followed by a 4-week Urolithin A treatment at a dosage of 50 mg per kg per day. The aim was to assess whether Urolithin A could restore mitophagy, thereby reducing mitochondrial dysfunction and improving cardiac health. [21]
The researchers found a strong link between obesity and reduced mitophagy in heart cells. In the Urolithin A-treated mice, mitophagy activity significantly improved, and symptoms of MC were reduced. When heart cells were exposed to palmitic acid (a fat), 5 μM of Urolithin A increased the production of autophagosomes (which engulf damaged mitochondria) while reducing autolysosomes (which break down autophagosomes). Urolithin A helped balance these processes, allowing better mitochondrial cleaning. Moreover, when mitophagy blockers or gene silencing techniques were used to inhibit Parkin (a key protein in mitophagy), the protective effects of Urolithin A were reduced, further proving that Urolithin A's benefits were tied to restoring mitophagy. [21]
While Rapamycin broadly stimulates autophagy, clearing a wide spectrum of cellular waste, Urolithin A is uniquely specialized in mitochondrial quality control. This specificity allows Urolithin A to address a key limitation in Rapamycin’s mechanism of action: the direct regulation of mitophagy. Although Rapamycin contributes indirectly to mitochondrial maintenance as part of its overarching autophagic effects, it lacks the precision and efficiency with which Urolithin A enhances mitophagy.
This distinction is particularly important because dysfunctional mitochondria play a central role in aging and age-related diseases. Urolithin A’s targeted action ensures that defective mitochondria are selectively removed and replaced, directly tackling one of the most significant contributors to cellular decline.
Synergistic Potential: Rapamycin and Urolithin A
When combined, Rapamycin and Urolithin A offer a potential synergistic approach to combating cellular aging. Their complementary mechanisms target fundamental processes underlying the decline in overall cellular function and mitochondrial specific dysfunction, creating a more comprehensive intervention than either compound alone.
- Rapamycin reactivates autophagy, the cell’s general quality-control and recycling system, clearing damaged cellular components.
- Urolithin A enhances mitophagy, optimizing mitochondrial quality and energy production, the core of cellular metabolism.
This dual action addresses aging on multiple levels: Rapamycin promotes systemic maintenance of cellular integrity, while Urolithin A safeguards and enhances the mitochondria, the cell’s energy generators. Together, they tackle the interconnected challenges of aging, from clearing senescent cells and protein aggregates to ensuring the integrity of the cell’s energy supply.
In the following sections, we will explore the implications of this dual approach and its potential to extend healthspan by addressing cellular aging from multiple angles. [22, 23, 24]
Mitigating Inflammation and Oxidative Stress
Chronic inflammation and oxidative stress are fundamental drivers of aging and the progression of age-related diseases. Imagine you have two muscle biopsy samples. You are told one biopsy is from an older person, and the other is from a younger person. Your assignment is to identify which sample belongs to the younger person and which to the older. How would you determine the difference?
It turns out there is a simple way to answer the question: look for inflammation. When you examine an older person's cell sample under a microscope, you would see much more inflammation—healthy cells interspersed with dysfunctional ones, bathing in a witch's brew of pro-inflammatory molecules. Inflammation is one of the most discernible hallmarks of aging. Scientists have even coined a term for this chronic, low-grade inflammation that develops with advanced age: inflamm-aging.
As we grow older, we lose the phenotype of the young person's biopsy—smooth, less inflamed tissue—and enter an inflammatory state. This shift is accompanied by a transition from functional to dysfunctional tissue, which, upon closer inspection, can be viewed as a hyper-functional state.
A key contributor to systemic inflammation is the accumulation of senescent cells in aging tissues. These damaged, non-dividing cells secrete a variety of pro-inflammatory cytokines and other molecules collectively known as the senescence-associated secretory phenotype (SASP). SASP includes inflammatory mediators such as IL-6, IL-1β, and TNF-α, which perpetuate chronic, low-grade inflammation [25].
Senescent cells become particularly harmful in the presence of pro-growth signals, such as elevated mTOR activity. Instead of dividing, senescent cells become hypertrophic (grow excessively without being able to divide), hyperactive, and secrete excessive amounts of inflammatory molecules, amplifying systemic inflammation. This hyperinflammatory state contributes to tissue damage, immune dysfunction, and the progression of age-related pathologies.
By inhibiting mTOR, Rapamycin reduces the hyperactivity of senescent cells and suppresses SASP secretion. Rapamycin also combats inflammation by reducing the burden of senescent cells and suppressing key inflammatory pathways. One critical mechanism involves the inhibition of NF-κB signaling, a major regulator of inflammation. By suppressing NF-κB activity, Rapamycin decreases the production of pro-inflammatory cytokines such as TNF-α and IL-6, effectively dampening systemic inflammation.
Additionally, by enhancing autophagy, Rapamycin facilitates the clearance of cellular debris that would otherwise trigger inflammatory responses. This dual action—reducing SASP secretion and promoting autophagy—mitigates the chronic inflammation associated with aging.[26]
An important 2015 study led by Judith Campisi at the Buck Institute for Research on Aging explored the role of mTOR in cellular senescence and its connection to SASP. Cellular senescence plays a paradoxical role in aging and cancer. While senescence serves as a tumor-suppressive mechanism by halting the proliferation of damaged cells, senescent cells also adopt a pro-inflammatory, pro-growth phenotype through the secretion of SASP factors. This dual role makes the regulation of senescence a critical area of study in aging and cancer biology.
To explore the role of mTOR in cellular senescence and the link between mTOR activity and the SASP, Campisi's lab exposed senescent human cells to rapamycin, which selectively inhibits mTOR complex 1. The study revealed several key findings:
Reduction in SASP Factors: Rapamycin suppressed the secretion of approximately 35% of SASP components, including major pro-inflammatory cytokines such as IL-6, a key driver of chronic inflammation. This finding is significant, as IL-6 plays a central role in propagating systemic inflammation and promoting the tumorigenic microenvironment associated with senescent cells. [27]
Rapid and Sustained Effects: In cell culture, a single 24-hour exposure to Rapamycin immediately following ionizing radiation reduced IL-6 secretion by ~80% for a full seven days—an effect comparable to continuous treatment for the same duration. This underscores Rapamycin’s efficiency in attenuating SASP even with short-term exposure, highlighting its potential for therapeutic applications. [27]
Tumor-Promoting Effects of Senescent Cells: To further investigate SASP’s role in vivo, the researchers grafted prostate tumor cells into mice alongside senescent cells. Tumor growth was significantly enhanced in the presence of senescent cells, confirming their pro-tumorigenic effects. However, when the senescent cells were pretreated with Rapamycin, their tumor-promoting effects were markedly reduced, providing direct evidence that mTOR signaling contributes to tumor progression in vivo. [27]
While Rapamycin targets inflammation through its effects on senescent cells and inflammatory signaling, Urolithin A complements this action by addressing oxidative stress. As we’ve established, oxidative stress arises primarily from the overproduction of reactive oxygen species, particularly by dysfunctional mitochondria.
These ROS cause widespread damage to DNA, proteins, and lipids, further exacerbating cellular dysfunction and aging. Urolithin A clears damaged mitochondria by enhancing mitophagy, reducing ROS production, and alleviating oxidative stress.
Additionally, Urolithin A boosts the activity of antioxidant enzymes such as superoxide dismutase (SOD) and catalase, which neutralize ROS and provide further protection against oxidative damage. Through these mechanisms, rapamycin reduces inflammation by targeting senescent cells and suppressing inflammatory pathways, while Urolithin A improves mitochondrial function and clears dysfunctional mitochondria to address oxidative stress at its source. [28]
Enhancing Cellular Maintenance and Energy Metabolism
Aging cells experience a gradual decline in energy production, driven largely by mitochondrial dysfunction. Mitochondria, the cell’s powerhouses, generate energy through oxidative phosphorylation within the electron transport chain (ETC). However, as mitochondria accumulate damage over time, the efficiency of the ETC deteriorates. This results in reduced ATP production, the cell’s primary energy currency, and an increase in the generation of ROS. This decline in mitochondrial function is a hallmark of aging and contributes to the loss of cellular energy output. [16]
Urolithin A directly addresses this mitochondrial decline by enhancing mitochondrial quality control through mitophagy. As we’ve discussed, by removing damaged mitochondria and replacing them with healthier, more functional ones, Urolithin A rejuvenates the mitochondrial pool and restores efficient ATP production.
Moreover, Urolithin A activates the expression of PGC-1α, a master regulator of mitochondrial biogenesis. This promotes the formation of new mitochondria, further bolstering cellular energy production. The effects of Urolithin A are particularly impactful in high-energy-demand tissues such as muscles and neurons, which are among the first to suffer from mitochondrial dysfunction during aging.[16]
While Urolithin A focuses on mitochondrial health, Rapamycin complements these effects by maintaining the overall cellular environment through autophagy. By promoting the clearance of damaged proteins, organelles, and other cellular debris, Rapamycin ensures that cells are free of toxic accumulations that could impair mitochondrial function. This creates an optimal environment for the healthy mitochondria generated through Urolithin A’s action. By preventing the buildup of dysfunctional components, Rapamycin supports cellular homeostasis and enhances the conditions necessary for sustained mitochondrial activity.
A Comprehensive Approach to Addressing Age-Related Diseases
Rapamycin and Urolithin A represent a potential complementary therapeutic strategy for addressing the cellular dysfunction underlying aging. By targeting distinct but interconnected mechanisms, they offer a more comprehensive intervention than either compound alone. Rapamycin broadly modulates cellular quality control through autophagy and inflammation reduction, while Urolithin A focuses on mitochondrial-specific challenges such as energy deficits and excessive ROS production.
Together, these interventions create a synergistic framework that not only mitigates the cellular decline associated with aging but also improves the resilience and functionality of tissues with high energy demands. This combined approach holds particular promise for addressing chronic diseases that disproportionately affect major organ systems, including the brain, muscles, cardiovascular system, and metabolic organs.
In the following sections, we explore how this dual therapy targets these critical systems, examining evidence from preclinical and clinical studies to highlight its potential for combating age-related diseases and promoting healthier aging.
Neurodegenerative Diseases
In the 20 years from 1999-2019, there has been an increased prevalence of Alzheimer's, with >6 million adults aged >60 suffering from the disease. Although there is a genetic component to this disease via the APO-e4 gene, changes to dietary, exercise, and other environmental influences may accelerate its progression.
Since 2019, Professor Matt Kaeberlein has been calling for clinical trials into Rapamycin as a therapeutic tool to prevent and slow Alzheimer's disease. He even wrote an article published in Science Translation Medicine titled "Rapamycin and Alzheimer's disease: Time for a clinical trial?" [29]
Rapamycin has shown promise in reducing the accumulation of protein aggregates, a hallmark of neurodegenerative diseases such as Alzheimer’s and Parkinson’s. In these conditions, misfolded proteins like amyloid-beta in Alzheimer’s and α-synuclein in Parkinson’s form aggregates that disrupt neuronal function and lead to cell death. By inhibiting mTORC1, Rapamycin induces autophagy, the cellular process responsible for clearing damaged proteins and organelles. This enhanced autophagy facilitates the removal of misfolded proteins, alleviating some of the neurodegenerative processes underlying these diseases. [29]
Rapamycin has a compelling pre-clinical record in mice showing outstanding benefits, including reducing amyloid-β(Aβ) deposition, reducing pathogenic tau phosphorylation and abundance of misfolded tau species, including neurofibrillary tangles, restoring cerebral blood flow, preserving blood-brain barrier integrity, and improving cognitive function. [29]
In Alzheimer’s disease models, mitochondrial dysfunction plays a significant role in disease progression. The mitochondrial membrane’s potential—essential for energy production—declines, while its permeability increases. These changes result in elevated production of reactive oxygen species (ROS) and the release of cytochrome C, a key trigger of programmed cell death (apoptosis) in neurons. These findings highlight the critical role of mitochondrial dysfunction in the onset and progression of Alzheimer’s disease, a concept increasingly supported by a growing body of research.
Recent studies have begun to explore the molecular processes by which Rapamycin alters brain metabolism, with mitochondrial function and particular mitophagy and quality control (i.e., the synthesis of new mitochondria and subsequent degradation of old/damaged mitochondria) playing a critical role.
In aged mice, the treatment with Rapamycin increases mitochondrial protein synthesis (i.e., biogenesis & building of new mitochondria). Utilizing state-of-the-art stable isotope tracer techniques, Professor Benjamin Miller's research group at Oklahoma Medical Research Foundation has observed no effect of Rapamycin on brain mitochondrial protein synthesis in younger mice [30].
However, in an older cohort, Rapamycin induced a nearly 40% increase in mitochondrial protein synthesis, with no distinct effect on mTOR, AMPK, or protein aggregates (i.e., insoluble amyloid-beta plaques (Aβ) and neurofibrillary tangles of tau protein) [30].
On the other side of mitochondrial quality control, others have sought to determine how Rapamycin can force the removal of damaged/dysfunctional mitochondria within the aging brain. Although no research group to date has used the powerful Mito-QC model to study mitophagy dynamics, several authors have probed the effects of Rapamycin on molecular markers associated with mitophagy in models of Alzheimer's [31].
An 8-week study investigating daily Rapamycin treatment (1 mg/kg/day) demonstrated significant benefits in cognitive function, including enhanced learning, memory, and synaptic plasticity, as well as increased expression of synapse-related proteins [31]. To explore Rapamycin’s effects on mitophagy, the researchers employed a method to assess the co-localization of key autophagy markers. Specifically, they examined TOM20, a protein located on the outer mitochondrial membrane, and LC3B, a marker of autophagosome formation. Greater co-localization of these proteins indicates increased mitochondrial-specific autophagy (mitophagy).
The study found that Rapamycin treatment led to a greater than 400% increase in mitophagy compared to the untreated group. This increase was accompanied by elevated expression of key proteins involved in mitophagy, including P62, Parkin, and LC3B, within the brain’s mitochondrial fraction. Notably, isolating mitochondria for analysis proved essential, as whole-brain tissue samples in the study showed a decline in mitophagy-related markers. These findings underscore the importance of precise methods for assessing mitophagy and highlight Rapamycin’s potential role in promoting mitochondrial health within the brain [31].
While Rapamycin addresses protein aggregates effectively, its impact on mitochondrial health in neurons is less direct. Neurons, particularly in the brain, have high energy demands, making mitochondrial dysfunction a critical factor in neurodegeneration. Urolithin A complements Rapamycin’s action by directly enhancing mitophagy. [32]
This process is critical in tissues like the brain and auditory cells, where mitochondrial dysfunction contributes to age-related diseases, including hearing loss. A notable study by Cho et al. (2022), titled Urolithin A attenuates auditory cell senescence by activating mitophagy, explored whether Urolithin A could protect auditory cells from aging-related decline by improving mitochondrial function. Hearing loss is often linked to aging, as the mitochondria in auditory cells—which convert sound into electrical signals for the brain—become less efficient over time. This decline in mitochondrial efficiency leads to oxidative stress, where ROS, harmful byproducts of cellular metabolism, accumulate and damage cells. Excess ROS causes mitochondrial damage, disrupting the cell's energy production and leading to cell death, a major contributor to hearing loss as we age. [33]
The study used a model in which HEI-OC1 auditory cells and cochlear explants (tissue samples from the inner ear) were exposed to hydrogen peroxide (H₂O₂) to mimic the oxidative stress that occurs during aging. Hydrogen peroxide generates high levels of ROS, which damage the cells' proteins, DNA, and mitochondria, accelerating the aging process. After inducing this oxidative stress, the researchers treated the cells with Urolithin A to test whether it could counteract the damage. [33]
The results were striking. The cells exposed to H₂O₂ showed significant increases in p53 and p21, two key markers of cellular aging. These proteins control the cell cycle and promote senescence (a state in which cells stop dividing and lose function). At the same time, the markers that promote mitophagy were reduced, suggesting that the cells' ability to clear out damaged mitochondria was impaired. [33]
However, after treatment with Urolithin A, the levels of p53 and p21 significantly decreased, indicating that the aging process was being reversed. Additionally, there was a marked increase in proteins associated with mitophagy and mitochondrial metabolism, meaning Urolithin A had reactivated the cell's ability to clean out dysfunctional mitochondria. This is crucial because when damaged mitochondria accumulate, they produce higher ROS, perpetuating the cycle of cellular damage and aging. [33]
The findings from the study strongly suggest that Urolithin A holds promise as a neuroprotective agent, particularly in combating age-related mitochondrial dysfunction in auditory cells. The study demonstrated that Urolithin A reverses cellular aging markers and restores mitochondrial function, leading to improved energy production and a decrease in harmful ROS accumulation. These protective effects of Urolithin A could have significant implications for preventing or slowing neurodegenerative conditions linked to mitochondrial dysfunction. [33]
Together, Rapamycin and Urolithin A offer a dual approach to targeting the underlying cellular dysfunction that underpins many neurodegenerative disorders. Rapamycin clears protein aggregates through autophagy, while Urolithin A ensures efficient mitochondrial turnover and energy production, addressing two of the primary drivers of these conditions.
Muscle Health and Sarcopenia
Age-related muscle loss, known as sarcopenia, is a significant contributor to reduced strength, endurance, and overall quality of life in older adults. This progressive decline in muscle mass and function results from a combination of factors, including mitochondrial dysfunction, chronic inflammation, and reduced regenerative capacity within muscle cells. Research on Urolithin A highlights its potential to combat sarcopenia by enhancing mitophagy [19].
A 2022 study by Liu et al., titled Effect of Urolithin A Supplementation on Muscle Endurance and Mitochondrial Health in Older Adults, investigated Urolithin A’s impact on muscle health in elderly individuals aged 65 to 90. Participants received either 1000 mg of Urolithin A or a placebo daily for four months. Researchers assessed muscle endurance, physical performance (via a 6-minute walk test), and biomarkers associated with mitochondrial and cellular health. Specific muscles tested included the tibialis anterior (TA) in the leg and the first dorsal interosseus (FDI) in the hand, which are crucial for mobility and grip strength [18].
The findings showed that participants receiving Urolithin A experienced significant improvements in muscle endurance compared to those in the placebo group. Muscle endurance was assessed by counting the number of muscle contractions before fatigue set in. Those given Urolithin A could perform more contractions, demonstrating better muscle endurance. This effect can be attributed to the enhanced mitochondrial function, as healthy mitochondria produce more ATP, reducing the energy deficit that leads to muscle fatigue. [18]
Additionally, Urolithin A reduced plasma levels of biomarkers such as acylcarnitines, ceramides, and C-reactive protein (CRP). Elevated levels of these molecules are often associated with poor mitochondrial function and increased inflammation. Acylcarnitines are byproducts of fatty acid metabolism, and their elevated presence indicates mitochondrial inefficiency, as the mitochondria cannot properly use fatty acids for energy. These acylcarnitine levels are an indication that the mitochondria work more efficiently to produce energy. [18]
The reduction in CRP, a marker of systemic inflammation, suggests that Urolithin A also mitigates the inflammatory processes contributing to muscle fatigue and degradation. Chronic inflammation is known to impair muscle function by disrupting cellular processes and promoting oxidative stress, so reducing CRP levels reflects a decrease in these harmful effects. [18]
The study also revealed that participants receiving Urolithin A walked further in the 6-minute walk test—an average of 60.8 meters compared to 42.5 meters for the placebo group. This improvement in walking distance indicates enhanced muscle endurance and physical performance, further emphasizing Urolithin A's role in improving mitochondrial function. [18]
Rapamycin addresses sarcopenia, the age-related loss of muscle mass and function, through two primary mechanisms distinct from Urolithin A: combating anabolic resistance and reducing chronic inflammation in muscle tissue. As we age, our bodies exhibit a reduced capacity to construct new muscle protein, even in the presence of anabolic stimuli like resistance exercise or protein intake. The precise molecular mechanisms behind anabolic resistance are still unclear. Emerging evidence from the lab of Dr. David J. Glass, MD, in a paper titled, "Partial Inhibition of mTORC1 in Aged Rats Counteracts the Decline in Muscle Mass and Reverses Molecular Signaling Associated with Sarcopenia" suggests that the dysregulation of the mTOR pathway may be a cause.
To grasp the significance of anabolic resistance, it is essential to comprehend its core concept. Anabolic resistance refers to the diminished ability of the body to construct fresh muscle protein, despite the presence of anabolic stimuli, such as resistance training and protein intake. This phenomenon is intricately intertwined with the process of aging, which brings us closer to the question of why anabolic resistance becomes a prevalent feature as the years pass.
One compelling theory proposes that chronic elevation of mTOR plays a pivotal role in the development of anabolic resistance. The idea is that the machinery responsible for controlling cellular size reaches its maximum capacity as we age, rendering us less responsive to protein intake or exercise. In essence, the heightened mTOR activity in aging individuals impedes their ability to further activate mTOR in response to anabolic stimuli—leading to what could be referred to as an mTOR insensitivity to stimuli.
Work conducted in Dr. Glass's laboratory offers compelling support for this hypothesis. Through experiments, his team observed a progressive increase in the basal (fasted) activity of RPS6, a downstream target of mTOR, across the lifespan—indicating an increase in mTOR activity with age.
The age-related increase in mTOR signaling coincided with a decrease in muscle mass. Though muscle loss at that age is not a surprise, the coincidence of this loss with the elevated mTOR activation was quite unexpected, given that it favors muscle growth and hypertrophy.
The same study administered rapamycin, a pharmacological agent known to inhibit mTOR, for a duration of six weeks. The outcomes were fascinating. The mTOR inhibition led to a restoration of mTOR signaling intermediates and a reversal of sarcopenia—the harmful loss of muscle mass, strength, and function that afflicts many older adults.
Sarcopenia, a gradual and progressive condition that primarily emerges in middle age, exacerbates with time, leaving individuals more susceptible to frailty and diminished quality of life. By attenuating mTOR activity to "youthful" levels through the use of rapamycin, it seems that we may be able to mitigate the effects of sarcopenia and rekindle the body's muscle-building capabilities.
The research team also examined how mTOR inhibition affected the appearance and health of the muscle tissue. The researchers used different doses of mTOR inhibitors, referred to as LD (low dose) and HD (high dose), to see which was more effective. The low-dose treatment turned out to be the key. It led to increased muscle mass in aged rats and more healthy muscle morphology, with other positive molecular changes supporting this growth.
To understand these changes, the scientists performed detailed analyses of the muscle tissue, using techniques like staining with hematoxylin and eosin (H&E). This allowed them to visualize the muscle fibers' structure, comparing healthy young rats with older, sarcopenic ones. They used histology samples of 9 month-year-old rats as an example of healthy tissue and compared them to the samples 24 month-year-old muscle samples to see the progression of aging on and off of rapamycin.
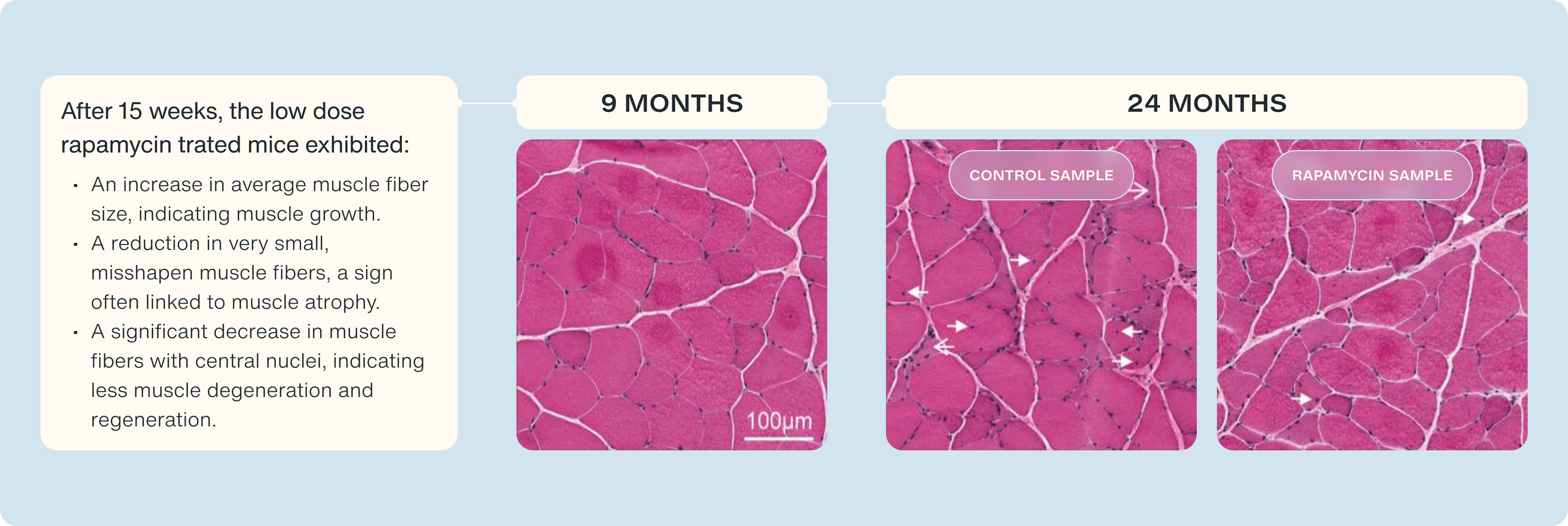
What did they find?
- Healthy Muscle: Tissue from 9-month-old rats showed normal, healthy muscle characteristics.
- Distressed Muscle: In older rats not treated with the mTOR inhibitor, they detected signs of distressed and degenerated muscle.
Improvement with mTOR Inhibition: In the low-dose RAD001-treated rats, they observed:
- An increase in average muscle fiber size, indicating muscle growth.
- A reduction in very small, misshapen muscle fibers, a sign often linked to muscle atrophy.
- A significant decrease in muscle fibers with central nuclei, indicating less muscle degeneration and regeneration. [34]
The high-dose mTOR inhibition treatment did not provide these benefits, emphasizing the importance of finding the correct dose. We see how small pulses of mTOR inhibition, help maintain or anabolic sensitivity and preserve our capacity to grow muscle.
Rapamycin also combats chronic inflammation—a significant contributor to muscle degeneration. Persistent inflammation impairs muscle regeneration and repair, exacerbating age-related muscle loss. By inhibiting mTORC1, Rapamycin suppresses inflammatory pathways such as NF-κB, which regulates the expression of pro-inflammatory cytokines like TNF-α and IL-6. These cytokines are linked to muscle wasting, and their reduction through Rapamycin’s action helps preserve muscle mass and function. [35]
The combination of Rapamycin and Urolithin A provides a synergistic approach to muscle health. Urolithin A enhances mitochondrial efficiency, promoting muscle endurance and energy production, while Rapamycin reduces inflammation, protecting muscle from degradation. This combined strategy effectively counters sarcopenia, helping maintain muscle strength and function as we age.
Metabolic Health
Rapamycin has demonstrated benefits in improving insulin sensitivity and regulating glucose metabolism, making it a valuable tool in addressing age-related metabolic diseases such as type 2 diabetes and obesity. By inhibiting mTORC1, Rapamycin reduces insulin resistance and promotes fat metabolism, helping to normalize glucose levels and decrease visceral fat accumulation. These effects can slow or prevent the progression of metabolic disorders, including obesity and type 2 diabetes. [36]
While Rapamycin enhances metabolic function, it does not directly improve mitochondrial efficiency. Urolithin A addresses this gap by promoting mitophagy in fat and muscle cells, restoring mitochondrial function, and increasing ATP production efficiency. Improved mitochondrial health reduces fat accumulation, particularly in the liver, and optimizes energy expenditure, thereby preventing conditions such as fatty liver disease and obesity. Additionally, Urolithin A reduces inflammation commonly associated with metabolic disorders, further amplifying its positive effects on metabolic health. [37]
The complementary actions of Rapamycin and Urolithin A provide a robust approach to metabolic health. Rapamycin improves insulin sensitivity and glucose metabolism, while Urolithin A enhances mitochondrial efficiency, reduces fat accumulation, and lowers inflammation. Together, they offer a comprehensive strategy for combating age-related metabolic diseases, including type 2 diabetes, obesity, and fatty liver disease.
Practical Considerations for Combined Therapy
Dosage and Administration
The dosing of Rapamycin and Urolithin A for longevity must balance efficacy with safety, emphasizing low doses that avoid significant side effects while maximizing therapeutic benefits.
Rapamycin is typically administered in low doses, generally under 10 mg per week, when used for longevity purposes. Most longevity protocols recommend doses ranging from 2–6 mg per week, often split into a single weekly dose or intermittent pulse dosing (e.g., once every 7–14 days). This dosing strategy aims to selectively inhibit mTORC1 while minimizing the inhibition of mTORC2, which is essential for maintaining glucose metabolism, lipid regulation, and tissue integrity. Higher doses or frequent dosing may increase the risk of side effects such as mild immune suppression or insulin resistance, though these effects vary among individuals. [38]
Urolithin A, on the other hand, is typically taken daily at doses of 250–500 mg in clinical studies. These doses have been shown to support mitochondrial health and function without significant adverse effects. By promoting mitophagy, Urolithin A helps remove dysfunctional mitochondria, improve energy production, and reduce oxidative stress. This makes it particularly beneficial for tissues with high metabolic demand, such as muscles and neurons, though energy levels or endurance improvements are not universally experienced. [39]
Monitoring and Safety
Careful monitoring is essential for individuals using Rapamycin and Urolithin A as part of a longevity protocol. Rapamycin’s suppression of mTORC1 can lead to immune modulation, which may increase susceptibility to mild infections, though serious immune suppression is rare at doses used for longevity. Other possible side effects include lipid profile changes, mild blood glucose elevations, or fatigue. Monitoring blood glucose, lipid levels, and immune markers over time can help identify emerging issues. [38, 39]
Urolithin A, with its mitochondrial-targeted effects, is generally well-tolerated. Its ability to enhance mitochondrial efficiency and reduce oxidative stress may support energy levels and potentially mitigate some of the fatigue or muscle-related side effects of Rapamycin. However, while Urolithin A has shown promise in clinical studies for improving mitochondrial function, the degree of benefit may vary based on an individual’s baseline mitochondrial health. [38, 39]
The liver and kidneys metabolize both compounds, so routine testing of liver enzymes and kidney function (e.g., serum creatinine and GFR) is recommended, especially during long-term use. Although Urolithin A has demonstrated protective effects on mitochondrial health, including in kidney cells, it is not a substitute for regular monitoring in individuals with pre-existing kidney or liver conditions. [38, 39]
For most individuals, combining Rapamycin with Urolithin A offers a promising but not guaranteed approach to supporting cellular health and slowing aspects of aging. The combination works synergistically, with Rapamycin targeting broad cellular repair and Urolithin A specifically supporting mitochondrial quality control. While significant side effects are uncommon when following recommended dosing protocols, individual variability underscores the importance of regular monitoring and personalized adjustments to maximize safety and efficacy.
The Future of Rapamycin and Urolithin A
The combination of Rapamycin and Urolithin A represents an innovative approach to tackling the complexities of aging. Instead of addressing symptoms alone, it targets the cellular mechanisms that underlie age-related decline. These compounds function synergistically to address two of the most significant contributors to aging: cellular debris accumulation and mitochondrial dysfunction. Together, they create a foundation for enhancing cellular resilience, supporting organ health, and potentially extending the health span.
What sets this combination apart is its duality. Rapamycin’s ability to activate autophagy allows it to remove damaged proteins and organelles that accumulate with age, reducing cellular toxicity and fostering a more efficient cellular environment. Meanwhile, Urolithin A’s targeted action on mitophagy rejuvenates mitochondria, ensuring cells' energy demands are met while minimizing the oxidative stress that drives aging. This partnership between a broad-spectrum and a precision-targeted therapy exemplifies the future of anti-aging strategies: integrated approaches that address multiple hallmarks of aging in tandem.
While the scientific foundation is promising, the path to practical application is still emerging. Key questions remain around optimal dosing, the long-term safety of sustained use, and how individual variability—shaped by genetics, microbiome composition, and lifestyle—might influence outcomes. Early studies suggest substantial potential, but large-scale clinical trials will be crucial to confirm efficacy, understand interactions, and refine protocols for widespread use.
Beyond its biological promise, this combination could have transformative implications for society. As populations worldwide confront the challenges of an aging demographic, therapies like Rapamycin and Urolithin A offer a vision for healthier aging—potentially reducing the burden of chronic diseases on healthcare systems, improving the quality of life for millions, and alleviating the economic strain of age-related conditions. By shifting the focus from reactive treatment to proactive cellular optimization, this combination signals a paradigm shift in how we approach aging itself.
- López-Otín, C., et al. (2013). The hallmarks of aging. Cell, 153(6), 1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
- Saxton, R. A., & Sabatini, D. M. (2017). mTOR signaling in growth, metabolism, and disease. Cell, 169(2), 361–371. https://doi.org/10.1016/j.cell.2017.02.004
- Nixon, R. A. (2013). The role of autophagy in neurodegenerative disease. Nature Medicine, 19(8), 983–997. https://doi.org/10.1038/nm.3232
- Johnson, S. C., et al. (2015). mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science, 347(6228), 1237–1241. https://doi.org/10.1126/science.1244360
- Blagosklonny, M. V. (2013). Rapamycin extends life- and health span because it slows aging. Aging, 5(8), 592–598.
- Mito T, Vincent AE, Faitg J, Taylor RW, Khan NA, Mcwilliams TG et al. Mosaic dysfunction of mitophagy in mitochondrial muscle disease. Cell Metab 2022; 34. doi:10.1016/j.cmet.2021.12.017.
- Ubaida-Mohien C, Spendiff S, Lyashkov A, Moaddel R, Macmillan NJ, Filion ME et al. Unbiased Proteomics, Histochemistry, and Mitochondrial DNA Copy Number Reveal Better Mitochondrial Health in Muscle of High Functioning Octogenarians. Elife 2022; 11. doi:10.7554/ELIFE.74335.
- Hodzic Kuerec, A., Lim, X. K., Khoo, A. L. Y., Sandalova, E., Guan, L., Feng, L., & Maier, A. B. (2024). Targeting aging with urolithin A in humans: A systematic review. Ageing Research Reviews, 85, 102406. https://doi.org/10.1016/j.arr.2024.102406
- Plummer JD, Johnson JE. Extension of Cellular Lifespan by Methionine Restriction Involves Alterations in Central Carbon Metabolism and Is Mitophagy-Dependent. Front Cell Dev Biol 2019; 7. doi:10.3389/FCELL.2019.00301/PDF.
- Palikaras K, Lionaki E, Tavernarakis N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. doi:10.1038/nature14300.
- Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med 2016; 22: 1428–1438.
- Ryu, D., et al. (2016). Urolithin A induces mitophagy and improves muscle function in aging. Nature Medicine, 22(8), 879–888.
- Andreux, P. A., et al. (2019). The mitophagy activator Urolithin A is safe and induces a molecular signature of improved mitochondrial health in humans. Nature Metabolism, 1(6), 595–603.
- Amico DD', Andreux PA, Valdés P, Singh A, Rinsch C, Auwerx J. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. 2021. doi:10.1016/j.molmed.2021.04.009.
- Singh A, Davide D’amico •, Pénélope •, Andreux A, Dunngalvin G, Kern • Timo et al. Direct supplementation with Urolithin A overcomes limitations of dietary exposure and gut microbiome variability in healthy adults to achieve consistent levels across the population. Eur J Clin Nutr 2022; 76: 297–308.
- Ryu D, Mouchiroud L, Andreux PA, Katsyuba E, Moullan N, Nicolet-Dit-Félix AA et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nature Medicine 2016 22:8 2016; 22: 879–888.
- Singh A, Amico DD’, Andreux LA, Aebischer P, Auwerx J, Rinsch C et al. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. doi:10.1016/j.xcrm.2022.100633.
- Liu S, D’Amico D, Shankland E, Bhayana S, Garcia JM, Aebischer P et al. Effect of Urolithin A Supplementation on Muscle Endurance and Mitochondrial Health in Older Adults: A Randomized Clinical Trial. JAMA Netw Open 2022; 5: e2144279–e2144279.
- Singh, R., et al. (2009). Autophagy regulates lipid metabolism. Nature, 458(7242), 1131–1135.
- Peiling Luan et al. ,Urolithin A improves muscle function by inducing mitophagy in muscular dystrophy.Sci. Transl. Med.13,eabb0319(2021).DOI:10.1126/scitranslmed.abb0319
- Huang, Jr., Zhang, Mh., Chen, Yj. et al. Urolithin A ameliorates obesity-induced metabolic cardiomyopathy in mice via mitophagy activation. Acta Pharmacol Sin 44, 321–331 (2023).
- Guertin, D. A., & Sabatini, D. M. (2005). An expanding role for mTOR in cancer. Trends in Molecular Medicine, 11(8), 353–361.
- Laplante, M., & Sabatini, D. M. (2012). mTOR signaling in growth control and disease. Cell, 149(2), 274–293.
- Selvarani, R., Mohammed, S., & Richardson, A. (2021). Effect of rapamycin on aging and age-related diseases-past and future. GeroScience, 43(3), 1135–1158.
- Tchkonia, T., Zhu, Y., van Deursen, J., Campisi, J., & Kirkland, J. L. (2013). Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. The Journal of Clinical Investigation, 123(3), 966–972.
- Cappoli, N., et al. (2019). The mTOR kinase inhibitor rapamycin enhances the expression and release of pro-inflammatory cytokine interleukin 6, modulating human microglial cell activation. EXCLI Journal, 18, 779–798. https://doi.org/10.17179/excli2019-1715
- Laberge RM, Sun Y, Orjalo AV, Patil CK, Freund A, Zhou L, Curran SC, Davalos AR, Wilson-Edell KA, Liu S, Limbad C, Demaria M, Li P, Hubbard GB, Ikeno Y, Javors M, Desprez PY, Benz CC, Kapahi P, Nelson PS, Campisi J. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015 Aug;17(8):1049-61. doi: 10.1038/ncb3195. Epub 2015 Jul 6. Erratum in: Nat Cell Biol. 2021 May;23(5):564-565. PMID: 26147250; PMCID: PMC4691706.
- Luan, P., et al. (2021). Urolithin A improves muscle function by inducing mitophagy in muscular dystrophy. Science Translational Medicine, 13(588), eabb0319.
- Kaeberlein M, Galvan V. Rapamycin and Alzheimer's disease: Time for a clinical trial? Sci Transl Med. 2019 Jan 23;11(476):eaar4289. doi: 10.1126/scitranslmed.aar4289. PMID: 30674654; PMCID: PMC6762017.
- Reid JJ, Linden MA, Peelor FF, Miller RA, Hamilton KL, Miller BF. Brain Protein Synthesis Rates in the UM-HET3 Mouse Following Treatment With Rapamycin or Rapamycin With Metformin. J Gerontol A Biol Sci Med Sci 2020; 75: 40.
- Wang H, Fu J, Xu X, Yang Z, Zhang T. Rapamycin Activates Mitophagy and Alleviates Cognitive and Synaptic Plasticity Deficits in a Mouse Model of Alzheimer's Disease. The Journals of Gerontology: Series A 2021; 76: 1707–1713.
- Burchell, V. S., et al. (2010). Targeting mitochondrial dysfunction in neurodegenerative disease: Part I. Expert Opinion on Therapeutic Targets, 14(4), 369–385.
- Cho, S. I., Jo, E. R., & Song, H. (2022). UA attenuates auditory cell senescence by activating mitophagy. Scientific reports, 12(1), 7704. https://doi.org/10.1038/s41598-022-11894-2
- Joseph GA, Wang SX, Jacobs CE, Zhou W, Kimble GC, Tse HW, Eash JK, Shavlakadze T, Glass DJ. Partial Inhibition of mTORC1 in Aged Rats Counteracts the Decline in Muscle Mass and Reverses Molecular Signaling Associated with Sarcopenia. Mol Cell Biol. 2019 Sep 11;39(19):e00141-19. doi: 10.1128/MCB.00141-19. PMID: 31308131; PMCID: PMC6751631.
- Jing, F., et al. (2017). Rapamycin alleviates inflammation and muscle weakness while altering the Treg/Th17 balance in a rat model of myasthenia gravis. Bioscience Reports, 37(4), BSR20170767.
- Reifsnyder, P. C., et al. (2016). Rapamycin treatment benefits glucose metabolism in mouse models of type 2 diabetes. Aging, 8(11), 3120–3130.
- Xia, B., et al. (2020). Urolithin A exerts antiobesity effects through enhancing adipose tissue thermogenesis in mice. PLOS Biology, 18(3), e3000688.
- Papadopoli, D., et al. (2019). mTOR as a central regulator of lifespan and aging. F1000Research, 8, F1000 Faculty Rev-998.
- Pereira, M. J., et al. (2012). mTOR inhibition with rapamycin causes impaired insulin signalling and glucose uptake in human subcutaneous and omental adipocytes. Molecular and Cellular Endocrinology, 355(1), 96–105.