Examining mTOR's Influence on Multiple Sclerosis: A Scientific Review of Rapamycin's Therapeutic Potential
Introduction
Multiple Sclerosis (MS) is a chronic autoimmune disease that affects the central nervous system, including the brain and spinal cord. It manifests with a diverse array of symptoms, including mobility challenges, vision impairments, and persistent fatigue.
Recent studies have cast the spotlight on the mammalian target of rapamycin (mTOR) pathway, which is believed to play a significant role in MS pathophysiology. The heightened activity of mTOR is gaining recognition for its potential to influence the course of MS, presenting new therapeutic opportunities to modulate this pathway.
A recent narrative review paper by Dr. Aigli Vakrakou from the University of Athens provides a comprehensive analysis of emerging approaches to the treatment of Multiple Sclerosis. The review provides a thoughtful analysis of mTOR's role within the cellular framework and its impact on the progression and symptoms of MS.
In our comprehensive Research Review, we delve into the pivotal role of mTOR inhibition in MS. We present findings on how this inhibition can bolster autophagy, potentially leading to improved clinical outcomes for MS sufferers. Moreover, we examine the influence of mTOR regulation on T cells and the ensuing impact on immune system equilibrium, with the aim of decreasing MS-related symptoms.
This discussion extends to the clinical application of mTOR inhibitors, where we dissect the current landscape of patient care, interpreting the nuanced benefits and considerations inherent in such a complex treatment approach. As we synthesize these topics, our review remains rooted in the latest empirical data and ongoing research efforts.
Through this synthesis, we strive to provide a balanced and insightful overview that respects the complexity of MS treatment and the careful, research-driven approach to understanding mTOR's role in it. [1]
Understanding the Origins and Effects of Multiple Sclerosis
Even though more than 1.8 million individuals worldwide are diagnosed with MS, the exact cause of the disease remains unclear, with scientists believing that it arises from a complex interplay of genetic, environmental, and immunological factors. While scientists do not fully understand the causes of MS, they have achieved a comprehensive understanding of the biological processes occurring in the brains of individuals affected by the condition.
To understand the pathology of MS, we have to first learn about myelin sheaths. The myelin sheath is the protective armor around our nerve fibers in the brain and spinal cord. Let's think of nerve fibers as wires that connect each neuron in the brain. The myelin sheaths are akin to the insulating material around those wires, facilitating efficient and rapid transmission of electrical signals.
However, in MS, the immune system—which typically targets and destroys pathogens—erroneously directs its attack against the myelin sheath. The immune system's attack leads to swelling and harm to the myelin sheath, which we call "demyelination." It's like scratching the surface of a wire – it doesn't work so well anymore. This demyelination leads to scar tissue, also called sclerosis.
With the myelin armor damaged, the messages that travel along our nerve fibers get disrupted. It's similar to a faulty phone line—messages can slow down, get distorted, or even stop altogether. This disruption in nerve communication leads to various symptoms like difficulty walking, trouble seeing, or fatigue.
The condition also involves the destruction of oligodendrocytes, the cells tasked with the creation and maintenance of the myelin sheath. When these cells are damaged, the natural repair of myelin is hindered, causing prolonged neurological impairment.
Moreover, MS is not just limited to demyelination; the disease can also lead to the degeneration of the actual nerve fibers, or axons. This kind of damage is crucial as it represents a more permanent form of neurological impairment since axons, unlike myelin, have a limited capacity for regeneration in the central nervous system.
Chronic inflammation, a typical feature of MS, can cause and exacerbate this axonal damage. Such persistent inflammatory conditions can ultimately alter the course of the disease from one where patients experience intermittent periods of symptom remission, to a progressive decline in neurological function without periods of recovery.
Unfortunately, the current treatments for MS cannot help the brain repair the damaged myelin or prevent further nerve damage. Therefore, scientists are searching for novel therapies. One such therapeutic approach is targeting the mTOR (mammalian target of rapamycin) pathway. The mTOR pathway plays a pivotal role in how our immune system functions and modulation of this pathway may lead to advances in treating MS. [1]
What is the mTOR Pathway?
Recent studies have delved into the mTOR pathway, exploring its potential as a therapeutic target in the battle against MS.
As a central regulator, mTOR coordinates a range of cellular functions—it monitors and responds to the presence or absence of nutrients, growth factors, and energy. When nutrients are plentiful and growth factors are present, mTOR spurs cells into action, promoting protein synthesis and cell growth. Conversely, in a nutrient-scarce environment, it can initiate autophagy, a process of cellular 'housekeeping' or 'cleaning,' where cells recycle components and clear out debris to maintain cellular efficiency.
This balancing act conducted by mTOR is essential for normal cellular function, but in the context of MS, it's thought that the pathway may play a role in the disease’s progression. How? Well, as we get older, mTOR may stay active all the time—opening the door to out-of-control cell growth that can lead to cancer and closing the door on cell repair. In MS, certain immune cells become overly active and attack the central nervous system.
Researchers hypothesize that by inhibiting mTOR, we might be able to dial back this overactivity, reducing the immune system's assault on the nervous system. When mTOR is inhibited, either through natural or pharmacological mechanisms, the pathway receives a signal to slow down or, in some instances, halt unhealthy growth and overactivity. Recently, mTOR inhibition has emerged as an approach for mitigating immune responses and offering potential treatments for diseases where the immune system goes awry, such as MS. By modulating this pathway, there's the potential not only to suppress harmful immune activity but also to influence the course of the disease. [2]
mTORs Role in Multiple Sclerosis: What is research saying?
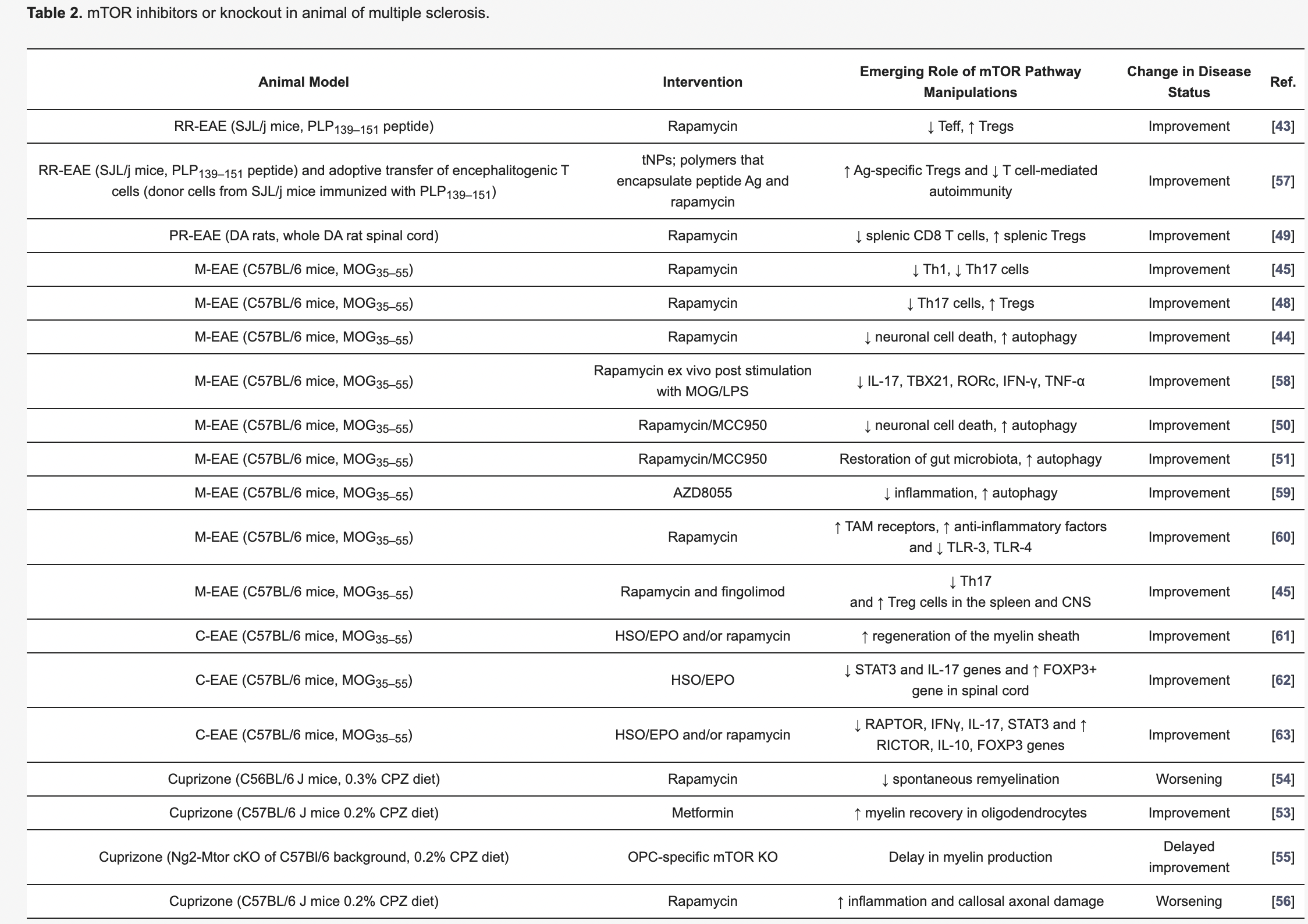
More than fifteen research studies in animals and five clinical trials in humans have examined the impact of mTOR inhibition on Multiple Sclerosis. These studies have used a variety of genetic modifications, medications, and diets to induce mTOR inhibition.
All of these studies have found an improvement in MS symptoms, such as reduced inflammation, enhanced neural repair, and slowed disease progression. In the proceeding section, we will review the specific mechanisms that mTOR inhibition influences and what implications this holds for treating MS. [1]
mTOR's Role in Modulating Immune Responses
In a healthy immune system, two types of immune cells, T and B cells, function as critical defenders against infections and external threats. But in Multiple Sclerosis, these cells tend to become overly aggressive and damaging. This leads to the immune system erroneously targeting the myelin sheath, a protective layer surrounding nerve fibers in the central nervous system.
mTOR plays a pivotal role in modulating immune cell behavior. It can either enhance or limit the activity of specific immune cells. Inhibiting mTOR, therefore, serves as a critical control mechanism, preventing certain immune cells (T Cells) from becoming overly active and causing diseases, akin to keeping a choir section from becoming too loud and discordant. One study, conducted by Dr. Hou of the Key Laboratory of Hebei Neurology, found that mTOR inhibition through the use of rapamycin not only decreased the number of T cells, but also decreased the symptoms of MS in animal models by more than 50%. [4]
Given that MS is partially an autoimmune disorder characterized by the immune system's misguided assault on nerve fiber protection, mTOR inhibition offers promise in managing the condition. Regulating the activity of immune cells, particularly T cells, mitigates their tendency to become overly aggressive, ultimately safeguarding the nervous system and contributing to the improvement of MS symptoms. [1]
The Influence of mTOR on Regulatory T Cells and Immune Balance
Inhibition of mTOR plays a vital role in MS by encouraging the development of regulatory T cells (Tregs). Tregs are a specific type of immune cell that act as peacekeepers within the immune system. Their primary role is to maintain control over the immune response and prevent it from becoming overly aggressive or harmful. One study, conducted by Dr. LaMothe of Selecta Biosciences, found a 16% increase in Tregs associated with mTOR inhibition. [3] Additionally, in MS, there is often an imbalance between Tregs and pro-inflammatory T cells, which exacerbates the autoimmune response and the damage it causes to the brain. Inhibition of mTOR helps rectify this imbalance by increasing the number of Tregs. This enables the immune system to regulate itself better and limit the excessive immune response observed in MS. Bolstering the presence of Tregs essentially helps restore a more harmonious and controlled immune environment, potentially reducing the severity of the disease and improving MS symptoms. [1]
mTOR's Role in Reducing Inflammation
In multiple sclerosis (MS), immune cells, especially T cells, invade the central nervous system (CNS) and create an inflammatory response. These immune cells release molecules that trigger inflammation, damaging the protective myelin sheath surrounding nerve fibers. This harmful process, known as demyelination, results in the development of scar tissue, or sclerosis, and contributes to the symptoms of MS. One promising avenue for managing the disease involves tackling this inflammation in the central nervous system. Inhibition of the mTOR pathway helps calm the overactive immune cells contributing to the inflammation. This can help reduce inflammation, providing relief from some of the symptoms of MS and slowing down the progression of the disease. [1]
mTOR's Role in Enhancing Autophagy
mTOR serves as a key overseer of autophagy, the body's inherent recycling program designed to dispose of cellular debris and faulty components. When the mTOR pathway is suppressed, there is a consequential boost in autophagy, which has implications for diseases like MS.
Research reveals that autophagy is a safeguard for brain and neural health, with divergent patterns of gene expression controlling autophagy observed in MS patients compared to those without the disease [5]. Defects in autophagy have been identified as contributing factors to MS pathology, suggesting that this process is integral to the condition's development [6].
Furthermore, impediments in autophagy may lead to the buildup of damaged mitochondria and an overproduction of Reactive Oxygen Species (ROS), both implicated in the myelin damage characteristic of MS [7, 8]. This deficit reduces the efficacy of microglial cells—our neurological janitors—leading to a pro-inflammatory state.
This balancing act extends to glial cells, such as astrocytes and oligodendrocytes. For instance, inhibiting a specific autophagy gene, Atg5, in astrocytes can trigger neuronal death. In oligodendrocytes, tasked with myelin production, reduced autophagy has been linked to a decline in myelin-rich axons and a diminishment of myelin sheath quality, reinforcing the beneficial aspects of autophagy in these cell types.
Encouragingly, studies indicate that bolstering autophagy could offer protective benefits for MS sufferers. Enhanced autophagic activity has been correlated with improvements in MS, as demonstrated by the positive outcomes from mTOR pathway inhibition [6]. Additionally, dietary strategies like caloric restriction, which induce a fasting-mimicking state, have shown promise in reducing disease severity and promoting remyelination in mouse models of MS, such as experimental autoimmune encephalomyelitis (EAE), and in humans with relapsing-remitting MS [9].
However, the picture is not uniformly optimistic. In specific cells of the nervous system, such as T cells, excessive autophagy has been associated with a heightened immune offensive against myelin, suggesting a detrimental effect in these contexts.
While the mechanisms of this paradox are still a topic of research, striking a delicate balance in modulating autophagy, perhaps through fine-tuning the mTOR pathway, is crucial for effectively managing the intricate interplay between autophagy and the progression of MS. [1]
mTOR's role in the Protection of Neurons
Inhibition of the mTOR pathway may also protect neurons and other essential cells in the brain. In addition to reducing inflammation and increasing autophagy, mTOR inhibition can also reduce oxidative stress. Oxidative stress is a chemical imbalance within our cells with an excess of harmful molecules called free radicals.
Inhibition of the mTOR pathway may protect neurons and other vital brain cells by performing several key functions: it reduces inflammation, thereby minimizing harm from an overactive immune response; it enhances autophagy, allowing cells to remove damaged components and maintain health; and it lessens oxidative stress, contributing to a more stable and less harmful cellular environment. This multifaceted approach holds promise for supporting neuronal health and treating neurodegenerative conditions like MS [1].
How to achieve mTOR inhibition?
The potential therapeutic benefits of mTOR inhibition have garnered significant attention in the field of medical research, particularly in the context of neurodegenerative conditions and immune-related diseases such as MS. So, what does this mean for individuals looking to access these benefits?
Achieving mTOR inhibition can be accomplished through various approaches and strategies, each offering unique advantages and challenges [2].
- Pharmacological Interventions: One of the most common methods to achieve mTOR inhibition is through pharmaceutical agents. Rapamycin and its analogs, collectively referred to as rapalogs, are well-established mTOR inhibitors used in clinical settings for specific conditions. These drugs work by directly binding to mTOR, inhibiting its activity, and recalibrating its activity to more youthful levels.
- Dietary and Nutritional Interventions: Emerging research has highlighted the impact of nutrition and dietary choices on mTOR activity. Caloric restriction and intermittent fasting are known to inhibit mTOR, while specific nutrients, such as amino acids like leucine, have been shown to activate mTOR [9]. Dietary modifications can provide a more natural and holistic approach to mTOR regulation.
- Lifestyle Modifications: Lifestyle factors, such as regular exercise and physical activity, have also been associated with mTOR inhibition. Cardiovascular exercise, in particular, has been shown to promote autophagy and reduce mTOR activity, offering a non-pharmacological means of achieving mTOR regulation.
- Combination Therapies: In some cases, a combination of approaches may be employed to achieve mTOR inhibition. This could involve a synergistic use of pharmaceutical agents, dietary adjustments, and lifestyle modifications tailored to an individual's specific health needs and goals. Combining strategies can enhance the effectiveness of mTOR inhibition while minimizing side effects.
Considerations
While the potential benefits of mTOR inhibition in the context of MS are intriguing, it's essential to acknowledge and address potential concerns and limitations associated with this approach. [1]
- Side Effects: Like many pharmaceutical interventions, mTOR inhibitors like rapamycin may have side effects. Therefore, the balance between the potential benefits and side effects must be carefully considered, particularly in the long-term use of these inhibitors.
- Individual Variability: The response to mTOR inhibition may vary from person to person. Factors such as genetics, the stage and severity of the disease, and the presence of other underlying health conditions can influence how an individual responds to treatment. Developing personalized treatment plans that consider these factors will be crucial for optimizing the effectiveness of mTOR inhibition in MS.
- Complexity of Autophagy: The dual role of autophagy in MS adds a layer of complexity to its modulation. While enhancing autophagy can be beneficial by clearing damaged cellular components, excessive autophagy may have detrimental effects. Striking the right balance in regulating autophagy is a challenge that requires further research and understanding.
- Long-Term Outcomes: The long-term effects of mTOR inhibition in MS still need to be fully understood. It is essential to investigate the potential consequences of prolonged mTOR inhibition, including its impact on the immune system, overall health, and any potential risks associated with extended treatments.
- Treatment Interactions: Many individuals with MS are already on existing treatments or therapies. It's crucial to explore the compatibility of mTOR inhibitors with these other treatments and assess any potential synergistic or adverse interactions.
Conclusion
mTOR inhibition through pharmacological agents, dietary interventions, or lifestyle changes may offer a promising avenue for Multiple Sclerosis treatment, presenting a new ray of hope for individuals grappling with this challenging condition. Scientists have found that the mechanisms of mTOR inhibition can help protect the myelin sheath surrounding our nerve fibers and offer protection to neurons and neural connections. Additionally, the potential benefits in symptom alleviation and disease progression make mTOR inhibition a compelling area of research and development with regards to MS. [1]
Nevertheless, the path forward is not without its complexities and considerations. It demands further in-depth exploration and careful investigation, particularly regarding individual variability, long-term effects, and the optimization of treatment strategies. The integration of mTOR inhibition into existing therapeutic approaches for MS is a nuanced task, necessitating collaboration among healthcare professionals, researchers, and patients. [1]
As this promising approach unfolds, it signifies a fresh chapter in the ongoing efforts to enhance the lives of those affected by Multiple Sclerosis, embodying the scientific community’s persistent commitment to finding innovative solutions and improving the management of this condition.
- Vakrakou AG, Alexaki A, Brinia ME, Anagnostouli M, Stefanis L, Stathopoulos P. The mTOR Signaling Pathway in Multiple Sclerosis; from Animal Models to Human Data. Int J Mol Sci. 2022 Jul 22;23(15):8077. doi: 10.3390/ijms23158077. PMID: 35897651; PMCID: PMC9332053.
- Mikhail V. Blagosklonny (2006) Aging and Immortality: Quasi-Programmed Senescence and Its Pharmacologic Inhibition, Cell Cycle, 5:18, 2087-2102, DOI: 10.4161/ cc.5.18.3288
- LaMothe RA, Kolte PN, Vo T, Ferrari JD, Gelsinger TC, Wong J, Chan VT, Ahmed S, Srinivasan A, Deitemeyer P, Maldonado RA, Kishimoto TK. Tolerogenic Nanoparticles Induce Antigen-Specific Regulatory T Cells and Provide Therapeutic Efficacy and Transferrable Tolerance against Experimental Autoimmune Encephalomyelitis. Front Immunol. 2018 Mar 2;9:281. doi: 10.3389/fimmu.2018.00281. PMID: 29552007; PMCID: PMC5840162.
- Hou, H., Miao, J., Cao, R. et al. Rapamycin Ameliorates Experimental Autoimmune Encephalomyelitis by Suppressing the mTOR-STAT3 Pathway. Neurochem Res 42, 2831–2840 (2017). https://doi.org/10.1007/s11064-017-2296-7
- Igci M, Baysan M, Yigiter R, Ulasli M, Geyik S, Bayraktar R, Bozgeyik İ, Bozgeyik E, Bayram A, Cakmak EA. Gene expression profiles of autophagy-related genes in multiple sclerosis. Gene. 2016 Aug 15;588(1):38-46. doi: 10.1016/j.gene.2016.04.042. Epub 2016 Apr 25. PMID: 27125224. https://pubmed.ncbi.nlm.nih.gov/27125224/
- Boyao, Y., Mengjiao, S., Caicai, B., Xiaoling, L., Zhenxing, L., and Manxia, W. (2019). Dynamic expression of autophagy-related factors in autoimmune encephalomyelitis and exploration of curcumin therapy. J. Neuroimmunol. 337:577067. doi: 10.1016/j.jneuroim.2019.577067 https://pubmed.ncbi.nlm.nih.gov/31629984/
- Chen, Y., McMillan-Ward, E., Kong, J., Israels, S. J., and Gibson, S. B. (2008). Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 15, 171–182. doi: 10.1038/sj.cdd.4402233
- Hassanpour, M., Hajihassani, F., Hiradfar, A., Aghamohammadzadeh, N., Rahbarghazi, R., Safaie, N., et al. (2020). Real-state of autophagy signaling pathway in neurodegenerative disease; focus on multiple sclerosis. J. Inflamm. 17, 1–8. doi: 10.1186/s12950-020-0237-8
- Choi, I. Y., Piccio, L., Childress, P., Bollman, B., Ghosh, A., Brandhorst, S., et al. (2016). A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 15, 2136–2146. doi: 10.1016/j.celrep.2016.05.009