Regulating Anabolic and Catabolic Signaling for Healthspan Optimization: Impacts of Rapamycin, Exercise, and Testosterone Replacement Therapy
Introduction
The pursuit of longevity has long fascinated science, with recent decades witnessing significant advancements in understanding the molecular underpinnings that influence healthspan—the disease-free and functional period of an individual’s life. Central to this pursuit is the study of metabolic pathways, particularly anabolic and catabolic signaling, which play pivotal roles in aging and the development of age-related diseases. This narrative review aims to dissect the complex interactions between these pathways, with a specific focus on the pharmacological modulation by rapamycin, the physiological impacts of exercise, and the therapeutic potential of testosterone replacement therapy.
Anabolic signaling is primarily associated with growth and repair processes, involving the synthesis of complex molecules from simpler ones. It is crucial for maintaining tissue integrity and promoting recovery from cellular stress. Conversely, catabolic signaling, which involves the breakdown of complex molecules to release energy, is essential for removing damaged organelles and proteins—a process vital for cellular survival and function. The mammalian target of rapamycin (mTOR) pathway, a key regulator of cellular growth, serves as a nexus for these processes, responding dynamically to the body's nutritional and energy statuses.
Emerging evidence suggests that the strategic modulation of these pathways, particularly through interventions such as the administration of rapamycin, tailored exercise regimens, and testosterone replacement therapy, can significantly enhance healthspan. Rapamycin, a potent mTOR inhibitor, has been shown to extend lifespan in various organisms by inducing a shift from anabolic to catabolic states, thereby enhancing autophagic activity and reducing cellular senescence. Exercise, by promoting anabolic signaling, counters muscle loss and improves metabolic health, which are crucial for maintaining functional capacity with age. Testosterone replacement therapy also plays a critical role in this context by bolstering anabolic processes that wane with age, thus supporting muscle strength and overall vitality.
This review explores the dual roles of these interventions in regulating anabolic and catabolic signaling and proposes a model for their integration to optimize healthspan. By understanding the mechanisms through which these pathways influence aging, we can better strategize interventions that not only extend lifespan but also enhance the quality of life in later years.
Understanding Metabolism
Think of metabolism like a city’s power grid. It’s the complex system of chemical reactions that keeps everything in a living organism running smoothly. We typically think about it in terms of converting macronutrients into cellular energy. It is divided into two principal categories: catabolism and anabolism. You’ve probably heard the term anabolic in the context of bodybuilding or muscle growth. However, before diving into anabolism, it's crucial to understand the benefits of the catabolic phase, particularly its role in cellular cleansing through a process known as autophagy.
Catabolism: The Destructive Phase
Catabolism, the breakdown phase of metabolism, involves the decomposition of complex molecules into simpler ones, thereby releasing energy. This energy is essential for various cellular functions. For example, during catabolism, large biomolecules such as proteins, fats, and carbohydrates are broken down into their smaller components: amino acids, fatty acids, and monosaccharides (simple sugars), respectively. The energy liberated during these breakdown processes is frequently captured in the form of adenosine triphosphate (ATP).
ATP is the energy currency of the cell.Your body uses ATP like cash to power pretty much everything it does, from lifting weights at the gym to digesting the burger you just ate. It’s crucial because it provides the energy boost that your cells need to build new proteins and store energy for later.
The major catabolic processes include:
- Glycolysis: During glycolysis, glucose—a six-carbon sugar molecule—gets split into two smaller molecules called pyruvate. This splitting process is like opening the canister to release the energy stored inside. Along the way, your body produces ATP (think of it as quick cash for immediate energy needs) and NADH (a kind of savings account for energy that’s used later in other energy-producing reactions).
- Proteolysis: In this process, proteins are broken down into their building blocks known as amino acids.
- Lipolysis: Lipolysis is the process by which triglycerides (the main form of fat in the body) are broken down into smaller molecules, specifically fatty acids and glycerol.
As we explore ways to manipulate metabolic pathways to enhance healthspan, understanding the balance between catabolism and anabolism is key. Now, let’s briefly turn our attention to anabolism and describe it in the simplest biochemical terms.
Anabolism: The Constructive Phase
Anabolism, is the constructive phase of metabolism. If you imagine your body as a construction site, anabolism is the phase where all the building happens. It takes simpler materials—kind of like bricks, cement, and steel beams—that have been broken down by catabolism (the body’s demolition team) and uses them to build complex structures like DNA, RNA, proteins, and complex carbohydrates.
Think of ATP as the electricity that powers the construction site. It's the essential energy that drives the assembly of these materials into new buildings—or in your body's case, into new cells and tissues. This construction is crucial not just for growth but also for repairing any wear and tear your body experiences.
Anabolism is critical to:
- Building proteins from amino acids, which are like assembling huge, complex machines in a factory.
- Synthesizing DNA and RNA, which is similar to laying down the blueprints that guide the whole operation.
- Creating complex sugars, which are like producing the energy packs that will power the city.
Anabolism keeps your body growing and repairing, ensuring everything runs smoothly and stays strong. Without it, your body wouldn’t be able to heal injuries, support your immune system, or even continue to grow.
Some examples of anabolic processes of biological significance include:
- Protein synthesis: In this process, proteins are built up by linking together amino acids.
- Lipogenesis: In this process, triglycerides are built by combining fatty acids with glycerol. The synthesized triglycerides are stored in adipose tissue, providing a reservoir of energy that can be mobilized during periods of fasting.
- Gluconeogenesis: In this process, glucose is formed from non-carbohydrate precursors like amino acids and glycerol. The process helps to maintain blood glucose levels, especially during periods of fasting or low carbohydrate intake.
The balance between catabolism and anabolism is meticulously regulated by cells. When energy levels are high, indicated by abundant ATP, cells generally favor anabolic pathways to synthesize and store complex molecules.
Conversely, when energy is scarce, as reflected by low ATP levels, catabolic pathways dominate to break down complex molecules and release energy. This regulatory mechanism ensures cellular efficiency and balance.
As we delineate in the proceeding section, we can leverage anabolic and catabolic pathways to optimize our healthspan, as they are both essential components of maintaining cellular health and overall tissue function as we age.
Levers to Utilize to Optimize Healthspan
To effectively achieve various health objectives, it is crucial to strategically manipulate metabolic pathways. We are going to consider these pathways as levers at our disposal to optimize our health objectives.
A key component in this strategy is autophagy, a catabolic process vital for cellular health. Autophagy involves the degradation and recycling of damaged organelles and proteins, which not only generates cellular energy but also prevents the accumulation of toxic cellular debris.
We are going to review the importance of autophagy in various contexts. This process is particularly significant in the context of neurodegenerative diseases such as Alzheimer's Dementia, where enhanced autophagy can prevent the buildup of amyloid plaques, a hallmark of the disease. Furthermore, autophagy plays a crucial role in mitigating the adverse effects of senescent cells, which contribute to aging and various diseases by promoting chronic inflammation and tissue dysfunction.
Stimulating autophagy is, therefore, a pivotal catabolic strategy for preserving cellular integrity and function.
Conversely, anabolism plays a beneficial role in many physiological contexts, such as muscle growth. Muscle hypertrophy, the increase in muscle size, results from anabolic processes where muscle proteins are synthesized more rapidly than they are degraded. This is vital for maintaining muscle mass and strength, particularly crucial as we age, supporting not only physical mobility but also metabolic health.
In the upcoming sections, we will delve deeper into the importance of timing and moderating these metabolic stimuli. The concept of metabolic cycling—or providing the body with alternating pulses of anabolic and catabolic stimuli—is central to this discussion. By oscillating between these states, we can enhance the sensitivity and efficiency of both metabolic pathways.
Rapamycin: Harnessing Catabolic Pathways for Longevity
Rapamycin is a pharmacological agent that has garnered considerable interest within the longevity research community for its multifaceted impact on cellular processes that are integral to healthspan. It targets senescent cells, stimulates autophagy, and recalibrates cellular growth to more youthful levels, making it one of the most intriguing molecules in the study of aging.
At the molecular level, rapamycin exerts its effects primarily through the inhibition of a key enzyme known as the mammalian target of rapamycin (mTOR). You can think of mTOR as the air-traffic controller for cellular growth. When cells receive signals that indicate an abundance of nutrients or growth factors, mTOR becomes activated and stimulates cellular protein synthesis and cell growth. This activation of mTOR promotes cell growth and division, which is essential for tissue repair and growth in developing organisms.
Evolutionarily, the role of mTOR makes sense—it enables organisms to grow and thrive when resources are plentiful. In the presence of sufficient nutrients, mTOR orchestrates the cellular machinery required for protein synthesis and growth, ensuring that cells capitalize on favorable energy conditions.
Conversely, in times of nutrient scarcity, mTOR activity is suppressed, triggering the cellular machinery responsible for autophagy. Autophagy, often likened to 'spring cleaning,' involves the breakdown and recycling of damaged cellular components and misfolded proteins. This not only clears the cell of dysfunctional elements but also helps to sustain cellular energy levels during periods of deprivation. The suppression of mTOR under these conditions is crucial as it shifts the cellular focus from growth to maintenance and survival, thereby conserving resources and optimizing cellular function during adverse conditions.
The Problem: Chronic Overactivation of mTOR
Chronic overactivation of mTOR underpins many age-related diseases. As we age, mTOR may become perpetually active, fostering uncontrolled cell growth that can culminate in cancer, while simultaneously impeding essential cell repair processes. This persistent activation leads to unhealthy cell growth that leads to the decline of our tissue.
Understanding Cellular Hyperfunctions—The Work of Mikhail Blagosklonny
The concept of cellular hyperfunctions in aging, popularized by Dr. Mikhail Blagosklonny, provides a framework to understand why mTOR overactivity becomes problematic as we age. This theory suggests that aging is less about the loss of cellular function or the "wear and tear" of tissues and more about the cellular 'hyper-functions' that drive pathological changes.
Blagosklonny identifies three hyperfunctional features critical to understanding the morphology and pathology of dysfunctional cells:
- Hyperplasia: This refers to an increase in the number of cells within an organ or tissue. Often, senescent cells secrete excessive amounts of mitogens that stimulate adjacent cells to proliferate, which can lead to pathological conditions such as cancer or benign tumors.
- Hypertrophy: An increase in the size of individual cells, leading to the enlargement of the organ or tissue they compose. Persistent or excessive hypertrophy can lead to cellular dysfunction, tissue damage, or disease.
- Hyperfunctionality: This occurs when cells or organs operate beyond normal or necessary levels. Factors such as excessive hormonal stimulation or aberrant signaling pathways can lead to hyperfunctionality, which may result in cellular damage, dysfunction, or disease.
As cells develop these hyperfunctions, they become oversized, stimulate excessive growth of unhealthy cells and tissues, and become hyperinflammatory. Such cellular states drive the acceleration of tissue dysfunction and aging in humans. Blagosklonny's counterintuitive perspective on aging—as a phenomenon driven by cellular overactivity—has important implications for strategies aimed at decelerating the aging process [1].
The Hyperfunction Metaphor
Blagosklonny effectively describes hyperfunction using the metaphor of driving a car. During the transition from childhood to adulthood, cellular activity accelerates as if driving on a highway. However, as aging progresses, it becomes necessary to decelerate to the "neighborhood speeds" of growth and repair. Cellular mechanisms or molecular brakes, such as quiescence, enable cells to rest and slow down. Quiescent cells halt division and reduce metabolic activity, yet they retain the capacity to reactivate and re-enter the cell cycle when necessary, allowing them to adapt to both rapid growth conditions and periods of rest [1].
As we age, our cellular ability to 'slow down' diminishes. Instead of a leisurely drive, our hyperactive cells race through the body at unsustainable speeds, causing widespread damage. This inappropriate cellular activity often manifests in age-related diseases such as cancer and osteoporosis [2].
The excessive activity of mTOR in aging drives these hyperfunctions in dysfunctional cells, representing a harmful, pathological level of anabolic cellular growth. It's important to clarify that we are not merely discussing growth that aids in tissue regeneration and other healthy anabolic processes. When mTOR is overactive, we witness the proliferation of unhealthy tissues, including senescent cells and potentially the growth of cancer cells [2].
Let’s review how this manifests in some common age-related chronic diseases.
mTOR Overactivity in Alzheimer’s Disease
Neurodegenerative diseases, particularly Alzheimer's disease, provide a compelling illustration of how mTOR (mammalian target of rapamycin) overactivity contributes to age-related pathologies. In Alzheimer's, the pathological accumulation of Tau proteins within neurons is a hallmark feature, leading to the formation of neurofibrillary tangles—a primary indicator of the disease [3]. These tangles are closely associated with neuronal death, which progressively impairs brain function.
The role of mTOR in this process is critical. Overactive mTOR signaling in neurons has been shown to increase the production of Tau proteins [4]. Normally, Tau helps stabilize microtubules in nerve cells; however, in Alzheimer's, abnormally phosphorylated Tau accumulates and leads to tangle formation, disrupting neuronal transport systems and ultimately causing cell death.
Furthermore, the excess Tau proteins provoke a significant immune response, characterized by inflammation that contributes to further neuronal damage. This inflammatory response not only accelerates the degeneration of brain tissue but also exacerbates the cognitive decline observed in Alzheimer’s patients [5].
Activation of mTOR in Alzheimer’s disease not only stimulates the production of Tau proteins but also affects various other cellular processes that can worsen the disease’s progression. For instance, mTOR overactivity impairs the cellular cleanup process, autophagy, which is crucial for removing defective proteins and organelles [6]. By hindering this vital function, overactive mTOR contributes to the accumulation of cellular debris, exacerbating the pathological features of Alzheimer’s.
mTOR Overactivity in Cancer
In 2023, Dr. Blagosklonny released a narrative review paper, titled “Cancer prevention with rapamycin.” One of the most infamous examples of aberrant growth and proliferation is cancer, and with this article, Blagosklonny combines what he knows about cancer with what he knows about aging. The pharmaceutical strategy that most directly addresses Blagosklonny’s theory of aging is mTOR inhibition.
Blagosklonny begins the article with preclinical data from dozens of mouse cancer models and experimental designs. Preclinical models are imperative to establish robust treatment strategies and mechanisms of action. Throughout the article, Blagosklonny references toxin exposure, such as Nicotine-derived nitrosamine ketone (NNK), as well as genetic models of cancer. In these genetic models, the mouse genome is purposefully altered, causing a huge increase in cancer chance, often reaching 100% cancer incidence throughout the experiment.
From all this preclinical data, Blagosklonny shows, impressively, that treatment with mTOR-inhibiting drugs slowed cancer progression in all stages, regardless of treatment start point. This means that, no matter how far along the cancer has progressed in the mice, there was a significant slowing with them [7].
Additionally, pre-treating mice at high risk of cancer prolonged cancer incidence, suggesting that this effect can begin as early as needed for high-risk candidates. Taking it one step further, inhibition of mTOR also delayed cancer in normal mice [7]. This fact is relatively unsurprising because rapamycin has been extensively shown to extend lifespan and slow aging in mice; however, it is interesting to note that the positive effect is also seen in a low-risk mouse population where some mice may never get cancer.
To clarify, even normal mice will develop cancer, and even though the progression and severity were slowed, rapalogs did not and do not cure cancer. However, in many cases, that will be all we want the treatment to do. Blagosklonny recognizes that this treatment, though widely effective, will not replace many of the necessary cancer treatments that currently exist and are still in development. Interestingly, there is already some clinical human data showing similar trends and giving high-risk people meaningful, cancer-free time.
In certain diseases, especially various types of cancer, this mTOR signaling can go awry. In fact, it's been noted that over 70% of cancers have an overactive mTOR signaling pathway [7]. Both animal studies and clinical patient data further affirm that a dysfunctional mTOR system plays a significant role in the development and growth of tumors.
This overactivation of the mTOR pathway can cause cells to grow and multiply unchecked, leading to the formation and progression of cancer. The components of the mTOR pathway are frequently found to be mutated in cancers, suggesting a clear link between this pathway and the disease.
Given the integral role that mTOR plays in the development of cancer, it makes sense that mTOR inhibitors like rapamycin would be studied to slow progression or onset of certain cancers. mTOR inhibitors like rapamycin work by interfering with the mTOR signaling pathway. They can slow down or halt cells at a certain stage in their life cycle, known as the G1 phase, effectively stopping them from multiplying. In addition, these inhibitors can promote apoptosis, a form of programmed cell death, which can help to reduce the number of cancerous cells in the body [7].
These inhibitors can also impact angiogenesis, which is the process of forming new blood vessels. Angiogenesis is critical for the growth of tumors because these newly formed vessels supply the cancer cells with oxygen and nutrients necessary for their growth and spread. Therefore, by interfering with angiogenesis, mTOR inhibitors can potentially starve tumors and slow down their growth. So, in essence, mTOR inhibitors act as a potential multifaceted approach to tackle cancer by not only directly targeting the growth of cancer cells but also affecting the supportive infrastructure (like blood vessels) that tumors need to thrive.
Blagosklonny's theory posits that excessive activity of the mTOR pathway is a critical factor in the progression of a wide range of age-related chronic diseases. This overactivity leads to tissue decline and dysfunction, ultimately contributing to disease development. Rapamycin, by moderating mTOR activity, can restore cellular growth signals to healthier levels, thereby mitigating the risk of cellular hyperfunctions that can become harmful over time. Beyond merely adjusting mTOR levels, rapamycin plays a crucial role in enhancing the cell’s ability to clear away toxic debris, supporting overall cellular health and longevity.
Rapamycin and Autophagy
Rapamycin's influence extends beyond merely reducing mTOR activity; it actively promotes autophagy. Autophagy, from the Greek meaning "self-eating," is a mechanism through which cells degrade and recycle their damaged components, such as misfolded proteins and dysfunctional organelles. This process is particularly vital during nutrient-deprived states, such as fasting, where cells must rely on internal reserves for energy.
To understand the importance of autophagy, let’s review its involvement in a number of age-related diseases.
Previously, we discussed how mTOR overactivity contributes to neurodegenerative disorders like Alzheimer’s disease, primarily through the accumulation of misfolded Tau proteins, which form neurofibrillary tangles and contribute to neuronal death. In contrast, the inhibition of mTOR by rapamycin can activate autophagy, allowing cells to effectively degrade and recycle these toxic protein aggregates [3].
The implications of disrupted mTOR activity on cellular clean-up processes are profound. Post-mortem analyses of brain samples from patients with various neurodegenerative diseases have frequently shown disruptions in autophagy. Specifically, autophagosomes, the cellular structures that sequester and degrade cellular debris, often appear to be overaccumulated and inadequately processed, suggesting a failure in the later stages of the autophagy pathway.
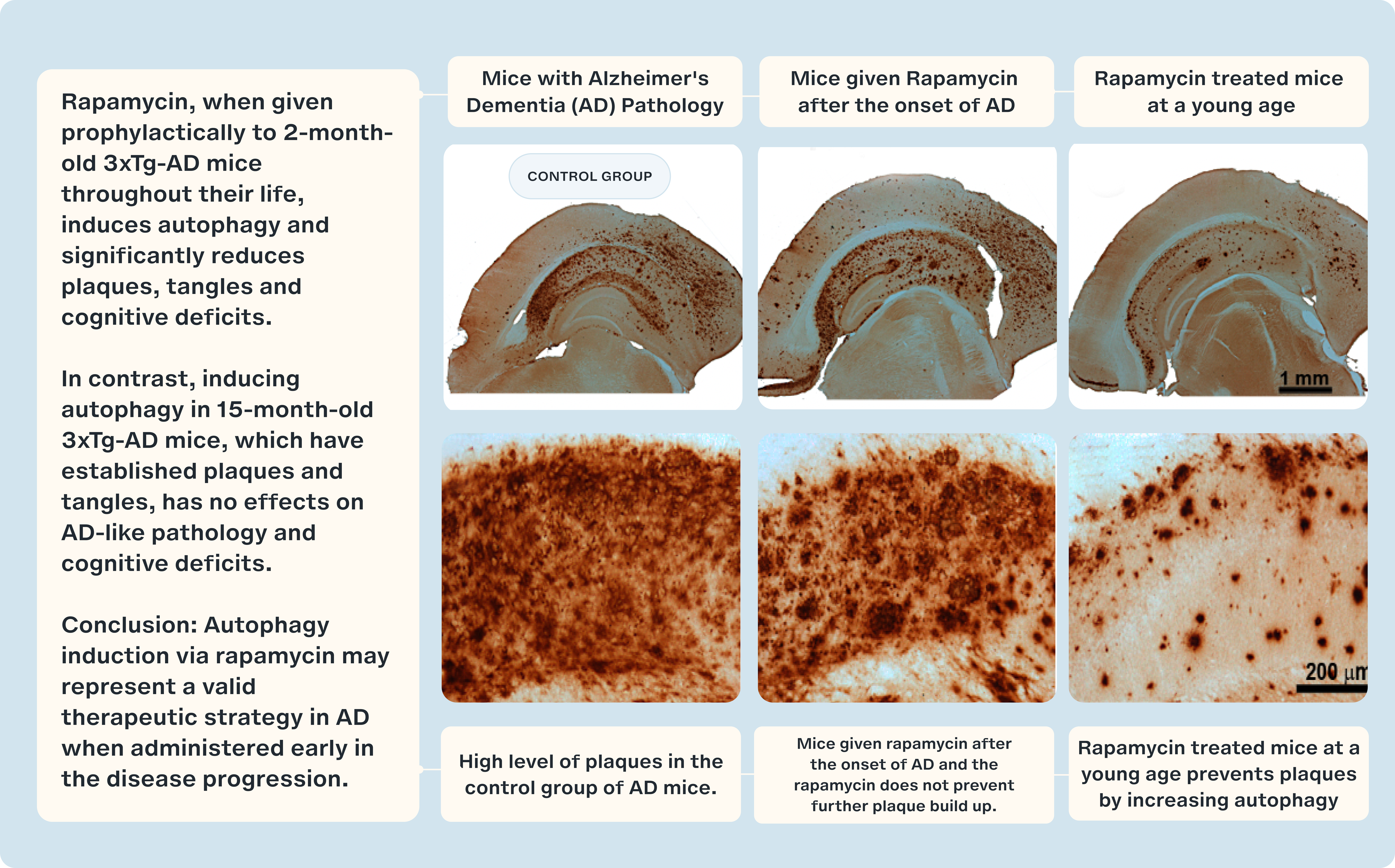
This observation has led researchers to hypothesize that enhancing autophagy could significantly improve the ability of cells to clear out misfolded proteins and other cellular debris [8]. By leveraging agents like rapamycin to increase autophagy, there is potential not only to alleviate symptoms but also to halt or perhaps reverse the progression of neurodegenerative diseases.
The role of autophagy in maintaining tissue health and functionality is evident across a spectrum of chronic diseases. When autophagy is impaired we see the decline of tissue function. Conversely, when autophagy pathways function optimally, there is a notable preservation of tissue health over time, highlighting the protective nature of this process.
A compelling example of autophagy's therapeutic potential is its role in cardiovascular health. A paper, titled "The Induction of Endothelial Autophagy and Its Role in the Development of Atherosclerosis" demonstrated that rapamycin, through its induction of autophagy, can significantly reduce lipid accumulation in the arteries of macrophages. This process is crucial for mitigating the development of atherosclerosis, a leading cause of cardiovascular diseases. By enhancing autophagy, rapamycin helps to clear lipid deposits from arterial walls, thus preventing the formation of atherosclerotic plaques, which are the primary contributors to heart attacks and strokes [9].
Emerging research underscores the potential of rapamycin to induce autophagy across various cancer cell lines, leading to promising therapeutic outcomes such as reduced tumor growth and increased sensitivity to chemotherapy. This mechanism is particularly significant in the context of cancer treatment, where autophagy can either suppress tumor development or enhance the efficacy of existing therapies.
The process by which rapamycin enhances autophagy involves the inhibition of the mTOR pathway, which is often upregulated in cancer cells. By inhibiting this pathway, rapamycin seems to put a brake on cellular processes that contribute to tumor growth and survival. Furthermore, the activation of autophagy by rapamycin can lead to the degradation of proteins and organelles that are essential for cancer cell survival, thereby sensitizing tumors to chemotherapy and potentially overcoming resistance mechanisms.
In the context of liver disease, particularly non-alcoholic fatty liver disease (NAFLD), rapamycin has demonstrated significant potential. NAFLD, characterized by excessive fat accumulation in the liver without alcohol consumption, can lead to more severe liver conditions like steatohepatitis and cirrhosis. A study by Lin et al. (2013) highlighted that rapamycin could induce autophagy in liver tissues, effectively reducing lipid accumulation and alleviating liver damage [10]. In their research, mice with NAFLD treated with rapamycin showed marked improvements in liver function, suggesting that enhancing autophagy could be a viable strategy to manage or even reverse the progression of fatty liver diseases.
Similarly, in muscular dystrophies—a group of genetic disorders marked by progressive muscle weakness and loss—rapamycin has been beneficial. These conditions often involve the accumulation of defective proteins and organelles that contribute to muscle degeneration. A study titled, "Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function" demonstrated rapamycin's ability to restore autophagy pathways in a mouse model of muscular dystrophy. By reactivating these pathways, rapamycin helped clear accumulated cellular debris, leading to an improvement in muscle function and strength [11].
The activation of autophagy through the inhibition of mTOR Complex 1 (mTORC1) signifies a pivotal shift from an anabolic state, which focuses on growth and reproduction, to a more catabolic state emphasizing maintenance and repair. This transition is crucial for longevity, particularly under conditions of stress or limited resources. It allows the organism to prioritize vital survival processes overgrowth, enhancing its ability to withstand environmental pressures and age-related physiological challenges.
Energy Metabolism
Rapamycin’s catabolic effects extend beyond the promotion of autophagy. By inhibiting mTOR, rapamycin shifts the cellular energy balance towards catabolic pathways. One such pathway is glycolysis, the process by which glucose is broken down to generate ATP, the cell's primary energy currency. This shift in energy metabolism can lead to the breakdown and release of energy stores, such as stored fats and sugars, to support essential cellular functions.
An illustrative study by Brown et al. (2007) explored the role of rapamycin in β-oxidation within the liver cells of rats. β-oxidation is a crucial metabolic process where fatty acids are broken down to produce acetyl-CoA, which enters the Krebs cycle to generate ATP [12]. This process is vital for energy production, especially when glucose levels are low.
The study found that rapamycin significantly increased the extent of β-oxidation in liver cells. This increase was believed to be closely linked to the enhanced autophagy induced by rapamycin. Autophagy targets various cellular components for degradation, including fatty acids [12]. By promoting the breakdown and recycling of these fatty acids, autophagy provides substrates for β-oxidation, thereby boosting the process.
The induction of autophagy by rapamycin facilitates the release of fatty acids from fat stores within liver cells. Once released, these fatty acids are channeled into β-oxidation, enhancing the cell's ability to produce energy during times of nutrient scarcity or increased energy demand. This mechanism highlights the interplay between autophagy and other catabolic pathways in maintaining cellular energy homeostasis.
Moreover, the increased catabolic activity not only supports energy production but also contributes to improved metabolic health. Enhanced β-oxidation can reduce the accumulation of fatty deposits in the liver, thereby mitigating the risk of fatty liver disease and other metabolic disorders. This is particularly relevant in the context of age-related metabolic decline, where the body's efficiency in managing energy stores and metabolic processes diminishes.
Overall, the catabolic effects of rapamycin, including its impact on autophagy and energy metabolism, underscore its potential as a therapeutic agent for promoting longevity and metabolic health. By facilitating the breakdown and recycling of cellular components and energy stores, rapamycin helps maintain cellular function and prevent metabolic dysregulation, which are critical factors in aging and age-related diseases.
Caloric Restriction in Catabolism
Caloric Restriction (CR) is a dietary intervention that involves reducing caloric intake, typically by 20-40% below normal levels, without malnutrition. It has been studied extensively for the benefits it offers in the longevity space. During CR, the body enters a state of increased catabolism, where it breaks down conserved energy stores to release energy. By mobilizing existing stores, the body is put under stress. This results in the activation of stress-responsive physiological pathways to mitigate oxidative stress and inflammation. Both oxidative stress and inflammation are linked to aging and age-related diseases.
The catabolic effects of caloric restriction (CR) have been widely studied and demonstrate significant impacts on metabolism. Research indicates that mice subjected to a diet with lower than normal caloric intake typically experience an increased activation of lipolysis, the process by which fats are broken down into fatty acids and glycerol for energy. Such dietary conditions also lead to elevated production of stress hormones, such as corticosterone, which help the body adapt to lower energy availability. These findings suggest that caloric restriction can activate crucial adaptive mechanisms that enhance metabolic efficiency and stress resilience.
These metabolic changes triggered by CR ultimately translated to improved lifespans in the mice, compared to control mice that were fed a regular diet. The study suggests that the catabolic effects of CR, such as enhanced fat breakdown and increased stress hormone production, contribute to the beneficial effects on longevity by promoting efficient energy use and maintenance of cellular health.
Specifically, the CR mice had reduced amounts of visceral fat (fat around the internal organs) and elevated levels of the stress hormone corticosterone compared to the control group. These changes were associated with extended lifespans in the mice. In a related study, Baumeier et al. (2015) investigated how CR and intermittent fasting affected the health of mice prone to developing diabetes. They placed some mice on restricted diets (either eating fewer calories or fasting every other day) and compared them to mice that could eat as much as they wanted. They found that the mice on restricted diets had fewer harmful fat molecules called diacylglycerols (DAGs) in their liver fat stores, as these molecules were being metabolically broken down into fatty acids. This reduction in harmful fat molecules made the mice more sensitive to insulin and protected against the development of diabetes. [13]
Overall, CR serves as an effective strategy for mitigating aging and age-related pathologies. It accomplishes this by activating catabolic pathways that release energy from energy stores. By putting the body in a state of calorie deficit, CR also induces stress and promotes the release of stress hormones like corticosterone. Collectively, this combined activation of catabolic processes and stress hormone release promotes anti-aging effects.
We have reviewed the benefits of pulsating stimuli for catabolism—particularly as it pertains to enhancing autophagy and the benefits of temporarily inhibiting mTOR. Now, let’s show how anabolic stimulation fits into our idea of optimizing healthspan.
Testosterone and Anabolism
Testosterone is a hormone primarily produced in the testes in males and in smaller amounts in the ovaries and adrenal glands in females. It belongs to a class of hormones called androgens, which are responsible for the development and maintenance of male characteristics. Testosterone plays a crucial role in various physiological processes, including the development of secondary sexual characteristics like facial hair, deepening of the voice, and muscle mass. Additionally, it influences bone strength, mood, cognition, and libido. In females, testosterone contributes to reproductive health and sexual function. For both sexes, testosterone is a key hormone with a wide array of effects.
Testosterone exerts its effects through a complex mechanism of action primarily mediated by its interaction with androgen receptors (ARs). ARs are specific protein structures that serve as the docking site for testosterone. Upon binding to ARs, testosterone forms a hormone-receptor complex, which then influences gene expression.
The regulation of gene expression by testosterone plays a crucial role in various aspects of growth and development. Testosterone influences the expression of genes involved in skeletal muscle development, leading to increased muscle mass and strength. This was demonstrated in a study where researchers investigated how testosterone influences the expression of genes in muscle cells. [14] The results of the study found that testosterone leads to an increase in the expression of several genes that code for proteins involved in the synthesis of ATP, which as described previously is our body’s main ‘energy currency.’ The study shows that testosterone facilitates energy production in skeletal muscle cells by upregulating proteins involved in the anabolic synthesis of ATP molecules.
In addition to the aforementioned benefits, testosterone significantly enhances physical performance. This is because the hormone promotes muscle protein synthesis, leading to increased muscle mass and strength. It also helps reduce fat mass, thereby improving the muscle-to-fat ratio, which is advantageous in many sports. Furthermore, testosterone plays a crucial role in red blood cell production. Red blood cells are a type of cell that carry oxygen to the working tissues and muscles of our bodies. In particular, they contain a compound called hemoglobin, which holds oxygen and delivers it through circulation to different body regions. [15]
The improvements in muscle mass and strength are particularly beneficial for strength and power athletes, such as weightlifters and sprinters. Enhanced oxygen delivery, on the other hand, is advantageous for endurance athletes like marathon runners. Notably, a dose-response relationship exists between testosterone levels and increases in muscle mass, strength, and circulating hemoglobin levels. The rise in hemoglobin levels due to testosterone allows more oxygen to be supplied to working muscles during physical exercise, further enhancing physical performance.
While testosterone can enhance athletic performance, elevated levels, especially when achieved through supplements or steroids, pose significant health risks. The steroids that help enhance testosterone levels are typically termed anabolic-androgenic steroids (AAS). Potential negative effects of these include liver damage, an increased risk of heart disease, hormonal imbalances, and changes in mood and behavior. In a recent study, researchers examined the hearts of 87 deceased men who tested positive for AAS and compared them to 173 control men. They found that the men who had used AAS had significantly larger heart muscles, a condition known as increased cardiac mass [15].
Another study focused on the hearts of four men who died suddenly and had a history of AAS use. This study found that their hearts exhibited ventricular hypertrophy (enlargement of the ventricles, the main pumping chambers of the heart), fibrosis (the thickening and scarring of connective tissue), and myocytolysis (breakdown of muscle cells). These changes are associated with an increased risk of heart disease and sudden cardiac death.[16]
Further research on the effects of AAS has looked at how these substances impact the electrical activity of the heart. Studies have shown that AAS users experience decreased cardiac electrical stability, meaning their hearts are more prone to abnormal rhythms that can lead to sudden death. [17] Therefore, while testosterone has undeniable benefits for physical performance, its misuse via AAS administration can lead to serious health complications.
While misuse of testosterone, especially through anabolic-androgenic steroids (AAS), poses significant health risks, the proper use under medical supervision can be beneficial for many individuals. For elderly individuals with low testosterone levels and reduced muscle mass, testosterone therapy can be essential. As people age, natural testosterone production declines, leading to conditions such as sarcopenia, which is characterized by decreased muscle mass and strength, increased fat mass, and reduced bone density. These changes can significantly impact quality of life, increasing the risk of falls, fractures, and overall frailty.
Testosterone replacement therapy in these individuals can help restore muscle mass and strength, enhance bone density, and improve physical function. Studies have shown that testosterone stimulates muscle protein synthesis by binding to androgen receptors in muscle cells, leading to increased muscle mass and strength. Additionally, testosterone influences the expression of genes involved in skeletal muscle development and energy production, facilitating better physical performance and energy levels.
Moreover, testosterone also supports bone health by stimulating the activity of osteoblasts, the cells responsible for bone formation, and inhibiting the activity of osteoclasts, the cells that break down bone tissue. This dual action helps increase bone density, reducing the risk of osteoporosis and fractures in the elderly.
Therefore, while it is crucial to avoid the misuse of testosterone due to its potential health risks, appropriate testosterone replacement therapy can provide significant benefits for those with medically diagnosed deficiencies, particularly in elderly populations suffering from sarcopenia and other related conditions.
If we excessively stimulate anabolic pathways through the utilization of exogenous hormones, resulting in supraphysiological levels, we are going to grow tissue. As we saw in the context of mTOR and our discussion of hyperfunctions, we are going to grow unhealthy tissue in the form of senescent cells and tumorigenic cells, as well as heighten the hyperfunctions of senescent cells. This is detrimental to optimizing healthspan. As we’ll see the key is to cycle normal physiological levels of anabolism, while also following up those cycles with pulses of catabolic stimuli.
Exercise and Anabolism
Exercise can also improve longevity outcomes by altering metabolic pathways. However, unlike CR, exercise primarily exerts its effects by targeting anabolic pathways. As people age, sarcopenia, or the age-related loss of muscle mass and strength, becomes more common. This condition can make it harder for individuals to move around and perform daily activities. Exercise combats sarcopenia by stimulating muscle protein synthesis, which helps build and maintain muscle mass and strength. Regular physical activity also enhances insulin sensitivity, cardiovascular health, and overall metabolic function, contributing to improved longevity and quality of life.
In an extensive review, titled "Role of Dietary Protein and Muscular Fitness on Longevity and Aging" underscored the importance of exercise in protecting against sarcopenia. Regular physical activity promotes the anabolic build-up of protein in aging muscle tissue, thereby improving physical performance and quality of life [18]. Fiatarone et al. (1994), which appeared in the prestigious New England Journal of Medicine. looked at whether exercise and nutrition could help improve muscle strength and function in elderly, frail nursing home residents. The researchers divided 100 participants into four groups: one group engaged in progressive resistance exercise training, the second group received multinutrient supplementation, the third group received both exercise training and supplementation, and the last group did not engage in either exercise or receive supplementation. The results showed that muscle strength increased by 113% in the exercise groups, compared to only 3% in the non-exercise groups. Gait speed, stair-climbing power, and physical activity levels also improved in the exercise groups, but not in the non-exercise groups. Muscle size slightly increased in the exercise groups but decreased in the non-exercise groups. Nutritional supplementation alone did not affect the outcomes. Overall, high-intensity resistance exercise was found to be an effective way to counteract muscle weakness and physical frailty in elderly nursing home residents, whereas nutritional supplementation alone, without exercise, did not improve these measures. [19]
The type of exercise also matters. Resistance training involves performing exercises that challenge the muscles to contract against some form of resistance (such as weights, resistance bands, or body weight). This type of training has been found to exert impressive anabolic effects. In fact, a single session of resistance training can lead to a two- to threefold increase in the rate of muscle protein synthesis, meaning that the muscles respond to the resistance training by rapidly building new muscle proteins, which drives muscle growth and repair. Bautmans et al. (2009) showed that elderly sedentary subjects can acquire more than 50% strength gain even after only six weeks of resistance training when performing two or three sessions with sufficiently high intensity. [20]
When resistance training is combined with dietary changes, anabolic muscle buildup and protection against wasting are even further amplified. Breen & Phillips (2013) evaluated the influence of dietary factors in muscle protein synthesis following exercise in elderly subjects. They found that the type of protein consumed can make a major difference. Milk contains two main types of protein: whey and casein. Whey protein is quickly absorbed into the bloodstream, causing a rapid increase in the amino acids (building blocks of proteins) available for building muscle. In contrast, casein protein is slower to be absorbed, leading to a more gradual and prolonged increase in amino acids. This means that whey protein is better at immediately stimulating muscle protein synthesis, especially when consumed after resistance exercise. Even though whey and casein proteins contain similar amounts of essential amino acids and are considered high-quality proteins, whey has a higher proportion of the amino acid leucine [21].
Overall, exercise, particularly resistance training, is crucial for countering sarcopenia and promoting healthy longevity. Unlike CR, exercise primarily benefits the body by stimulating anabolic pathways that build up muscle. Studies show resistance training has powerful anabolic effects, increasing muscle strength by over 100% in frail elderly individuals. Just a single session can double or triple muscle protein synthesis, driving muscle growth and repair. Combining resistance exercise with optimal nutrition, such as whey protein supplements, further amplifies this anabolic response. By maintaining and improving muscle mass and function, regular high-intensity resistance exercise is a key intervention for supporting independent, healthy aging. Its anabolic effects are critical for promoting longevity.
Managing Your Anabolic and Catabolic Signals
Understanding the balance between anabolic and catabolic pathways is crucial. Each pathway plays a vital role, yet when either is overstimulated, it can lead to adverse health outcomes.
Overstimulating Anabolic Pathways
Consider the case of someone using supraphysiological levels of testosterone to enhance muscle growth. While this approach might initially seem beneficial by promoting the growth of muscle tissue, it also has unintended consequences. Elevated testosterone levels can indiscriminately stimulate the growth of various types of cells, not just the desired muscle cells. This can lead to the proliferation of senescent cells and potentially tumorigenic cancer cells [22].
The mechanism behind these effects involves the mTOR pathway, which we've discussed extensively. By artificially boosting anabolic activity through external testosterone, mTOR's activity is enhanced, not just in muscle cells but across a range of cell types. This global stimulation of mTOR can lead to what is known as cellular hyperfunction—a state where cells grow and function excessively [23]. Senescent cells, which are typically in a state of growth arrest but still metabolically active due to being damaged, can become even more harmful under these conditions. They can secrete pro-inflammatory cytokines and other factors that degrade tissue quality and function and spread senescence to other cells, thus accelerating the aging process [24].
The Consequences of Excessive Anabolism
Therefore, excessive anabolism, while beneficial for muscle growth, can paradoxically speed up the aging process by fostering environments conducive to cellular malfunction and malignancy. This underscores the importance of maintaining a careful balance in signaling pathways to ensure that the stimulation of growth does not inadvertently promote harmful cellular behaviors. Managing these signals effectively not only supports the growth of healthy tissue but also minimizes the risks associated with overactive anabolic pathways.
Overstimulating Catabolic Pathways
A substantial body of research supports the health benefits of caloric restriction, which is known to extend healthspan, largely through the inhibition of the mTOR pathway. Caloric restriction activates several catabolic processes that help clear damaged cells and promote cellular efficiency. However, maintaining a constant caloric deficit can prevent the body from experiencing the necessary anabolic stimulation that is essential for muscle growth and overall robust health. Muscle tissue, known for its metabolic and functional benefits, is crucial for maintaining strength and vitality, particularly as we age.
The Risks of Prolonged Catabolic States
Prolonged engagement in catabolic states can lead to significant health issues, such as cachexia. Cachexia is a severe pathological condition characterized by dramatic weight loss, muscle wasting, and general weakness, often seen in individuals with chronic diseases like cancer, chronic obstructive pulmonary disease (COPD), and HIV/AIDS. This condition is driven by overactive catabolic pathways, including proteolysis (the breakdown of proteins) and lipolysis (the breakdown of fats), which lead to the excessive degradation of muscle and fat stores [25].
As people age, the risk of developing conditions associated with cachexia increases, complicating the management of various chronic diseases and significantly impairing quality of life. Therefore, while catabolic processes are crucial for recycling cellular components and adapting to low-nutrient conditions, their overactivation can be detrimental [25].
Balancing Catabolic and Anabolic Signals
The challenge of balancing catabolic and anabolic processes is crucial for maintaining health, especially in older adults. Excessive anabolism can lead to conditions like obesity and type 2 diabetes, while excessive catabolism can lead to muscle and weight loss, as seen in cachexia. The key to optimizing healthspan is to achieve a dynamic equilibrium between these two processes, allowing for the benefits of both without the risks associated with their extremes.
This balance raises several theoretical questions: How can one effectively manage the interplay between catabolism and anabolism, especially when certain activities and substances distinctly promote one over the other? Understanding the triggers and regulators of these metabolic pathways is essential for developing targeted interventions that can enhance health outcomes while minimizing the risk of associated diseases.
Thinking in Pulses: Balancing Anabolism and the Cellular Benefits of Canabalism
Imagine we want to maximize muscle growth while also being able to tap into the benefits of autophagy and mTOR inhibition through either the utilization of rapamycin or caloric restriction. We know for example that while rapamycin is in your system you are going to be inhibiting mTOR and diminishing some level of protein synthesis. Alternatively, if you were fasting, the lack of nutrients would signal to mTOR that we do not have sufficient amount of building blocks to grow or fuel growth. This mTOR inhibition is beneficial for promoting autophagy but can blunt the protein synthesis needed for muscle growth. Therefore, engaging in resistance training during periods of strong mTOR inhibition may not yield the full benefits of anabolic stimuli.
Strategic Cycling of Catabolic and Anabolic Stimuli
The key to leveraging these processes lies in the strategic cycling of catabolic and anabolic stimuli. This approach ensures that the periods of mTOR inhibition do not overlap significantly with anabolic activities like resistance training, allowing each process to occur effectively without interference from the other.
A practical example of this strategy was shared by Professor Matt Kaeberlein, who discussed his own regimen with rapamycin. After monitoring his blood levels, Kaeberlein noted that his rapamycin concentration dropped from 20.4ng/ml to 2.9ng/ml within 24 hours of administration, classifying him as a fast metabolizer. Based on this metabolism rate, he could schedule his resistance training outside of this 24-hour window on days when he does not take rapamycin, thereby minimizing the drug's impact on muscle growth.
Healthspan’s founder, Daniel Tawfik’s approach involves scheduling resistance training from Monday to Friday, engaging in different activities over the weekend, and taking rapamycin on Saturdays. This timing ensures that the peak effects of rapamycin—particularly its inhibitory impact on anabolic muscle adaptations—are avoided during critical training periods.
The key, therefore, would be to understand how you metabolize rapamycin. A serum sirolimus test can provide insights into how quickly one metabolizes rapamycin, helping to optimally time resistance training and rapamycin doses to maximize the benefits of both muscle growth and enhanced autophagy.
We often talk about why rapamycin is dosed on an intermittent basis, typically once every 7 to 14 days. This is largely to optimize for mTORC1 sensitivity and avoid mTORC2 inhibition. However, we also want to allow for enough of an mTOR rebound to allow for an unmitigated anabolic bounce. We want our pulse of autophagy stimulation while allowing mTOR to rebound and get the benefits of anabolism.
When we zoom out beyond our individual workouts, we also see a trend as we get older in which we become less sensitive to anabolic stimuli.
Rapamycin and Muscle Preservation as We Age
As we age, our bodies exhibit a reduced capacity to construct new muscle protein, even in the presence of anabolic stimuli like resistance exercise or protein intake. The precise molecular mechanisms behind anabolic resistance are still unclear. Emerging evidence from the lab of Dr. David J. Glass, MD, in a paper titled, "Partial Inhibition of mTORC1 in Aged Rats Counteracts the Decline in Muscle Mass and Reverses Molecular Signaling Associated with Sarcopenia" suggests that the dysregulation of the mTOR pathway may be a cause.
To grasp the significance of anabolic resistance, it is essential to comprehend its core concept. Anabolic resistance refers to the diminished ability of the body to construct fresh muscle protein, despite the presence of anabolic stimuli, such as resistance training and protein intake. This phenomenon is intricately intertwined with the process of aging, which brings us closer to the question of why anabolic resistance becomes a prevalent feature as the years pass.
One compelling theory proposes that chronic elevation of mTOR plays a pivotal role in the development of anabolic resistance. The idea is that the machinery responsible for controlling cellular size reaches its maximum capacity as we age, rendering us less responsive to protein intake or exercise. In essence, the heightened mTOR activity in aging individuals impedes their ability to further activate mTOR in response to anabolic stimuli—leading to what could be referred to as an mTOR insensitivity to stimuli.
Work conducted in Dr. Glass's laboratory offers compelling support for this hypothesis. Through experiments, his team observed a progressive increase in the basal (fasted) activity of RPS6, a downstream target of mTOR, across the lifespan—indicating an increase in mTOR activity with age.
The age-related increase in mTOR signaling coincided with a decrease in muscle mass. Though muscle loss at that age is not a surprise, the coincidence of this loss with the elevated mTOR activation was quite unexpected, given that it favors muscle growth and hypertrophy.
The same study administered rapamycin, a pharmacological agent known to inhibit mTOR, for a duration of six weeks. The outcomes were fascinating. The mTOR inhibition led to a restoration of mTOR signaling intermediates and a reversal of sarcopenia—the harmful loss of muscle mass, strength, and function that afflicts many older adults.
Sarcopenia, a gradual and progressive condition that primarily emerges in middle age, exacerbates with time, leaving individuals more susceptible to frailty and diminished quality of life. By attenuating mTOR activity to "youthful" levels through the use of rapamycin, it seems that we may be able to mitigate the effects of sarcopenia and rekindle the body's muscle-building capabilities.
The research team also examined how mTOR inhibition affected the appearance and health of the muscle tissue. The researchers used different doses of mTOR inhibitors, referred to as LD (low dose) and HD (high dose), to see which was more effective. The low-dose treatment turned out to be the key. It led to increased muscle mass in aged rats and more healthy muscle morphology, with other positive molecular changes supporting this growth.
To understand these changes, the scientists performed detailed analyses of the muscle tissue, using techniques like staining with hematoxylin and eosin (H&E). This allowed them to visualize the muscle fibers' structure, comparing healthy young rats with older, sarcopenic ones. They used histology samples of 9 month-year-old rats as an example of healthy tissue and compared them to the samples 24 month-year-old muscle samples to see the progression of aging on and off of rapamycin.
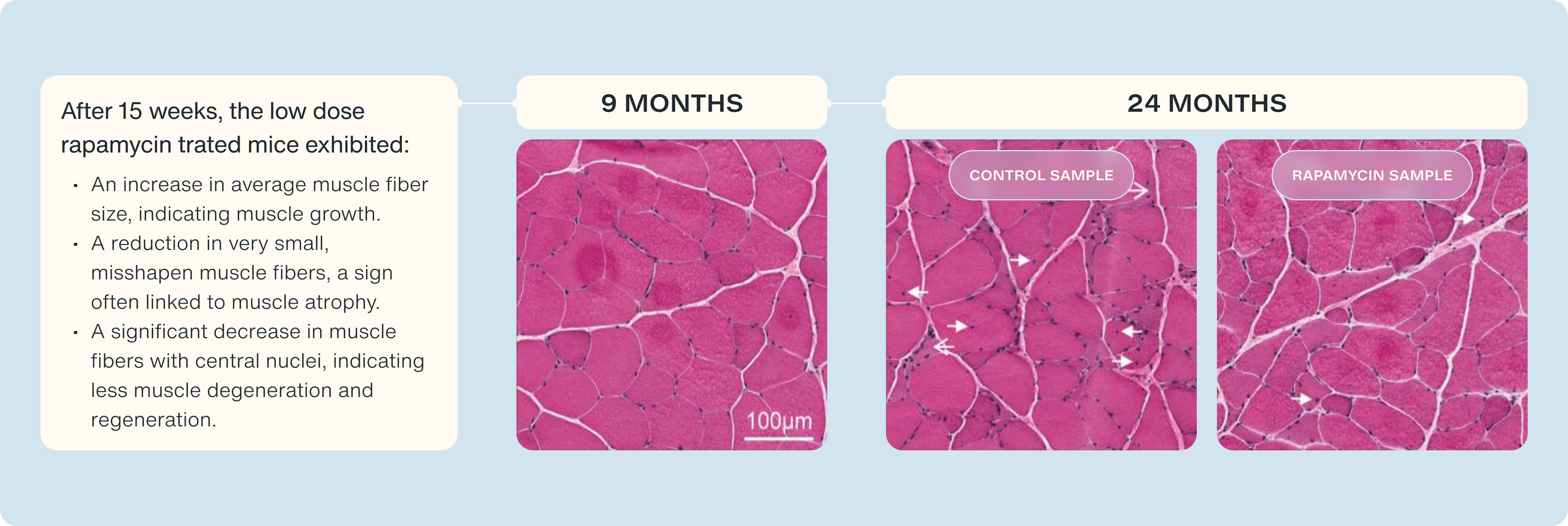
What did they find?
- Healthy Muscle: Tissue from 9-month-old rats showed normal, healthy muscle characteristics.
- Distressed Muscle: In older rats not treated with rapamycin, they detected signs of distressed and degenerated muscle.
Improvement with rapamycin: In the low-dose RAD001-treated rats, they observed:
- An increase in average muscle fiber size, indicating muscle growth.
- A reduction in very small, misshapen muscle fibers, a sign often linked to muscle atrophy.
- A significant decrease in muscle fibers with central nuclei, indicating less muscle degeneration and regeneration.
The high-dose rapamycin treatment did not provide these benefits, emphasizing the importance of finding the correct dose. We see how small pulses of mTOR inhibition, help maintain or anabolic sensitivity and preserve our capacity to grow muscle.
Cross Signals: Exogenous Hormones (Testosterone) and Rapamycin
As testosterone replacement therapy (TRT) becomes more widespread, questions arise about how the anabolic effects of testosterone might interact with the catabolic effects of rapamycin, particularly regarding mTOR inhibition. While empirical data are lacking, a theoretical exploration can provide insights into potential outcomes of these combined therapies.
Interactions at Physiological and Supraphysiological Levels
When testosterone is used as replacement therapy to restore normal physiological levels in men with low testosterone, the interaction with rapamycin presents a complex scenario. Theoretically, normalizing testosterone levels could support the body's natural growth and maintenance functions without significantly conflicting with the intermittent mTOR inhibition induced by rapamycin. In this context, testosterone may not necessarily negate the benefits of rapamycin; instead, it could complement rapamycin's role by maintaining essential anabolic functions, such as muscle maintenance and recovery, which are crucial for overall health.
Conversely, if testosterone is administered at supraphysiological levels to aggressively promote muscle growth, it may indeed counteract the effects of mTOR inhibition by rapamycin. High levels of testosterone can excessively activate anabolic pathways, potentially overwhelming the catabolic processes induced by rapamycin and leading to a state where cellular signals are mixed, or "crossed." This could diminish the effectiveness of both agents, as the body receives conflicting signals about whether to build up or break down cellular components.
Cycling Rather Than Concurrent Use
Given these complexities, a strategic approach might involve cycling between these therapies rather than using them concurrently. By timing the administration of rapamycin and testosterone so that their active phases do not overlap, one might optimize the benefits derived from each. For instance, employing rapamycin during periods of rest or lower physical activity could maximize its catabolic and autophagic benefits, while testosterone could be used during active training periods to enhance muscle synthesis and strength.
Looking Ahead
As we explore the intersection of catabolic and anabolic processes through compounds like Rapamycin and testosterone, several promising future research areas emerge, poised to unlock the secrets to healthier longevity. Long-term randomized controlled trials are essential to assess the safety and efficacy of combining Rapamycin and testosterone, evaluating various dosing regimens, treatment durations, and potential side effects. Understanding the long-term impacts through these studies will help develop safe and effective guidelines. Moreover, in-depth mechanistic studies are needed to elucidate the molecular interactions of Rapamycin and testosterone, focusing on specific cellular pathways influenced by each compound and how these pathways converge or diverge when used together. This knowledge will be crucial for optimizing treatment protocols and minimizing adverse effects.
Investigating how lifestyle factors such as diet, exercise, and stress management interact with Rapamycin and testosterone treatments can provide a holistic approach to longevity. Studies could examine how different diets or exercise regimens influence the efficacy of these compounds. Developing age-specific protocols that consider the unique metabolic and physiological changes associated with aging is also crucial. Younger individuals may benefit from different dosing schedules and lifestyle interventions compared to older adults, enhancing the effectiveness of treatments across age groups.
- Blagosklonny MV. TOR-driven aging: speeding car without brakes. Cell Cycle. 2009 Dec 15;8(24):4055-9. doi: 10.4161/cc.8.24.10310. Epub 2009 Dec 9. PMID: 19923900.
- Mikhail V. Blagosklonny (2006) Aging and Immortality: Quasi-Programmed Senescence and Its Pharmacologic Inhibition, Cell Cycle, 5:18, 2087-2102, DOI: 10.4161/ cc.5.18.3288
- Granholm AC, Hamlett ED. The Role of Tau Pathology in Alzheimer's Disease and Down Syndrome. J Clin Med. 2024 Feb 27;13(5):1338. doi: 10.3390/jcm13051338. PMID: 38592182; PMCID: PMC10932364.
- Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010 Apr 23;285(17):13107-20. doi: 10.1074/jbc.M110.100420. Epub 2010 Feb 23. PMID: 20178983; PMCID: PMC2857107.
- Oddo S. The role of mTOR signaling in Alzheimer disease. Front Biosci (Schol Ed). 2012 Jan 1;4(3):941-52. doi: 10.2741/s310. PMID: 22202101; PMCID: PMC4111148.
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010 Apr 1;5(4):e9979. doi: 10.1371/journal.pone.0009979. Erratum in: PLoS One. 2011;6(11). doi:10.1371/annotation/05c1b976-7eab-4154-808d-0526e604b8eb. PMID: 20376313; PMCID: PMC2848616.
- Blagosklonny MV. Cancer prevention with rapamycin. Oncotarget. 2023 Apr 14;14:342-350. doi: 10.18632/oncotarget.28410. PMID: 37057884; PMCID: PMC10103596.
- Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PloS One. 2011;6(9):e25416.
- Hua Y, Zhang J, Liu Q, Su J, Zhao Y, Zheng G, Yang Z, Zhuo D, Ma C, Fan G. The Induction of Endothelial Autophagy and Its Role in the Development of Atherosclerosis. Front Cardiovasc Med. 2022 Mar 23;9:831847. doi: 10.3389/fcvm.2022.831847. PMID: 35402552; PMCID: PMC8983858.
- Lin CW, Zhang H, Li M, Xiong X, Chen X, Chen X, Dong XC, Yin XM. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol. 2013 May;58(5):993-9. doi: 10.1016/j.jhep.2013.01.011. Epub 2013 Jan 20. PMID: 23339953; PMCID: PMC3634371.
- Bibee KP, Cheng YJ, Ching JK, Marsh JN, Li AJ, Keeling RM, Connolly AM, Golumbek PT, Myerson JW, Hu G, Chen J, Shannon WD, Lanza GM, Weihl CC, Wickline SA. Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function. FASEB J. 2014 May;28(5):2047-61. doi: 10.1096/fj.13-237388. Epub 2014 Feb 5. PMID: 24500923; PMCID: PMC3986846.
- Brown NF, Stefanovic-Racic M, Sipula IJ, Perdomo G. The mammalian target of rapamycin regulates lipid metabolism in primary cultures of rat hepatocytes. Metabolism. 2007;56:1500–1507.
- Baumeier, C., Kaiser, D., Heeren, J., Scheja, L., John, C., Weise, C., Eravci, M., Lagerpusch, M., Schulze, G., Joost, H. G., Schwenk, R. W., & Schürmann, A. (2015). Caloric restriction and intermittent fasting alter hepatic lipid droplet proteome and diacylglycerol species and prevent diabetes in NZO mice. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1851(5), 566–576. https://doi.org/10.1016/j.bbalip.2015.01.013
- Handelsman, D. J., Hirschberg, A. L., & Bermon, S. (2018). Circulating Testosterone as the Hormonal Basis of Sex Differences in Athletic Performance. Endocrine reviews, 39(5), 803–829. https://doi.org/10.1210/er.2018-00020
- Far, H. R., Ågren, G., & Thiblin, I. (2012). Cardiac hypertrophy in deceased users of anabolic androgenic steroids: An investigation of autopsy findings. Cardiovascular Pathology, 21(4), 312–316.
- Montisci, M., El Mazloum, R., Cecchetto, G., et al. (2012). Anabolic androgenic steroids abuse and cardiac death in athletes: Morphological and toxicological findings in four fatal cases. Forensic Science International, 217(1-3), e13–e18.
- Sculthorpe, N., Grace, F., Jones, P., & Davies, B. (2010). Evidence of altered cardiac electrophysiology following prolonged androgenic anabolic steroid use. Cardiovascular Toxicology, 10(4), 239–243.
- Strasser B, Volaklis K, Fuchs D, Burtscher M. Role of Dietary Protein and Muscular Fitness on Longevity and Aging. Aging Dis. 2018 Feb 1;9(1):119-132. doi: 10.14336/AD.2017.0202. PMID: 29392087; PMCID: PMC5772850.
- Fiatarone, M. A., O'Neill, E. F., Ryan, N. D., Clements, K. M., Solares, G. R., Nelson, M. E., Roberts, S. B., Kehayias, J. J., Lipsitz, L. A., & Evans, W. J. (1994). Exercise training and nutritional supplementation for physical frailty in very elderly people. The New England Journal of Medicine, 330(25), 1769–1775. https://doi.org/10.1056/NEJM199406233302501
- Bautmans, I., Van Puyvelde, K., & Mets, T. (2009). Sarcopenia and functional decline: Pathophysiology, prevention and therapy. Acta Clinica Belgica, 64, 303–316.
- Breen L, Stokes KA, Churchward-Venne TA, Moore DR, Baker SK, Smith K, Atherton PJ, Phillips SM. Two weeks of reduced activity decreases leg lean mass and induces "anabolic resistance" of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab. 2013 Jun;98(6):2604-12. doi: 10.1210/jc.2013-1502. Epub 2013 Apr 15. PMID: 23589526.
- Basaria S, Wahlstrom JT, Dobs AS. Clinical review 138: Anabolic-androgenic steroid therapy in the treatment of chronic diseases. J Clin Endocrinol Metab. 2001 Nov;86(11):5108-17. doi: 10.1210/jcem.86.11.7983. PMID: 11701661.
- Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013 Sep;280(17):4294-314. doi: 10.1111/febs.12253. Epub 2013 Apr 17. PMID: 23517348.
- Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013 Mar;123(3):966-72. doi: 10.1172/JCI64098. Epub 2013 Mar 1. PMID: 23454759; PMCID: PMC3582125.
- Roubenoff, R. (1999). The pathophysiology of wasting in the elderly. The Journal of Nutrition, 129(1S Suppl), 256S–259S.