Targeting the Root Causes of Weight Gain: A Systems Framework for Metabolic Health, Muscle Preservation, and Longevity
Mitochondrial Efficiency Drives Energy Balance and BMR: Basal metabolic rate (BMR) accounts for 60-70% of daily energy expenditure, powering essential processes like protein synthesis, ion transport, and mitochondrial activity. Mitochondria serve as metabolic engines, converting nutrients into ATP with varying efficiency. Optimal mitochondrial function maximizes energy utilization, while mitochondrial dysfunction leads to energy loss, reduced ATP production, and oxidative stress, promoting fat storage over energy expenditure.
Hormonal Resistance Disrupts Energy Homeostasis: Chronic overconsumption of refined carbohydrates drives insulin resistance, where cells “tune out” insulin signals, leading to excess glucose storage as fat. Similarly, leptin resistance arises in obesity, where the brain fails to recognize satiety signals despite high leptin levels. Meanwhile, chronic stress and poor sleep amplify ghrelin secretion, sending false hunger cues and exacerbating overeating and fat accumulation.
Adipose Tissue Acts as a Metabolic "Command Center" but Becomes Dysregulated in Obesity: Adipose tissue is not merely an energy storage site but functions as a dynamic endocrine organ, secreting signaling molecules (adipokines and cytokines) that regulate energy balance, insulin sensitivity, and immune responses. However, in obesity, adipocytes produce elevated levels of pro-inflammatory cytokines (e.g., TNF-α, IL-6), triggering chronic, low-grade inflammation. This miscommunication disrupts metabolic regulation, perpetuating insulin resistance, fat accumulation, and systemic dysfunction.
Chronic Inflammation in Adipose Tissue Has Systemic Consequences: Expanding adipose tissue attracts pro-inflammatory macrophages (M1 phenotype) that amplify inflammation, creating a self-reinforcing loop of mitochondrial dysfunction, reduced fatty acid oxidation, and excess lipid accumulation. These inflammatory signals ripple through the body, impairing insulin signaling in the liver and skeletal muscle, leading to hyperglycemia, dyslipidemia, and increased risk of conditions such as type 2 diabetes, cardiovascular disease, and non-alcoholic fatty liver disease (NAFLD).
Physical inactivity impairs energy regulation by reducing AMPK activation, a key cellular energy sensor that promotes fatty acid oxidation and mitochondrial biogenesis. Without regular movement to “flip the switch,” AMPK remains inactive, slowing the body’s energy systems. This inactivity reduces the ability to burn fat efficiently, increasing the likelihood of surplus energy being stored as triglycerides, which over time exacerbates weight gain and metabolic dysfunction.
GLP-1 and Semaglutide Improve Blood Sugar and Promote Weight Loss: GLP-1 is a hormone that regulates blood sugar and appetite by stimulating insulin release, inhibiting glucagon, and slowing gastric emptying to promote satiety. GLP-1 receptor agonists like Semaglutide mimic these effects, supporting significant weight loss, improved glycemic control, and reduced caloric intake through mechanisms like prolonged fullness and stabilized blood sugar.
Semaglutide Demonstrates Long-Term Efficacy in Weight Management and Metabolic Health: Clinical trials, such as the STEP program, show that Semaglutide leads to durable weight loss (e.g., 15 kg over 68 weeks) and improves key metabolic markers like HbA1c, lipid profiles, and blood pressure. Its comprehensive effects, combined with increasing accessibility through oral formulations, solidify Semaglutide as a leading therapy for weight management and cardiometabolic health.
GLP-1 RAs Effectively Reduce Fat Mass but Also Lead to Lean Mass Loss: While GLP-1 receptor agonists (GLP-1 RAs) significantly reduce fat mass and improve metabolic health, clinical data show that 20-30% of the weight lost comes from lean body mass, including muscle tissue. This unintended muscle loss raises concerns about its long-term impact on metabolic function, physical performance, and overall health.
Body Composition Studies Highlight Variability in Fat and Lean Mass Loss: DEXA scans provide precise measurements of changes in body composition, showing that earlier studies reported up to 40% lean mass loss during weight reduction with GLP-1 RAs. However, more recent trials indicate improved outcomes, with up to 85% of weight loss coming from fat mass and minimal lean tissue loss (~11%), suggesting progress in promoting more targeted fat reduction.
Muscle is Essential for Metabolic Health and Energy Balance: Skeletal muscle acts as a metabolic furnace, driving resting energy expenditure through its high mitochondrial density and serving as the primary site for insulin-mediated glucose uptake. Loss of muscle mass reduces basal metabolic rate (BMR), slows energy expenditure, and diminishes glucose-buffering capacity, increasing the risk of weight regain and counteracting the metabolic benefits of GLP-1 RAs.
Muscle Loss Impacts Physical Functionality and Long-Term Health: Beyond its metabolic role, muscle provides strength, mobility, and balance, supporting independence and quality of life, particularly in aging individuals. The loss of lean muscle accelerates sarcopenia, leading to frailty, reduced mobility, and increased risk of falls and fractures, which can be further exacerbated by GLP-1 RA-induced muscle loss. Preserving muscle is critical for maintaining metabolic stability and physical resilience across the lifespan.
Caloric Deficits and Lack of Exercise Drive Muscle Loss with GLP-1 RAs: GLP-1 RAs suppress appetite, creating a chronic caloric deficit that leads to a catabolic state where the body breaks down both fat and muscle for energy. The absence of resistance exercise further exacerbates muscle loss, as the body lacks the stimulus needed to maintain lean mass during weight reduction.
Insufficient Protein Intake and Hormonal Changes Exacerbate Muscle Breakdown: Reduced appetite caused by GLP-1 RAs often leads to inadequate protein consumption, impairing the body’s ability to balance muscle protein synthesis and breakdown. Elevated cortisol levels during caloric deficits further enhance proteolysis (muscle breakdown), while delayed gastric emptying may reduce amino acid absorption, hindering muscle repair and maintenance.
Weight Gain Is a Symptom of Deeper Systemic Imbalances: Weight gain often reflects underlying issues such as insulin resistance, mitochondrial dysfunction, chronic stress, and exposure to environmental disruptors. Focusing solely on weight as a health metric can obscure the true drivers of metabolic dysfunction, inflammation, and energy regulation imbalances.
Background
Weight loss is often framed as a matter of discipline—a simple equation of consuming fewer calories and increasing physical activity. While these lifestyle interventions are essential and often the first line of defense, they sometimes fail to address the complex, underlying factors driving weight gain. Metabolic dysfunction, hormonal imbalances, chronic inflammation, and behavioral patterns can undermine even the most rigorous diet and exercise regimens. In these cases, we turn to pharmacological options to supplement lifestyle changes and tackle these deeper biological issues.
The rise of GLP-1 receptor agonists has transformed weight management, offering dramatic results for those who have struggled with traditional methods. These drugs, initially developed for diabetes, are now widely used for weight loss and have become one of the most discussed pharmacological interventions in recent years. Yet, their popularity has overshadowed critical questions about their long-term implications. Can these drugs promote weight loss, overall health, and longevity? Do they address the root causes of weight gain, or do they merely mask the symptoms?
In this research review, we will explore the multifaceted nature of weight regulation, analyzing why traditional methods sometimes fall short and how pharmacological interventions like GLP-1 receptor agonists fit into the broader picture. We will also critically examine the limitations of these medications, particularly in the context of longevity and sustainable health outcomes. By reframing weight gain as a symptom of systemic dysregulation, this review provides a holistic perspective on achieving sustainable metabolic health, preserving muscle mass, and promoting longevity.
Beyond Calories: The Science of Weight Gain
You may be familiar with the idea that weight gain stems from an energy imbalance—when calorie intake surpasses the energy the body expends. While this concept appears straightforward, it is governed by a complex interplay of physiological, cellular, and biochemical mechanisms. At its core, weight gain manifests as an increase in adipose tissue, the body’s primary energy reservoir.
Excess calories are converted into triglycerides through lipogenesis and stored within specialized cells known as adipocytes. Initially, these adipocytes accommodate surplus energy by expanding in size, a process called hypertrophy. If the energy surplus persists, pre-adipocytes are recruited and differentiated into new fat cells, leading to hyperplasia, or an increase in adipocyte number.
However, this energy storage process is far from uniform and is influenced by a combination of genetic predisposition, hormonal signaling (such as insulin, leptin, and cortisol), and environmental factors like diet, physical activity, and exposure to obesogenic chemicals.
At the foundation of this equation lies basal metabolic rate (BMR)—the energy the body requires to sustain essential physiological processes at rest. In the next section, we’ll explore how BMR influences overall energy utilization, the role mitochondria play in converting energy to fuel, and how inefficiencies in these processes can tip the scales toward weight gain and metabolic dysfunction.
Basal Metabolic Rate and Energy Utilization
Approximately 60-70% of daily energy expenditure arises from the basal metabolic rate (BMR). Think of BMR as the “idling energy” needed to keep the body’s engine running even when it’s parked. This baseline energy powers critical processes such as sustaining ion gradients across cell membranes, synthesizing and repairing proteins, and maintaining mitochondrial activity.
At the cellular level, mitochondria serve as the body’s metabolic engines, driving energy production by converting macronutrients into adenosine triphosphate (ATP)—the primary fuel that powers cellular functions. Much like a car engine, the efficiency of mitochondrial function determines how effectively “fuel” (nutrients) is converted into usable energy (ATP), with minimal “waste” in the form of harmful oxidative byproducts. When mitochondria operate optimally, they ensure energy is utilized efficiently rather than being stored as fat, establishing them as a cornerstone of energy balance and body composition. [1]
However, not all engines run at peak performance. BMR varies significantly among individuals. A key determinant of this variability is mitochondrial efficiency. When functioning efficiently, mitochondria produce ATP with minimal energy loss, akin to a well-tuned engine achieving optimal fuel economy. In contrast, mitochondrial dysfunction disrupts this process. Inefficient oxidative phosphorylation—where mitochondria produce ATP—results in greater energy loss, reduced ATP production, and increased oxidative stress through the generation of reactive oxygen species (ROS). Critically, this “metabolic inefficiency” favors energy storage over utilization, subtly shifting the balance toward fat accumulation over time. [1, 2]
Excess energy is stored in adipose tissue, primarily in the form of triglycerides. Initially, this occurs through hypertrophy, where existing adipocytes expand like overfilled reservoirs. Once their storage capacity is maxed out, adipocyte hyperplasia occurs—new fat cells are generated to handle the surplus. While this process is an evolutionary adaptation designed to store energy for times of scarcity, persistent caloric excess drives excessive fat accumulation, particularly in visceral adipose tissue. This is particularly relevant to the standard American diet, which constantly floods the system with a caloric surplus. More than simply leading to fat accumulation, this leads to a cascade of metabolic health harm—triggering insulin resistance, chronic inflammation, and other hallmarks of metabolic dysfunction. [1, 2]
The role of mitochondria as metabolic engines will remain central to our discussion. Their efficiency—or lack thereof—not only influences BMR and energy utilization but also shapes body composition and long-term metabolic health. However, mitochondrial function does not operate in isolation. It is tightly regulated by intricate networks of hormonal signals and cellular pathways that act as metabolic traffic controllers, determining how energy is allocated, stored, or expended.
To better understand how these processes shape body composition and fat accumulation, let’s turn our focus to the critical roles of hormonal regulation and cellular signaling—two key drivers that orchestrate metabolism and influence energy balance at every level of the body.
Hormonal Regulation and Cellular Signaling
Hormones such as insulin, leptin, and ghrelin act as critical biochemical messengers, orchestrating the regulation of appetite, energy storage, and expenditure to maintain energy homeostasis. These hormones function like a well-coordinated traffic control system, directing signals between the brain, peripheral tissues, and metabolic pathways to ensure that energy intake aligns with expenditure. When this signaling system becomes disrupted, the result is metabolic gridlock—a breakdown that often underlies disorders and challenges in weight management. [3]
Insulin, a peptide hormone secreted by pancreatic beta cells in response to elevated blood glucose levels, serves as the body’s gatekeeper of energy regulation. It facilitates glucose uptake into cells, where glucose is either immediately burned for energy or stored as glycogen in liver and muscle tissue—much like a well-managed fuel supply.
However, chronic overconsumption of carbohydrates, particularly refined sugars and starches, leads to persistently elevated insulin levels. Over time, this constant "knocking" on the cells’ doors overwhelms the system. Initially, insulin acts like a key, allowing glucose into cells for energy or storage. But with chronically high insulin levels, cells begin to "tune out the knocking," as if the lock no longer responds. This diminished response—known as insulin resistance—leaves glucose trapped in the bloodstream, disrupting energy balance and triggering downstream effects like fat accumulation and metabolic dysfunction. [3]
At the cellular level, insulin resistance disrupts normal glucose homeostasis, leaving excess glucose to accumulate in the bloodstream. Unable to enter cells efficiently, surplus glucose is diverted into de novo lipogenesis—akin to sending unused fuel to long-term storage tanks, where it becomes triglycerides housed in adipose tissue. Over time, insulin resistance acts like a traffic bottleneck, slowing the flow of glucose while fat synthesis accelerates behind the scenes. Key lipogenic enzymes, such as acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS), intensify activity to funnel excess energy into fat stores.
This creates a vicious metabolic cycle, where expanding fat reserves (via adipocyte hypertrophy) generate inflammatory signals, further straining the system. Much like an overburdened warehouse struggling with incoming deliveries, metabolic processes are overwhelmed, exacerbating insulin resistance and advancing metabolic dysfunction. This vicious cycle contributes to the growth of adipose tissue and the development of metabolic conditions such as type 2 diabetes and non-alcoholic fatty liver disease (NAFLD). [3]
While insulin governs glucose uptake and energy storage, another key player in the regulation of energy balance is leptin—a hormone intricately linked to fat stores and appetite control. Leptin is secreted by adipocytes. In doing so it signals to the hypothalamus—a critical energy-regulating center in the brain—that fat stores are sufficient. This rise of leptin instructs the hypothalamus to suppress appetite and increase energy expenditure to prevent excessive energy storage. In this way, leptin acts as a satiety signal, ensuring energy intake aligns with the body’s needs.
However, in individuals with excess adiposity, leptin levels remain chronically elevated due to the abundance of fat tissue. Paradoxically, the brain becomes less responsive to these elevated signals, a condition known as leptin resistance. Much like insulin resistance, leptin resistance represents a breakdown in the communication loop between tissues—in this case, the brain’s inability to properly interpret satiety cues. As a result, the hypothalamus fails to suppress appetite and stimulate energy expenditure, creating a metabolic environment that promotes continued energy intake and further fat accumulation.
The underlying mechanisms of leptin resistance are multifactorial and remain an active area of research. Current evidence suggests a role for chronic inflammation, particularly within the hypothalamus, which may disrupt leptin receptor signaling. Additionally, elevated levels of free fatty acids, oxidative stress, and inflammatory cytokines appear to interfere with leptin transport across the blood-brain barrier and impair downstream signaling pathways within hypothalamic neurons. [3]
While leptin signals satiety and energy sufficiency, its counterpart, ghrelin, plays an opposing role in energy regulation by stimulating hunger and energy conservation. Ghrelin, often referred to as the “hunger hormone,” is primarily secreted by specialized cells in the stomach and acts on the hypothalamus to stimulate appetite and promote energy storage. Ghrelin levels rise during periods of fasting, peaking just before meals to signal the body to initiate food intake. Once food is consumed, ghrelin secretion declines, providing a counterbalance to leptin, which signals satiety and promotes energy expenditure. This interplay between ghrelin and leptin helps maintain energy balance under normal conditions.
Ghrelin's effects extend beyond appetite stimulation: it also promotes fat storage by influencing energy conservation mechanisms. Think of ghrelin as a fuel gauge in the body—it signals when energy reserves are running low and prompts the system to refuel by increasing hunger and slowing energy expenditure. However, chronic dysregulation of this "fuel gauge" can throw the system off balance. Irregular eating patterns, chronic stress, and sleep deprivation are known to elevate ghrelin levels or heighten sensitivity to its signals. In these cases, the body receives overly loud hunger cues, prolonging the drive to eat and creating a physiological environment that encourages overconsumption and weight gain.
Conversely, suppressed ghrelin signaling—seen in certain metabolic or post-surgical conditions—resembles a fuel gauge that fails to register empty, impairing appetite regulation. This can lead to reduced caloric intake and disruptions in energy balance, as the body’s ability to respond appropriately to energy needs is diminished. Beyond its immediate role in hunger, ghrelin also interacts with pathways involved in reward processing and energy metabolism, underscoring its broader influence on feeding behavior and fat storage. [3]
The interplay between insulin, leptin, and ghrelin highlights the delicate balance of hormonal regulation in energy metabolism—a balance that resembles a three-way communication system designed to manage fuel availability, storage, and use. When functioning properly, insulin acts as the energy gatekeeper, leptin serves as the satiety signal, and ghrelin operates as the fuel gauge, together ensuring energy intake, storage, and expenditure remain synchronized.
In individuals with obesity, this communication system often breaks down. Insulin resistance and leptin resistance frequently coexist, diminishing the body’s ability to recognize satiety cues and regulate glucose uptake, exacerbating appetite dysregulation and fat accumulation. At the same time, chronic stress and inadequate sleep can amplify ghrelin secretion, akin to a fuel gauge sending false “empty” signals, while impairing leptin sensitivity and further driving hunger and energy storage. Addressing hormonal dysregulation through lifestyle interventions, such as dietary modifications, stress management, and sleep quality improvement, is essential for restoring metabolic balance and supporting healthy weight management.
Adipose Tissue as an Endocrine Organ
Adipose tissue is far more than an inert energy storage depot; it functions as a dynamic endocrine organ that plays a central role in metabolic regulation. Much like a command center, it communicates with the rest of the body by secreting a variety of bioactive molecules known as adipokines and cytokines. These signaling molecules influence energy balance, insulin sensitivity, and immune responses, ensuring the body adapts to changing metabolic demands.
However, when adipocytes expand in response to chronic excess energy intake, this communication system shifts into overdrive, producing elevated levels of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). This heightened signaling creates a state of chronic, low-grade inflammation, a hallmark of obesity.
TNF-α, for instance, acts like a disruptive interference signal, impairing insulin signaling by targeting the insulin receptor substrate (IRS) pathway. This interference reduces glucose uptake into cells, exacerbating insulin resistance. Similarly, IL-6 contributes to metabolic dysfunction by dysregulating both glucose and lipid metabolism, further tipping the balance toward energy storage and metabolic imbalance.
In this way, what begins as a normal adaptation to energy surplus can turn into a miscommunication network, where inflammatory signals override metabolic regulation, perpetuating a cycle of insulin resistance, inflammation, and fat accumulation. [2]
As adipose tissue expands, it becomes a hotbed of immune activity, attracting macrophages, key immune cells that adapt to their surrounding environment. In lean individuals, most macrophages in adipose tissue exhibit an anti-inflammatory M2 phenotype, functioning like maintenance workers that support tissue repair and balance. However, in obesity, macrophages shift toward a pro-inflammatory M1 phenotype, acting more like alarm responders. These activated M1 macrophages release additional cytokines, including tumor necrosis factor-alpha (TNF-α) and monocyte chemoattractant protein-1 (MCP-1), which recruits even more immune cells. This infiltration sets off a feed-forward loop of inflammation, progressively worsening local tissue dysfunction and contributing to systemic metabolic disturbances. [2]
The inflammatory environment within expanding adipose tissue also creates cellular stress, placing significant strain on mitochondrial function. Elevated levels of reactive oxygen species (ROS)—highly reactive byproducts of chronic inflammation—act like corrosive exhaust, damaging mitochondrial DNA and proteins. This damage impairs the mitochondria's ability to efficiently generate energy through oxidative phosphorylation, much like an engine losing power due to wear and tear. With reduced mitochondrial efficiency, the capacity for fatty acid oxidation declines, causing excess lipids to accumulate further in tissues. [2]
This cycle of inflammation, mitochondrial dysfunction, and reduced energy expenditure forms a self-reinforcing loop—akin to a system stuck in a downward spiral—that accelerates the progression of obesity and metabolic disease.
Beyond its localized effects, the dysfunction of adipose tissue sends ripples throughout the body, creating systemic consequences. Chronic inflammatory signals originating from adipose tissue act like disruptive broadcasts, interfering with normal metabolic processes in peripheral tissues such as the liver and muscle. In the liver, this inflammatory signaling contributes to insulin resistance, reducing glucose uptake and driving hyperglycemia. In skeletal muscle, a primary site of glucose utilization, impaired insulin signaling compounds this effect, leading to systemic glucose imbalances and dyslipidemia with elevated circulating triglycerides and fatty acids.
Additionally, adipose tissue overproduces adipokines such as leptin and resistin, which act as misaligned signals, further disrupting energy balance and exacerbating metabolic dysregulation. Leptin, once a reliable indicator of energy sufficiency, becomes ineffective due to leptin resistance, while resistin is linked to impaired glucose metabolism and insulin signaling. These metabolic disruptions act in concert, underscoring how adipose tissue dysfunction serves as a metabolic hub that drives obesity-related diseases.
The consequences are far-reaching: this systemic imbalance lays the groundwork for the development of type 2 diabetes, cardiovascular disease, and non-alcoholic fatty liver disease (NAFLD). Thus, inflamed and dysfunctional adipose tissue behaves less like a passive energy depot and more like a malfunctioning control center, driving inflammatory and metabolic disturbances throughout the body. [2]
Environmental and Lifestyle Drivers of Energy Dysregulation and Metabolic Dysfunction
Modern environments are filled with factors that disrupt the intricate balance of energy regulation at the cellular level, acting like constant stressors on the body’s metabolic machinery. Diets dominated by processed, calorie-dense foods flood the system with excess glucose and lipids, much like overfilling a reservoir with poor-quality fuel. While encouraging fat storage, these foods are often devoid of the essential micronutrients needed to support critical processes such as mitochondrial efficiency and cellular repair. This nutrient imbalance promotes adiposity and compromises the body’s ability to maintain optimal cellular function.
The absence of physical activity compounds these metabolic challenges by reducing the activation of AMP-activated protein kinase (AMPK), a key cellular energy sensor. AMPK functions like a master switchboard for energy regulation, activating pathways that promote fatty acid oxidation and mitochondrial biogenesis—processes that enable cells to efficiently burn fat and generate energy. [2]
Without regular movement to “flip the switch”, AMPK remains inactive, and the body’s energy systems slow down. This inactivity is akin to leaving a power plant idle, reducing its capacity to burn fuel effectively. As a result, cells become less efficient at utilizing fatty acids for energy, leading to an increased tendency to store surplus energy as triglycerides. Over time, this shift tips the energy balance toward weight gain and exacerbates metabolic dysfunction. [2]
Chronic stress and poor sleep, both hallmarks of modern life, place additional strain on the body by elevating levels of cortisol, a hormone that plays a central role in energy regulation. Under prolonged stress, cortisol acts like a misguided traffic director, rerouting fat storage toward visceral adipose tissue—the fat surrounding internal organs.
Unlike subcutaneous fat, visceral fat behaves like a metabolic troublemaker, releasing pro-inflammatory cytokines that act as distress signals. These signals impair insulin signaling and compromise mitochondrial function, much like smoke clogging the gears of a finely tuned machine. The resulting inflammatory cascade disrupts energy homeostasis, driving insulin resistance and amplifying fat accumulation. Over time, this feedback loop exacerbates metabolic dysfunction, further tipping the balance toward weight gain. [2]
These biological disruptions are often compounded by socioeconomic barriers. Limited access to fresh, nutrient-dense foods and safe environments for physical activity creates a systemic “obstacle course” that makes it increasingly difficult to achieve and maintain a healthy weight. Together, these interconnected factors form a complex web of energy dysregulation, underscoring the need for holistic approaches that address not only biology but also the broader societal determinants of metabolic health.
Epigenetics and Nutrient-Gene Interactions
Emerging research underscores the profound influence of the epigenetic layer—a dynamic system of molecular modifications that regulates gene activity without altering the underlying DNA sequence—on weight regulation and metabolic health. If DNA is the body’s instruction manual, epigenetic modifications function like annotations or bookmarks, telling cells which genes to read, highlight, or silence in response to internal and external cues. These modifications fine-tune how the genetic code is expressed, allowing for adaptability without permanent changes to the genome itself. [1]
The key mechanisms driving epigenetic regulation include DNA methylation, histone modification, and non-coding RNAs:
- DNA methylation involves the addition of methyl groups to specific DNA regions, effectively “locking” genes into silence, much like placing a padlock on certain chapters of the genetic instruction manual.
- Histone modification, on the other hand, alters how tightly DNA is wound around histone proteins. Loosely packed regions are “open for reading”, enabling gene activation, while tightly packed regions are effectively “closed”, suppressing gene expression.
- Non-coding RNAs, like microRNAs, act as post-transcriptional regulators, guiding how much protein is produced from a given gene—akin to fine-tuning the volume on a speaker.
Environmental factors such as dietary patterns, stress, and exposure to toxins act as epigenetic influencers, triggering these mechanisms to regulate genes involved in lipid metabolism, inflammation, and hunger signaling. For example, diets rich in highly processed foods and poor in micronutrients can induce changes like hypermethylation or histone acetylation that impair metabolic pathways. [1]
On a cellular level, nutrient deficiencies in essential cofactors such as magnesium, zinc, and B vitamins disrupt these epigenetic processes by interfering with mitochondrial function—the energy powerhouses of cells. Imagine attempting to run a complex engine without critical parts; the result is an inefficient energy system that promotes fat storage while limiting energy expenditure.
What makes the epigenetic layer particularly impactful is its persistence and heritable potential. These modifications are not only maintained over an individual’s lifetime but can also be passed across generations. For instance, poor nutrition or chronic stress during critical developmental windows can imprint epigenetic marks that influence metabolic health in offspring. In essence, the environment "writes notes" on our genes that shape energy regulation and fat storage, leaving a biological legacy that can extend far beyond a single generation. This underscores the enduring impact of lifestyle and environmental factors on body weight, energy regulation, and overall health, highlighting the importance of addressing these influences through thoughtful interventions. [1]
The Complexity of Weight Regulation
Weight gain is far more intricate than simply eating too much or exercising too little. It reflects a cascade of cellular, hormonal, and systemic processes influenced by genetic predisposition, environmental conditions, and behavioral patterns. Adipose tissue dynamics, mitochondrial function, hormone signaling, and inflammatory responses all converge to create a complex web of interactions that drive weight regulation. Understanding and addressing these factors holistically—through a focus on cellular health, hormonal balance, and lifestyle optimization—is essential for managing and preventing weight gain effectively.
Weight Loss Interventions
Understanding the mechanisms behind weight gain naturally leads to the discussion of interventions designed to address it. The most popular of these interventions can be broadly categorized into lifestyle modifications and pharmacological treatments. Lifestyle modifications remain the cornerstone of weight management, focusing on dietary adjustments, physical activity, stress reduction, and sleep optimization. A nutrient-dense diet, rich in whole foods, provides essential cofactors like magnesium, zinc, and B vitamins, supporting mitochondrial function and reducing the likelihood of epigenetic disruptions. Exercise enhances mitochondrial biogenesis, promotes fatty acid oxidation, and improves insulin sensitivity, while stress management and adequate sleep mitigate hormonal imbalances, such as elevated cortisol and ghrelin levels, that contribute to weight gain.
While lifestyle modifications remain the most critical piece in addressing the root causes of weight gain, in recent years pharmacological interventions have become much more popular. These often target specific mechanisms implicated in weight regulation. Some medications enhance insulin sensitivity or modulate hunger hormones to create a more favorable energy balance. Others, such as GLP-1 receptor agonists or semaglutide, mimic the action of incretin hormones, reducing appetite and slowing gastric emptying. GLP-1 receptor agonists (GLP-1 RAs) are among the most popular tools in recent years. While these drugs show remarkable results in facilitating weight loss, their effects are not without complexity, especially when it comes to preserving overall health and metabolic function.
GLP-1 Receptor Agonists
GLP-1 (Glucagon-Like Peptide-1) is a hormone released by specialized cells in the small intestine in response to a meal. It plays a crucial role in regulating blood sugar and appetite by acting on the pancreas, stomach, and brain. GLP-1 stimulates insulin release, facilitating glucose uptake into cells and lowering blood sugar levels. Simultaneously, it inhibits glucagon, a hormone that raises blood sugar levels, helping to maintain glucose homeostasis. Additionally, GLP-1 slows gastric emptying and promotes satiety, reducing appetite and contributing to weight management. [4, 8]
GLP-1 receptor agonists are synthetic drugs that mimic the effects of natural GLP-1. These agents are widely used for managing type II diabetes and have gained prominence in weight-loss treatments. Unlike endogenous GLP-1, receptor agonists resist enzymatic degradation, making them longer-lasting and more effective. Since the first FDA-approved GLP-1 receptor agonist, Exenatide, in 2005, the field has expanded, with Semaglutide emerging as one of the most impactful drugs. Backed by trials such as SUSTAIN, PIONEER, STEP, and OASIS, Semaglutide has demonstrated broad applications in managing glucose levels, reducing appetite, and promoting weight loss. [4]
Semaglutide achieves its effects through a multifaceted approach. By binding to GLP-1 receptors, it stimulates insulin secretion and suppresses glucagon release, improving glycemic control. It also delays gastric emptying, prolonging the feeling of fullness and reducing overall caloric intake. For example, in a placebo-controlled trial, participants taking Semaglutide retained ~37% of their meal in the stomach four hours after ingestion, compared to complete gastric clearance in the placebo group. These effects collectively support significant weight loss by reducing hunger and stabilizing blood sugar levels. [5]
In addition to its effects on appetite and digestion, Semaglutide may modestly enhance energy expenditure. Studies in rodent models suggest that it stimulates hypothalamic neurons involved in metabolic regulation, increasing thermogenesis—the process of burning energy as heat. This combination of reducing energy intake and potentially increasing energy expenditure underscores its comprehensive role in weight management. [6, 7]
Clinical trials have demonstrated the durability of Semaglutide’s benefits over extended periods. For instance, the STEP program showed that participants taking 2.4 mg weekly sustained an average weight loss of 15kg over 68 weeks, alongside improvements in metabolic markers like HbA1c, lipid profiles, and blood pressure. These outcomes highlight Semaglutide’s utility as a weight-loss drug and a tool for improving overall cardiometabolic health. [9]
Despite its efficacy, some patients experience mild gastrointestinal side effects, such as nausea and vomiting, particularly during the initial weeks of treatment. These effects are generally transient and resolve as the body adapts. The development of oral formulations, as seen in the PIONEER trials, further enhances accessibility, offering a convenient alternative to injections. With its strong clinical track record, Semaglutide stands as a leading innovation in weight management therapies. [10]
Balancing the Scales: GLP-1 RAs, Fat Loss, and the Risk of Lean Mass Reduction
GLP-1 receptor agonists (GLP-1 RAs) have transformed weight management by significantly reducing fat mass and improving markers of metabolic health. However, a critical caveat has emerged: these drugs cannot adequately discriminate between fat and lean body mass during weight loss. Some clinical data reveal that 20-30% of the weight lost with GLP-1 RAs comes from lean body mass, including muscle tissue. This unintended consequence is concerning, as it has implications for long-term health, metabolic functionality, and physical performance.
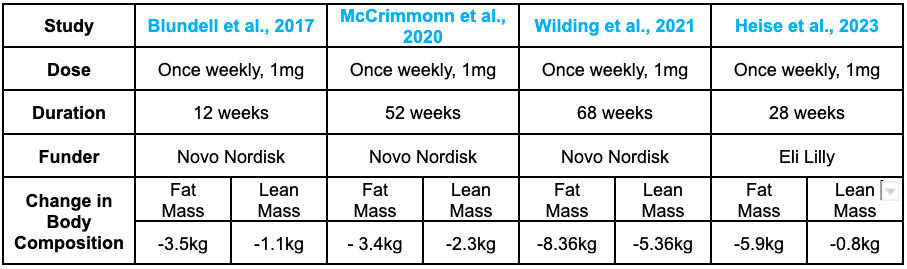
While many GLP-1 RA clinical trials do not measure changes in muscle mass, some studies have assessed body composition using DEXA scans, which stand for Dual-Energy X-ray Absorptiometry. DEXA is a highly accurate imaging technique commonly used to differentiate between fat mass, lean mass (such as muscle), and bone density by measuring how much X-ray is absorbed by different tissues in the body. This allows for a more precise understanding of how weight loss from GLP-1 RAs affects fat and lean tissue separately, providing valuable insights into how these drugs influence body composition beyond just total body weight.
In one recent study utilizing a DEXA scan, initial body composition data from a short-term (12-week) Semaglutide treatment showed a 5 kg change in body mass, with 70% (3.5 kg) of that from fat mass and 22% (1.1 kg) from lean mass. This study highlights the shift toward fat loss while revealing some lean muscle mass loss. Data from the 2020 Novo Nordisk SUSTAIN trial showed a reduction of 5.7 kg in total body mass, with 3.4 kg (60%) from fat mass and 2.3 kg (40%) from lean muscle mass following 52 weeks of Semaglutide at a dose of 1 mg once per week. This trial further underscores that GLP-1 RAs can result in significant fat loss, though muscle loss is also evident. [11]
A follow-up STEP trial led by Professor John Wilding observed a 15.3 kg reduction in body weight, with 8.36 kg (58%) from fat mass and 5.26 kg (34.3%) from lean muscle mass. This suggests that, while fat loss is the primary effect, lean mass loss is still a substantial factor. More recent clinical trials have shown a 6.9 kg reduction in total body mass, with 5.9 kg (85%) from fat mass and only 0.8 kg (11%) from lean mass. These findings indicate that, in newer trials, GLP-1 RAs appear to promote more targeted fat loss with minimal impact on lean tissue. [12]
The Impact of Muscle Mass on Basal Metabolic Rate and Long-Term Energy Regulation
Muscle is a metabolically active tissue that plays a pivotal role in maintaining energy and glucose balance. At rest, skeletal muscle acts like a metabolic furnace, accounting for a substantial proportion of the basal metabolic rate (BMR) due to its high mitochondrial density. These mitochondria serve as cellular power plants converting nutrients into energy and driving resting energy expenditure.
When muscle mass is lost, the “furnace” burns at a lower capacity. This reduction in BMR leads to a decline in resting energy expenditure, creating a metabolic slowdown that favors energy conservation over energy use. The resulting metabolic environment makes individuals more susceptible to weight regain, a process often described as “metabolic adaptation”. In the context of GLP-1 RAs, where significant weight loss is achieved, the unintended loss of lean muscle mass reduces the body’s energy-burning machinery, potentially undermining the long-term sustainability of fat loss. [13]
In addition to its role in energy expenditure, muscle serves as the primary reservoir for insulin-mediated glucose uptake, particularly following meals. Like a metabolic sponge, skeletal muscle absorbs circulating glucose, helping buffer blood sugar levels and maintain glycemic control. A reduction in muscle mass diminishes this glucose-buffering capacity. This is especially concerning for individuals already using GLP-1 RAs to manage diabetes, as the loss of muscle may counteract the drug’s intended benefits of improved glycemic control. [13]
Beyond its metabolic functions, muscle is indispensable for physical functionality and overall quality of life. It provides the strength and structural support needed for mobility, balance, and independence, especially as individuals age. It is a key component of maintaining optimal healthspan. The loss of lean muscle mass during weight loss accelerates the progression of sarcopenia—an age-related condition characterized by muscle wasting and weakness. Sarcopenia is akin to a gradual erosion of the body’s foundation, contributing to frailty, reduced mobility, and an elevated risk of falls and fractures.
For older adults or individuals with pre-existing muscle deficits, the muscle loss associated with GLP-1 RAs can amplify these vulnerabilities, compromising both functional capacity and overall health. Preserving muscle mass is therefore critical—not only for sustaining metabolic stability but also for maintaining physical resilience and independence across the lifespan. [13]
The Causes of Muscle Loss with GLP-1 RAs
Muscle loss associated with GLP-1 RAs arises from both physiological and behavioral factors, with significant contributions from the caloric deficits induced by these medications. In most, if not all, studies on Semaglutide, the effects are analyzed independently of exercise, which means that the observed loss of lean mass is partly due to the lack of physical activity, particularly resistance exercise. Additionally, the chronic negative energy balance created by GLP-1 RAs, suppressing appetite and reducing caloric intake, leads to a catabolic state where the body draws on fat and muscle storage for energy. When caloric intake falls below what is needed to maintain lean body mass, muscle protein breakdown increases, leading to net muscle loss. [14]
This catabolic state is further exacerbated by hormonal changes. Elevated cortisol levels, often observed in response to caloric deficits, enhance proteolysis—the breakdown of muscle proteins—to supply amino acids for energy production. While this physiological adaptation is essential for short-term survival, it can deplete muscle mass, undermining long-term health. Dietary protein intake plays a critical role in maintaining muscle mass during weight loss. GLP-1 RAs often reduce appetite, which in turn can lead to insufficient protein consumption and hinder the body’s ability to preserve muscle. Adequate protein intake, typically 1.2–2.0 grams per kilogram of body weight per day, is essential for balancing muscle protein synthesis and breakdown. Additionally, the delayed gastric emptying caused by GLP-1 RAs may impair the absorption of amino acids, reducing their availability for muscle repair and maintenance. Without sufficient dietary protein, muscle loss is inevitable in weight loss with GLP-1 RAs. [14]
Addressing Muscle Loss During GLP-1 RA Therapy
Mitigating muscle loss during GLP-1 RA therapy requires a proactive approach that incorporates dietary and lifestyle interventions. Ensuring adequate protein intake is critical for maintaining muscle protein synthesis. A target of 1.2–2.0 grams of protein per kilogram of body weight per day can help offset the catabolic effects of a caloric deficit. Distributing protein evenly across meals and incorporating high-quality protein sources can further support muscle preservation. [15]
Exercise, particularly resistance training, is essential to counteract muscle loss. Resistance training promotes muscle hypertrophy and stimulates anabolic signaling pathways, helping to preserve lean mass during weight loss. Incorporating structured exercise programs into GLP-1 RA treatment plans can significantly reduce the risk of muscle atrophy while enhancing the medication’s overall benefits. [15]
Education and awareness are also vital for patients using GLP-1 RAs. Emphasizing the importance of maintaining muscle mass and providing guidance on diet and exercise can empower patients to make informed choices. Incorporating strategies to support muscle health ensures that weight loss achieved through GLP-1 RAs is sustainable and does not compromise long-term metabolic and physical well-being.
Potential for Misuse and Overuse
GLP-1 RAs are primarily indicated for the treatment of type II diabetes and, in higher doses, for weight loss in individuals with obesity or weight-related medical conditions. However, the significant weight-loss effects have led to their use—and misuse—by individuals without a medical need. When used inappropriately for aesthetic weight loss, these medications may exacerbate muscle loss due to inadequate caloric and protein intake, particularly in individuals already within a healthy weight range.
The off-label use of GLP-1 RAs among people without clinical obesity presents risks, as these medications are not designed to target fat exclusively. The loss of lean body mass, including muscle, can impair metabolic health, lower basal metabolic rate, and increase the risk of regaining weight after discontinuation. Moreover, their appetite-suppressing effects may encourage extreme caloric restriction, further amplifying the risk of malnutrition and muscle catabolism.
Healthcare providers must be vigilant in prescribing GLP-1 RAs, ensuring they are used appropriately and with safeguards such as dietary support and exercise plans. Public awareness campaigns are also essential to address misconceptions about these medications and discourage misuse.
Systemic Metabolic Imbalances: The True Drivers Behind Weight Gain and Chronic Disease
Weight gain is often viewed as the root cause of various health issues, but in reality, it is more accurately a symptom of deeper physiological, behavioral, and environmental imbalances. Focusing solely on weight as a metric of health can obscure the underlying factors driving metabolic dysfunction, inflammation, and other conditions. It is essential to address these root causes to achieve meaningful and sustainable health outcomes rather than simply targeting the number on the scale.
Weight gain frequently coexists with conditions like insulin resistance, type II diabetes, and metabolic syndrome. These conditions reflect systemic disruptions in cellular energy regulation rather than weight itself being the cause. Insulin resistance, for example, occurs when muscle and liver cells fail to absorb glucose effectively, even in the presence of elevated insulin levels. This leads to chronic hyperinsulinemia, which promotes fat storage by driving lipogenesis and increasing enzyme activity in fatty acid synthesis. Over time, adipocytes expand and proliferate, disrupting normal metabolic signaling and reinforcing insulin resistance. [2, 3]
Mitochondrial dysfunction further exacerbates these metabolic disruptions. Mitochondria in muscle and liver cells become inefficient at utilizing glucose and fatty acids for energy, leading to ectopic fat accumulation in non-adipose tissues. This disrupts cellular signaling pathways, reduces energy expenditure, and perpetuates fat storage. Interventions targeting mitochondrial inefficiencies, such as dietary modifications and physical activity, are critical for restoring metabolic balance and reversing these processes. [2]
Chronic stress disrupts the body’s energy regulation systems by altering the hypothalamic-pituitary-adrenal (HPA) axis and driving sustained cortisol elevation. Cortisol influences energy partitioning, increasing glucose production in the liver and promoting fat storage, particularly in visceral fat depots. Visceral fat, in turn, releases inflammatory cytokines that impair insulin sensitivity and disrupt energy homeostasis. Cortisol also affects the brain’s reward systems, altering dopamine pathways and increasing cravings for calorie-dense, highly palatable foods. Simultaneously, stress-induced changes in gut microbiota composition can exacerbate metabolic dysfunction by impairing appetite regulation and nutrient metabolism. These disruptions create a feedback loop of stress, inflammation, and fat accumulation that is difficult to break. [2]
Environmental and behavioral factors further compound these challenges. Modern diets dominated by processed, calorie-dense foods reduce gut microbial diversity and promote inflammation, disrupting gut-brain communication and contributing to overeating. Exposure to endocrine-disrupting chemicals, such as bisphenol A (BPA), interferes with hormonal pathways critical for regulating fat storage and energy balance. These chemicals mimic or block the actions of key metabolic hormones, creating imbalances that predispose individuals to weight gain. Psychological stress and limited access to nutrient-dense foods reinforce unhealthy eating patterns, leading to the habitual consumption of energy-dense foods that exacerbate metabolic dysfunction. [2]
Treating weight as the primary target of intervention often leads to short-term solutions that fail to address the deeper drivers of metabolic dysfunction. Extreme caloric restriction, for instance, can induce rapid weight loss but often depletes lean body mass, including muscle, which is vital for long-term metabolic health. Muscle loss lowers basal metabolic rate and increases the likelihood of weight regain, undermining the sustainability of such approaches. Similarly, reliance on pharmacological interventions like GLP-1 receptor agonists without complementary lifestyle changes can fail to sustain metabolic improvements. These medications may suppress appetite and induce weight loss, but without adequate protein intake or resistance training, they can inadvertently exacerbate muscle loss. [2]
By reframing weight gain as a symptom rather than the core problem, interventions can shift toward addressing systemic issues like metabolic dysfunction, chronic inflammation, and environmental stressors. This holistic perspective ensures that health improvements extend beyond the scale, promoting sustainable metabolic health and overall well-being.
A Nuanced Approach to Weight Loss
At Healthspan, we understand that improving long-term health requires addressing the root causes of weight gain—metabolic dysfunction, hormonal imbalances, chronic inflammation, and lifestyle factors—rather than focusing solely on the number on the scale. Weight is a symptom, not the problem, and achieving sustainable health outcomes involves looking beyond quick fixes or temporary solutions.
No single medication is a “miracle drug,” and while certain therapies may offer short-term benefits, our focus is on promoting longevity and enhancing overall well-being. Our approach emphasizes a holistic strategy that supports mitochondrial health addresses chronic inflammation, and regulates metabolic dysfunction. This includes evidence-based protocols such as rapamycin or Urolithin A, which target cellular pathways involved in aging and metabolic health, alongside anti-inflammatory practices that can help restore balance at the systemic level.
In addition to these advanced protocols, lifestyle interventions are central to our philosophy. Regular exercise remains a cornerstone of metabolic health, promoting mitochondrial biogenesis, improving insulin sensitivity, and preserving lean body mass. Dietary modifications, such as incorporating nutrient-dense whole foods and maintaining adequate protein intake, are essential for cellular repair and balance energy. Stress management practices, improved sleep hygiene, and mindfulness strategies address the often-overlooked behavioral and environmental factors contributing to weight gain. Together, these interventions create a comprehensive framework for achieving sustainable weight management and improving healthspan.
As we look ahead to the next year, our mission at Healthspan is to guide and support our patients who are concerned about their weight and metabolic health through this transformative process. We are dedicated to helping individuals build holistic, personalized protocols tailored to their unique needs and goals. This will include introducing innovative lifestyle approaches, integrating cutting-edge protocols, and offering tools to empower our patients on their journey toward optimal health.
Stay tuned as we continue to develop and expand our offerings, delivering solutions that align with our vision of longer, healthier, and more fulfilling lives.
- Carmelinda Ruggiero, E. Jeffrey Metter, Vojtech Melenovsky, Antonio Cherubini, Samer S. Najjar, Alessandro Ble, Umberto Senin, Dan L. Longo, Luigi Ferrucci, High Basal Metabolic Rate Is a Risk Factor for Mortality: The Baltimore Longitudinal Study of Aging, The Journals of Gerontology: Series A, Volume 63, Issue 7, July 2008, Pages 698–706, https://doi.org/10.1093/gerona/63.7.698
- Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. 2021 Mar 1;320(3):C375-C391. doi: 10.1152/ajpcell.00379.2020.
- Korek E, Krauss H, Gibas-Dorna M, Kupsz J, Piątek M, Piątek J. Fasting and postprandial levels of ghrelin, leptin and insulin in lean, obese and anorexic subjects. Gastroenterology Review/Przegląd Gastroenterologiczny. 2013;8(6):383-389. doi:10.5114/pg.2013.39922.
- Lau J, Bloch P, Schä L, Pettersson I, Spetzler J, Kofoed J et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. ACS Publications 2015; 58: 7370–7380.
- Kapitza C, Dahl K, Jacobsen JB, Axelsen MB, Flint A. Effects of semaglutide on beta cell function and glycaemic control in participants with type 2 diabetes: a randomised, double-blind, placebo-controlled trial. doi:10.1007/s00125-017-4289-0.
- Weghuber D, Barrett T, Barrientos-Pérez M, Gies I, Hesse D, Jeppesen OK et al. Once-Weekly Semaglutide in Adolescents with Obesity. N Engl J Med 2022; 387: 2245.
- Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Rønne J, Alanentalo T, Baquero AF et al. Semaglutide lowers body weight in rodents via distributed neural pathways. 2020. doi:10.1172/jci.insight.133429.
- Jensterle M, Ferjan S, Ležaič L, Sočan A, Goričar K, Zaletel K et al. Semaglutide delays 4-hour gastric emptying in women with polycystic ovary syndrome and obesity. Diabetes Obes Metab 2023; 25: 975–984.
- Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. New England Journal of Medicine 2021; 384: 989–1002.
- Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, Jeppesen OK, Christiansen E, Hertz CL, Haluzík M; PIONEER 1 Investigators. PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison With Placebo in Patients With Type 2 Diabetes. Diabetes Care. 2019 Sep;42(9):1724-1732. doi: 10.2337/dc19-0749
- Mccrimmon RJ, Catarig A-M, Frias JP, Lausvig NL, Le Roux CW, Thielke D et al. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: a substudy of the SUSTAIN 8 randomised controlled clinical trial. doi:10.1007/s00125-019-05065-8.
- Heise T, DeVries JH, Urva S, Li J, Pratt EJ, Thomas MK et al. Tirzepatide Reduces Appetite, Energy Intake, and Fat Mass in People With Type 2 Diabetes. Diabetes Care 2023; 46: 998.
- Lee W. Advances in Muscle Research in Health and Disease. Cells. 2024 Oct 14;13(20):1694. doi: 10.3390/cells13201694
- Blundell J, Finlayson G, Axelsen M, Flint A, Gibbons C, Kvist T et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab 2017; 19: 1242–1251.
- Pasiakos SM, Vislocky LM, Carbone JW, Altieri N, Konopelski K, Freake HC et al. Acute energy deprivation affects skeletal muscle protein synthesis and associated intracellular signaling proteins in physically active adults. J Nutr 2010; 140: 745–751.