.png?u=https%3A%2F%2Fimages.ctfassets.net%2Fzvzqa1d1gh0f%2F68YrPbjn5g7t8jFrx2OLuQ%2Fb206487615608c4585c6e309d8dd9567%2Flabs_2__1_.png&a=w%3D55%26h%3D55%26fm%3Dpng%26q%3D75&cd=2025-06-12T20%3A31%3A13.529Z)
Longevity Pro Panel
Longevity Pro Panel offers the most advanced holistic analysis of vital health systems, paving the way for a healthier, longer life.
Alzheimer’s disease is often framed around two culprits—amyloid plaques and tau tangles—but new evidence shows the story is far more complex. In a recent Brain Research study, scientists explored how aerobic exercise affects not just amyloid and tau, but also iron accumulation, myelin health, and the “crosstalk” between neurons and glial cells in an aged rat model of sporadic Alzheimer’s. The results were eye-opening: exercise reduced hallmark pathologies, preserved healthy neurons, and shifted brain immune cells toward a low-inflammation, supportive state. In this review, we break down the cellular and molecular findings of this study, explain how exercise reshapes the brain’s internal networks, and discuss what this could mean for multi-target, low-cost strategies to slow disease progression.
Alzheimer's
Exercise
Cognitive Health
mitochondrial health
20 mins
By: Dr. Jennifer Szu
Alzheimer’s disease is a devastating progressive neurological disease that robs individuals of their memory, language, and other cognitive functions, including attention and problem-solving. According to the Alzheimer’s Association, over 7 million Americans are living with Alzheimer’s disease, and the numbers are rapidly rising.
Despite decades of research and billions of dollars invested, treatment strategies have largely centered on pharmaceuticals targeting amyloid plaques or tau tangles. Yet these approaches have yielded only modest benefits, and they often overlook the influence of lifestyle factors, including physical activity, on disease biology. Growing evidence suggests that exercise may directly modulate the cellular and molecular pathways involved in Alzheimer’s, offering a low-cost, accessible intervention that could complement medical therapies.
Beyond amyloid and tau, Alzheimer’s pathology involves a more complex network of changes, including myelin degeneration, iron dysregulation, and disrupted communication between neurons and glial cells. Understanding how these processes interact is critical for developing more effective prevention and treatment strategies.
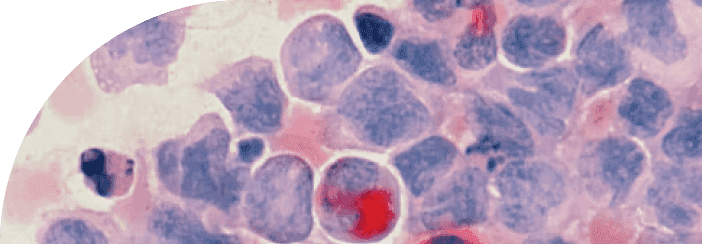
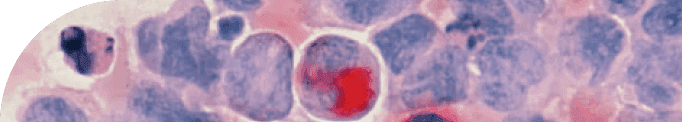
In line with this perspective, a recent study published in Brain Research investigated how the beneficial effects of physical exercise influence three major pathological features of Alzheimer’s disease: amyloid beta plaques, neurofibrillary tangles, and iron accumulation. In aged rats, the researchers quantified neurofibrillary tau tangles, amyloid protein plaques, myelin degeneration, and iron deposits, then correlated these measures to assess the crosstalk between neuronal and glial cell populations. Building on these findings, this review will explore how aerobic physical activity influences neuronal health, microglial activity, oligodendrocyte function, and hallmark Alzheimer’s pathology, with the aim of clarifying the cellular mechanisms through which exercise may slow or modify disease progression.
While several hypotheses regarding the etiology of Alzheimer’s disease have been proposed, the exact sequence of events that drives its pathogenesis remains unclear. Most research has centered on three interrelated mechanisms, each representing a hallmark feature of the disease: accumulation of amyloid beta plaques, formation of neurofibrillary tangles, and buildup of iron.
1. Accumulation of amyloid beta plaques
It is well established that one of the cardinal features of Alzheimer’s disease is the accumulation of extracellular amyloid plaques, which are composed of amyloid beta peptides [1]. Amyloid beta peptide is derived from amyloid precursor protein, which is involved in several functions, including neuroprotection, cell adhesion, synapse formation, and neuronal development.
The amyloid hypothesis is a prevailing theory that proposes that amyloid plaques are the main cause of Alzheimer’s disease. This model also considers the formation of neurofibrillary tangles (discussed below) as part of a cascade that drives memory loss, confusion, and the progressive cognitive and personality decline observed in patients [2].
Deposition of amyloid plaques occurs most prominently in the hippocampus, neocortex, and brain vasculature [2]. When these deposits accumulate within cerebral blood vessel walls, the condition is known as cerebral amyloid angiopathy, which can lead to serious outcomes such as cerebral hemorrhage, stroke, and cognitive decline. Notably, amyloid deposition in the vasculature may occur before plaques form within the brain parenchyma itself, suggesting that vascular dysfunction could be an early step in Alzheimer’s pathology [3].
2. Formation of neurofibrillary tangles
Another hallmark of Alzheimer’s disease is the presence of intraneuronal neurofibrillary tangles, which are composed of tau, an axon-enriched microtubule-associated protein. Tau protein is found mainly in neurons, primarily in the axonal compartment. In healthy cells, tau binds to and stabilizes microtubules, maintaining the structural integrity of the cell. Microtubules are key structural components of cells that appear as hollow tubes and serve as tracks for motor proteins, facilitating the intracellular transport of various cargos, including vesicles and organelles. [1]
In Alzheimer’s disease, tau protein becomes hyperphosphorylated (i.e., having excessive phosphate groups attached to it). The hyperphosphorylation of tau disrupts the microtubule assembly, leading to tau protein aggregating into bundles of filaments (also known as neurofibrillary tangles) inside the cell [4]. The prevailing theory suggests that the accumulation of neurofibrillary tangles causes neuronal dysfunction and death; however, the relationship of neurofibrillary tangles to cell death is not well understood [5]. Preclinical studies have demonstrated that neurofibrillary tangles are not the direct cause of cell death [6,7,8].
3. Buildup of iron
Iron plays various essential roles in the brain, including oxygen transport, myelin production, neurotransmitter synthesis, and metabolism. Maintenance of iron homeostasis is critical for normal brain functions, as excessive concentrations of iron can lead to neurotoxicity, oxidative damage, and cell death [9].
Accumulation of iron in the brain is part of normal aging, with iron mainly bound within ferritin and neuromelanin. Interestingly, the buildup of iron is not uniform throughout the brain, with select regions of the brain having higher concentrations of iron. The basal ganglia have the richest iron concentration, while lower concentrations of iron are found in the cortical gray matter, white matter, midbrain, and cerebellum [10]. However, accumulation of iron in these specific brain regions that far exceeds the normal amount of iron during healthy aging occurs in various neurodegenerative diseases, including Alzheimer’s disease. Elevated levels of iron have been found in the hippocampus of Alzheimer’s disease brains, suggesting that increased iron may exacerbate Alzheimer’s neuropathology even before the manifestation of a clinical syndrome [11].
Oligodendrocytes are a type of glial cell in the brain whose primary function is to produce the myelin sheath that wraps around the axons of neurons (or nerve fibers). The myelin sheath is composed primarily of fatty substances, mainly lipids. The composition of the myelin sheath acts not only as a protective layer around the nerve fiber but also as an insulator, allowing electrical signals to transmit quickly and efficiently along the axons [12]. Interestingly, oligodendrocytes are also the main iron-containing cells in the brain, where they store iron in the form of ferritin. In fact, iron is required for the production of myelin, where it acts as a cofactor for enzymes involved in making cholesterol and phospholipids, key components of myelin [13].
In Alzheimer’s disease, breakdown of myelin can release a considerable amount of the stored iron, leading to a buildup of iron in the brain, resulting in further myelin loss and neurodegeneration. Excessive iron also contributes to more amyloid beta deposition and the formation of neurofibrillary tangles. This imbalance of iron homeostasis in Alzheimer’s disease can trigger oxidative stress reactions, damage cell structure and function, ultimately killing the cells in the brain [14]. Thus, oligodendrocyte dysfunction is one crucial factor in the pathogenesis of Alzheimer’s disease.
Microglia are the immune residents of the central nervous system. They become activated and release inflammatory factors in response to stress or insults to the brain to promote neuroinflammation. Microglial cells are also involved in iron homeostasis, where they actively bind and internalize iron [15]. Indeed, iron accumulation in microglia has been detected in the frontal cortex and hippocampus of Alzheimer’s disease brains. Increased iron intake by microglia leads to further release of inflammatory factors, causing increased neuroinflammation [16].
Before the recent study examined in depth later in this review, much of what was known about exercise’s role in Alzheimer’s disease came from a patchwork of preclinical and clinical findings. These earlier studies suggested that physical activity could influence each of the three core pathological processes (amyloid beta accumulation, tau pathology, and iron dysregulation), but the evidence varied in scope, methods, and consistency.
1. Amyloid beta
Preclinical studies have consistently shown that physical activity can reduce the load of amyloid plaques in the brain and improve cognitive function. In one study, mice with Alzheimer’s disease were subjected to low and high-intensity exercise training on the treadmill. Exercise training not only significantly reduced the buildup of amyloid plaques in the cortex and the hippocampus but also markedly improved cognitive abilities such as learning and memory [20].
2. Tau
Animal models also suggested that exercise could lower tau pathology. In a transgenic mouse model overexpressing human mutant tau, long-term treadmill training significantly reduced hyperphosphorylated tau in both the spinal cord and hippocampus [21]. However, short-term treadmill exercise in other models paradoxically increased neurofibrillary tangles and neuroinflammation [22]. In human observational studies, high physical activity has been associated with lower plasma amyloid beta levels, hinting that exercise might reduce tau pathology indirectly by first limiting amyloid accumulation [23].
3. Iron
In animal studies of Alzheimer’s disease, the amount of iron levels in the brain after exercise remains conflicting. In one study, voluntary running in a transgenic mouse model of Alzheimer’s disease significantly reduced the levels of cortical ferritin, the main iron storage protein, compared to sedentary mice. However, the total level of iron remains unchanged in both the sedentary and exercise mice [24] In contrast, treadmill exercise attenuated the total iron levels in the brain in mouse models of Alzheimer’s disease [25]. Such discrepancies underscore the need for more detailed, integrative research to clarify how exercise impacts iron homeostasis in the Alzheimer’s brain.
Given the limitations of prior research—which often examined amyloid beta, tau, or iron in isolation and frequently relied on transgenic mouse models—Gutierre et. al designed a study to provide a more integrative view of how physical exercise influences multiple Alzheimer 's-related pathologies within a more clinically relevant model. Most preclinical Alzheimer’s studies use genetically modified mice that overproduce amyloid plaques, tau tangles, or exhibit chronic neuroinflammation via heightened microglial activity. While valuable, these models do not fully replicate the more gradual, multifactorial processes seen in sporadic Alzheimer’s disease in humans.
To address this gap, the researchers used aged rats (18 months old) without any genetic modifications. This model more closely mimics the sporadic form of Alzheimer’s disease, where pathology emerges from the combined effects of aging and environmental factors rather than a single genetic driver.
The primary objective was to measure and correlate the accumulation of amyloid beta, tau, and iron in the hippocampal formation of aged rats, and to determine how an aerobic exercise intervention might alter these pathological features. A secondary aim was to examine the “crosstalk” between specific brain cell types, providing insights into how neurons, microglia, and oligodendrocytes interact under exercise and sedentary conditions.
The study design involved dividing rats into two groups: a control (sedentary) group and an exercise group. Rats in the exercise group were first familiarized with the treadmill for three consecutive days (5 minutes/day at 8 m/min, 0% incline). Trainability was rated on a scale, and only animals rated three or higher were included; in practice, all animals were rated as good runners, and none were excluded.
The aerobic training program lasted 8 weeks, with sessions 5 times per week. Each session began with a 5-minute warmup at 8 m/min, followed by a gradual progression: initial training at 12 m/min for 10 minutes, increasing over time to 15 m/min for 30 minutes in later sessions. The sedentary control group did not participate in any treadmill activity.
Gutierre and colleagues quantified not only tau tangles, amyloid plaques, and iron accumulation, but also oligodendrocyte ferroptosis—a regulated form of cell death triggered by excessive iron and lipid peroxidation. Lipid peroxidation refers to the chemical damage of fats in cell membranes caused by oxidative stress, which weakens the cell’s structure and can ultimately cause it to die. The authors also measured the abundance and characteristics of various hippocampal neuronal and glial cell populations. This cell-type–specific analysis is vital because Alzheimer’s pathology does not affect all brain cells in the same way; different cells can either protect against or accelerate disease progression. Specifically, they examined pyramidal and granule neurons, microglia, and oligodendrocytes, providing a detailed view of how exercise might influence the hippocampal microenvironment.
The hippocampus is a region deep within the brain that contains multiple types of brain cells, including neurons and glial cells. In general, neurons are nerve cells capable of transmitting electrical and chemical signals for complex information processing—in other words, they are the “wiring” that carries messages between different parts of the brain and body. Glial cells, by contrast, are non-neuronal and cannot conduct electrical impulses. Instead, they play a support role—feeding, insulating, and protecting neurons, as well as helping regulate brain chemistry. For example, oligodendrocytes—a type of glial cell—enhance neuronal communication by producing the myelin sheath [12]. Myelin is a fatty covering that wraps around axons (the long projections of neurons), allowing electrical signals to travel quickly and efficiently. Without myelin, nerve impulses slow down or fail entirely. Microglia, another type of glial cell, can influence neuronal interactions through synaptic modulation, actively pruning or strengthening synapses [26]. Pruning is a process where unused or weak connections between neurons are removed, making brain circuits more efficient, similar to trimming a tree to help it grow stronger branches.
Functionally, the hippocampus is essential for memory formation, learning, and spatial navigation—the brain’s ability to remember places and navigate through environments. These functions are tightly regulated by its constituent cells, including pyramidal and granule neurons. Structurally, the hippocampus resembles a seahorse and is composed of three major regions: the dentate gyrus, the hippocampus proper (or cornu ammonis, CA), and the subiculum. The cornu ammonis is further subdivided into CA1, CA2, CA3, and CA4 [27]. Pyramidal cells—named for their pyramid-like shape—are excitatory neurons found in all four CA layers and the subiculum, with the highest concentration in CA1. They are crucial for spatial navigation and episodic memory [28]. Episodic memory refers to the recollection of specific events or experiences, like remembering what you ate for breakfast yesterday.
Granule cells, located in the dentate gyrus, are often called the “gateway” to the hippocampus because they regulate information flow from the entorhinal cortex into the hippocampus. The entorhinal cortex acts like a hub, linking the hippocampus with other brain regions involved in memory and navigation. Granule cells relay input from the entorhinal cortex to pyramidal neurons in the CA3 region, playing a key role in memory formation, memory retrieval, and the ability to discriminate between similar memories [29]. This discrimination process—known as pattern separation—helps you remember details that distinguish one event from another, even if they are very similar.
Oligodendrocytes and microglia also have pivotal roles in hippocampal learning and memory. Oligodendrocytes contribute to both short- and long-term memory, as well as memory consolidation, partly through activity-dependent myelin remodeling [30]. This means that the myelin they produce can adapt and change based on how often specific brain pathways are used, fine-tuning the speed and efficiency of signal transmission. Microglia influence the strength and quality of memory by actively pruning synaptic connections [31], removing unnecessary neural links while preserving and reinforcing the most important ones, helping the brain store memories more efficiently.
Gutierre and colleagues determined that aerobic exercise produced broad neuroprotective effects in aged rats, improving the health of multiple hippocampal cell types while reducing hallmark Alzheimer’s pathologies.
Pyramidal and Granule Neurons: Exercise group rats had about 2.5 times more normal pyramidal and granule neurons than sedentary controls, while the number of neurons affected by hyperphosphorylated tau (neurofibrillary tangles) was roughly four times lower. This pattern indicates that exercise not only shields neurons from tau-related damage but also supports the preservation and potentially regeneration of healthy neurons.
Microglia: Phagocytic (activated) microglia were 2–3 times less abundant in the exercise group, while non-phagocytic microglia were 1.5–2 times more numerous. This shift suggests a reduced inflammatory profile, likely because there was less amyloid beta and tau pathology for microglia to clear. Fewer iron-containing microglia were also observed, implying that exercise may help limit harmful iron accumulation.
Oligodendrocytes: The exercise group showed about twice as many normal oligodendrocytes and half as many iron-loaded oligodendrocytes compared to sedentary controls. These results point to improved oligodendrocyte health, reduced toxic iron overload, and better support for myelin maintenance.
Amyloid Beta: The volume of senile plaques was approximately 4.5 times lower in the exercise group, highlighting a robust effect of physical activity in reducing amyloid beta deposition.
Tau – Neurofibrillary Tangles: Neurofibrillary tangle volume was about 2.5 times lower in the exercise group, underscoring exercise’s role in limiting tau pathology.
The findings show that regular aerobic exercise in aged rats not only reduces amyloid beta, tau tangles, and iron-related damage but also promotes healthier populations of neurons, microglia, and oligodendrocytes, together supporting a more resilient hippocampal environment.
Building on these key findings, the authors next examined how exercise influenced the “crosstalk”—the functional communication and mutual influence—between neurons, glial cells, and the core pathological markers of Alzheimer’s disease (amyloid, tau, and iron). This analysis was important because Alzheimer’s pathology does not occur in isolation; the health or dysfunction of one cell type can directly alter the activity of others, creating feedback loops that either accelerate or slow disease progression.
In both exercise and control groups, there was a measurable numerical relationship between normal pyramidal and granule neurons. However, in sedentary rats, this relationship shifted toward pathology: an increased number of tau-affected pyramidal neurons was accompanied by a rise in tau-containing phagocytic microglia. Phagocytic microglia are in an activated state, engulfing and breaking down debris, but in Alzheimer’s, they are often chronically stimulated by accumulating pathology, which can fuel inflammation. This pairing suggests a mutual relationship—more tau pathology in neurons leads to more microglial activation, which in turn may contribute to ongoing tissue stress.
In sedentary animals, both pyramidal and granule neurons showed higher tau pathology, which was mirrored by elevated phagocytic microglia activity. In contrast, exercise reduced tau pathology in both neuronal populations and lowered the need for microglial activation. With less pathology to respond to, microglia were more often in their non-phagocytic (resting) state, which is associated with routine maintenance and support functions rather than inflammation. This shift indicates that exercise alters cellular crosstalk in a way that restores a healthier balance between neurons and microglia.
Exercise also appeared to protect the physiological relationship between axons and their myelin sheath. Healthy oligodendrocytes, which produce myelin, were more abundant in the exercise group, while iron-overloaded (damaged) oligodendrocytes were fewer. Because myelin is essential for rapid and efficient nerve signal conduction, this preservation supports overall neuronal function. Additionally, reduced amyloid and tau pathology in the exercise group meant less stress on myelin-producing cells, helping maintain axonal integrity.
The findings suggest that aerobic exercise can shift cell-to-cell communication networks in the hippocampus from a pathological “vicious cycle”—where neuronal injury, glial activation, and protein accumulation feed each other—toward a physiological “virtuous cycle” that supports healthier interactions, lower inflammation, and better structural integrity.
Building on the analysis of cellular crosstalk, the authors next examined how specific cell populations were correlated with one another and with AD-related pathologies. These correlations help reveal whether relationships between neurons, glia, and pathological markers reflect healthy, coordinated brain function or instead indicate dysfunction and chronic stress.
In the control (sedentary) group, several correlation patterns pointed to impaired cellular function. A negative correlation was observed between the number of iron-containing oligodendrocytes and the number of non-phagocytic microglia. In practical terms, this means that the more oligodendrocytes were overloaded with iron, the fewer resting microglia were present. Since non-phagocytic microglia can transition into an active, debris-clearing state when needed, their reduced numbers suggest that microglia in sedentary animals may be dysfunctional and less able to respond to toxic iron buildup. Another negative correlation emerged between the number of iron-containing oligodendrocytes and the total number of phagocytic microglia, the activated form responsible for engulfing iron, amyloid plaques, and neurofibrillary tangles. This further supports the idea that microglia in the sedentary group are impaired and unable to effectively clear damaging substances from the brain. Interestingly, a positive correlation was found between non-phagocytic and phagocytic microglia, suggesting a state of chronic immune activation in which some microglia remain in a resting state while others are persistently active. However, the persistence of pathology despite this activation pattern indicates that even when engaged, these microglia are ineffective at controlling the toxic accumulation of iron, amyloid beta, and tau proteins.
In the exercise group, the correlations painted a markedly different picture, consistent with a healthier and more balanced brain environment. A positive correlation was found between the number of normal pyramidal neurons and the number of iron-containing phagocytic microglia. This suggests that in exercising rats, active microglia were successfully clearing harmful substances such as excess iron, thereby protecting neurons and supporting their survival. Another positive correlation was observed between the number of normal pyramidal neurons and the number of non-phagocytic microglia, indicating that healthier neurons were associated with a greater proportion of microglia in their resting, homeostatic state. This pattern reflects a low-inflammation environment in which microglia can focus on regular maintenance rather than constant activation. A similar positive correlation was found between the number of dentate granule cells and the number of non-phagocytic microglia, reinforcing the idea that exercise promotes both neuronal preservation and a stable, well-regulated microglial population.
Together, these findings highlight that exercise not only alters the abundance of healthy versus pathological cells but also reshapes the functional relationships between them. In sedentary animals, correlations suggest a breakdown in microglial function and persistent, ineffective immune activation. In contrast, exercising animals exhibit correlation patterns consistent with coordinated cellular activity, adequate clearance of pathological markers, and preservation of healthy neurons—all features of a neuroprotective brain environment.
The authors’ findings demonstrate that physical exercise plays a protective role in Alzheimer’s disease by restoring healthy cellular relationships in the brain. In sedentary animals, crosstalk between neurons and glial cells shifted into a pathological state that contributed to disease progression. Tau and amyloid pathology spread between pyramidal and granule neurons and was linked to increased activity of phagocytic microglia. While microglia in their activated, phagocytic state can have a beneficial role—engulfing amyloid beta plaques produced in the brain—this capacity is finite. In later stages of Alzheimer’s disease, excessive plaque production overwhelms their clearance ability, leading to impaired function and the release of inflammatory molecules that drive chronic neuroinflammation [30].
Exercise reversed many of these pathological patterns. In the exercise group, tau pathology was lower, levels of phagocytic microglia were reduced, and numbers of non-phagocytic microglia increased, reflecting a shift toward a more homeostatic environment with normal neuron–glia communication. One of the clearest examples of restored cellular relationships was between axons and oligodendrocytes, which improved neuronal integrity and reduced degeneration. Both animal and human studies support these findings: in a mouse model of Alzheimer’s disease, long-term treadmill exercise maintained intact myelination on neuronal axons and slowed cognitive decline [32], while in humans, intense aerobic exercise such as walking, cycling, or dancing induced myelination in brain regions vulnerable to age-related demyelination [33]. Maintaining myelin along neuronal axons is critical for rapid signal transmission and effective cellular communication, which are essential for preserving cognitive function.
TAKE HOME POINTS
Physical activity enhances neuronal protection: Aerobic exercise significantly increased the number of neurons in the hippocampus, reflecting a neuroprotective effect that may help reduce cognitive decline.
Exercise shifts microglia toward a low-inflammation state: Fewer phagocytic (activated) microglia were observed after exercise, indicating reduced neuroinflammation and lower Alzheimer’s related pathology, including iron, amyloid plaques, and tau proteins.
Iron homeostasis in glial cells improves with exercise: Exercise reduced the number of iron-containing oligodendrocytes, increasing the proportion of healthy myelin-producing cells. This supports efficient neuronal communication, with positive effects on learning and memory.
Healthy cellular crosstalk is restored: Exercise promotes a homeostatic brain environment, fostering positive, protective interactions between neurons and glial cells.
Citations
Latest Longevity Research Straight to your Inbox
Sign up for The Longevity Blueprint, a weekly newsletter from Healthspan analyzing the latest longevity research.
Sign up for The Longevity Blueprint, a weekly newsletter from Healthspan analyzing the latest longevity research.