The Crucial Role of Inflammation in Exercise-Induced Reduction of Cellular Senescence
High-Intensity Exercise Reduces Cellular Senescence: The review highlights that high-intensity interval training (HIIT) significantly decreases markers of cellular senescence, such as p16^INK4a, in muscle tissue. This reduction is more pronounced shortly after exercise and suggests that vigorous physical activity can effectively diminish the accumulation of dysfunctional "zombie cells" that contribute to aging and chronic diseases.
Acute Inflammation is Essential for Anti-Aging Benefits: The acute inflammatory response triggered by high-intensity exercise plays a crucial role in reducing cellular senescence. This inflammation facilitates the clearance of senescent cells and promotes tissue repair and regeneration. Suppressing this natural response through anti-inflammatory interventions may impede these beneficial processes.
Anti-Inflammatory Interventions May Blunt Exercise Benefits: The use of nonsteroidal anti-inflammatory drugs (NSAIDs), cold water immersion, antihistamines, and excessive antioxidant supplementation around exercise sessions can diminish the positive effects of exercise on cellular senescence and adaptation. These interventions suppress acute inflammation and oxidative stress, which are integral signals for muscle adaptation and anti-aging effects.
Individuals with Higher Baseline Senescence Benefit Most: The research indicates that individuals with higher initial levels of senescence and inflammation markers experience the most significant reductions in these markers following high-intensity exercise. This suggests that HIIT may be particularly beneficial for populations with greater physiological stress or age-related cellular changes, potentially extending their healthspan.
Embracing Natural Inflammatory Responses Enhances Healthspan: Allowing the body's natural inflammatory and oxidative stress responses to proceed post-exercise is essential for maximizing the anti-aging benefits of physical activity. By embracing these natural processes, we can enhance mitochondrial function, promote muscle hypertrophy, reduce chronic inflammation, and ultimately extend our healthspan.
Background
Imagine you have two muscle biopsy samples before you. One is from an older individual, and the other from a younger person. Your task is to determine which sample belongs to whom. How would you distinguish between them?
It turns out there's a straightforward way to answer this question: look for inflammation. Under a microscope, the older person's muscle tissue would reveal a landscape marred by inflammation—healthy cells interspersed with dysfunctional ones, all immersed in a mixture of pro-inflammatory molecules. Inflammation is one of the most discernible hallmarks of aging. Scientists have even coined the term "inflammaging" to describe the chronic, low-grade inflammation that develops with advanced age.
As we grow older, we lose the youthful phenotype characterized by smooth, less inflamed tissue, and instead enter a state marked by increased inflammation. This shift accompanies a transition from functional to dysfunctional tissue.
"Inflammation"—it's a term that often triggers concern. We're frequently warned about its association with a myriad of health issues, from cardiovascular diseases like heart attacks and strokes to metabolic disorders such as diabetes, and even neurodegenerative conditions like Alzheimer's and Parkinson's disease. Chronic inflammation—a prolonged and persistent immune response—is not merely a symptom but a driving force in the progression of these ailments. This continuous state of low-grade inflammation can damage cells and tissues, disrupt normal physiological functions, and accelerate the aging process at the cellular level.
While chronic inflammation can be harmful, acute inflammation serves a crucial role. It's the body's immediate response to injury or infection, mobilizing immune cells, cytokines, and other molecules to the affected area. This rapid response initiates healing and protects us from further harm. Without acute inflammation, wounds wouldn't heal, and infections could become life-threatening.
Interestingly, inflammation is intricately tied to exercise. During physical activity, especially intense or prolonged sessions, our bodies experience a form of controlled stress. Muscle fibers undergo microtears, and metabolic byproducts accumulate. In response, the body mounts an acute inflammatory reaction, releasing cytokines like interleukin-6 (IL-6) and recruiting macrophages to repair and strengthen muscle tissue. This process not only heals but also adapts our muscles for future demands.
What makes exercise remarkable is its dual role. Post-exercise, the body initiates anti-inflammatory processes. Regular physical activity enhances the production of anti-inflammatory cytokines and upregulates antioxidant enzymes like superoxide dismutase (SOD) and glutathione peroxidase. These molecules combat oxidative stress and reduce chronic inflammation, contributing to better overall health and resilience against diseases.
The anti-inflammatory effects of exercise may also play a pivotal role in aging. Chronic inflammation and oxidative stress contribute to the accumulation of senescent cells—often referred to as "zombie cells." These are cells that have ceased to divide and function properly but resist programmed cell death. Instead, they linger in the body, releasing pro-inflammatory factors collectively known as the senescence-associated secretory phenotype (SASP). The SASP can disrupt tissue function and promote chronic inflammation, creating a feedback loop that accelerates aging and age-related diseases.
The buildup of senescent cells is recognized as one of the "hallmarks of aging," a concept highlighting the fundamental processes that contribute to biological aging. Reducing the accumulation of these cells, or mitigating their harmful effects, is a burgeoning area of research with significant implications for extending healthspan—the period of life spent in good health.
Exercise emerges as a promising strategy to combat cellular senescence. While evidence in humans is still developing, animal studies suggest that physical activity can reduce senescent cell burden and modulate the SASP. Questions remain about the optimal intensity and type of exercise needed to achieve these benefits and whether certain inflammatory responses are necessary for the anti-senescent effects.
A recent study published in the journal Aging delves into these questions. The researchers investigated how different exercise intensities affect senescent cell accumulation and inflammatory markers in humans. Their findings could illuminate new pathways through which exercise promotes healthy aging and inform guidelines for physical activity tailored to maximize longevity benefits.
Study Design: Investigating the Intersection of Exercise, Inflammation, and Cellular Senescence
To explore how exercise intensity and inflammation affect cellular aging, researchers conducted a controlled study involving 12 healthy men aged between 20 and 26 years. Importantly, these participants were not regular exercisers, which ensured that the exercise intervention would be a significant new stimulus for their bodies.
Study Structure
The study utilized a randomized crossover design, meaning each participant experienced both conditions in a random order, with a three-week washout period in between to prevent carryover effects. To ensure study validity, all participants were instructed to abstain from substances that could potentially affect inflammation (such as anti-inflammatory medications, nutritional supplements, and vaccinations), for at least four weeks before and during the study. All participants were instructed to maintain their usual levels of physical activity and dietary habits throughout the experimental period.
The two experimental conditions were:
- Exercise with Placebo: Participants performed a high-intensity interval training (HIIT) session and ingested a placebo capsule. The placebo group would not have any interference of inflammation after their bout of HIIT training.
- Exercise with Ibuprofen: Participants performed the same HIIT session but ingested 400 mg of ibuprofen 2 hours before exercise, and again at 3 and 8 hours post-exercise, totaling 1200 mg for the day.
High-Intensity Interval Training (HIIT) Protocol
The exercise protocol was designed to elicit a strong physiological response:
- Warm-up: 3 minutes of cycling at 50 Watts to prepare the cardiovascular system and muscles.
- Intervals: 20 seconds of intense cycling at 120% of each individual's maximal power output (averaging 233 Watts), followed by 20 seconds of rest.
- Repetitions: This cycle was repeated 15 times, resulting in approximately 10 minutes of high-intensity exercise.
HIIT is known for causing significant muscle stress and acute inflammation due to its demanding nature, making it ideal for studying the body's inflammatory and repair responses.
Ibuprofen Intervention
Ibuprofen, a nonsteroidal anti-inflammatory drug (NSAID), was used to assess the role of inflammation in exercise-induced cellular changes. By inhibiting cyclooxygenase enzymes (COX-1 and COX-2), ibuprofen reduces the production of pro-inflammatory prostaglandins. Administering ibuprofen allowed researchers to observe how blunting the inflammatory response might affect markers of cellular senescence.
Muscle Biopsies and Biomarker Analysis
To evaluate the cellular impact, muscle biopsies were taken from the vastus lateralis (a muscle in the thigh) at three time points:
- Before Exercise: Establishing baseline levels for each participant.
- 3 Hours Post-Exercise: Capturing immediate cellular responses to the exercise and any initial effects of ibuprofen.
- 24 Hours Post-Exercise: Observing longer-term changes and recovery processes.
The muscle samples were analyzed for the expression levels of two key genes:
- p16^INK4a: This gene is a well-recognized marker of cellular senescence. Higher expression indicates an increased presence of senescent cells, which are cells that have stopped dividing but remain metabolically active, often contributing to chronic inflammation.
- CD11b: A marker indicative of immune cell infiltration, specifically macrophages and neutrophils. Elevated levels suggest increased inflammation and immune activity within the muscle tissue.
By comparing the conditions—with and without ibuprofen—the researchers aimed to understand:
- The Role of Acute Inflammation: Determining whether the inflammatory response to intense exercise is necessary for reducing cellular senescence markers.
- Impact on Senescent Cells: Observing if blunting inflammation with ibuprofen affects the exercise-induced changes in p16^INK4a expression to gauge its impact on levels of cellular senescence.
- Immune Cell Involvement: Assessing how immune cell infiltration (CD11b levels) correlates with changes in cellular senescence.
By manipulating the inflammatory response through ibuprofen administration, the researchers could directly test whether acute inflammation is a necessary trigger for the reduction of cellular senescence markers induced by high-intensity exercise. This approach allows them to discern if suppressing inflammation alters the beneficial effects of exercise on senescent cells and to understand the role of immune cell activity in mediating these effects. Essentially, the study design isolates the impact of inflammation on exercise-induced cellular adaptations, providing insights into how inflammation and immune responses contribute to healthy aging.
Results: High-Intensity Exercise Reduces Markers of Cellular Senescence
The study's findings reveal significant effects of high-intensity interval training (HIIT) on cellular senescence markers in muscle tissue, specifically p16^INK4a.
In the placebo condition, where participants performed HIIT without any anti-inflammatory intervention, there was a notable decrease in the expression levels of p16^INK4a:
- 3 Hours Post-Exercise: p16^INK4a levels decreased by 82% compared to baseline. This substantial reduction indicates a rapid response in reducing senescent cell markers shortly after intense exercise.
- 24 Hours Post-Exercise: The levels remained lower than baseline, with a 62% decrease. Although slightly less than the initial reduction, this sustained decrease suggests lasting benefits from a single session of HIIT.
These results suggest that high-intensity exercise can effectively reduce markers associated with cellular aging in the short term, which is beneficial for muscle regeneration and overall cellular health.
When participants ingested ibuprofen—a nonsteroidal anti-inflammatory drug (NSAID)—alongside the HIIT session, the results differed slightly:
- 3 Hours Post-Exercise: p16^INK4a levels decreased but were not as low as in the placebo condition. This indicates that ibuprofen may attenuate the immediate reduction of senescence markers induced by exercise.
- 24 Hours Post-Exercise: p16^INK4a levels decreased to levels similar to those observed in the placebo condition. By this time point, the effect of exercise on reducing senescence markers was comparable, regardless of ibuprofen intake.
The data suggest that while high-intensity exercise alone significantly reduces markers of cellular senescence shortly after activity, the ingestion of ibuprofen may blunt this immediate effect. However, the long-term benefits appear to equalize within 24 hours.
- Role of Inflammation: The initial lesser reduction in p16^INK4a with ibuprofen suggests that the body's acute inflammatory response to exercise is partly responsible for the immediate decrease in senescence markers. Inflammation might facilitate processes that eliminate or repair senescent cells.
- Delayed Effects: By 24 hours post-exercise, the reduction in p16^INK4a levels in the ibuprofen condition catches up to the placebo condition. This implies that while ibuprofen delays the early benefits, it does not completely inhibit the exercise-induced reduction of senescence markers over a longer period.
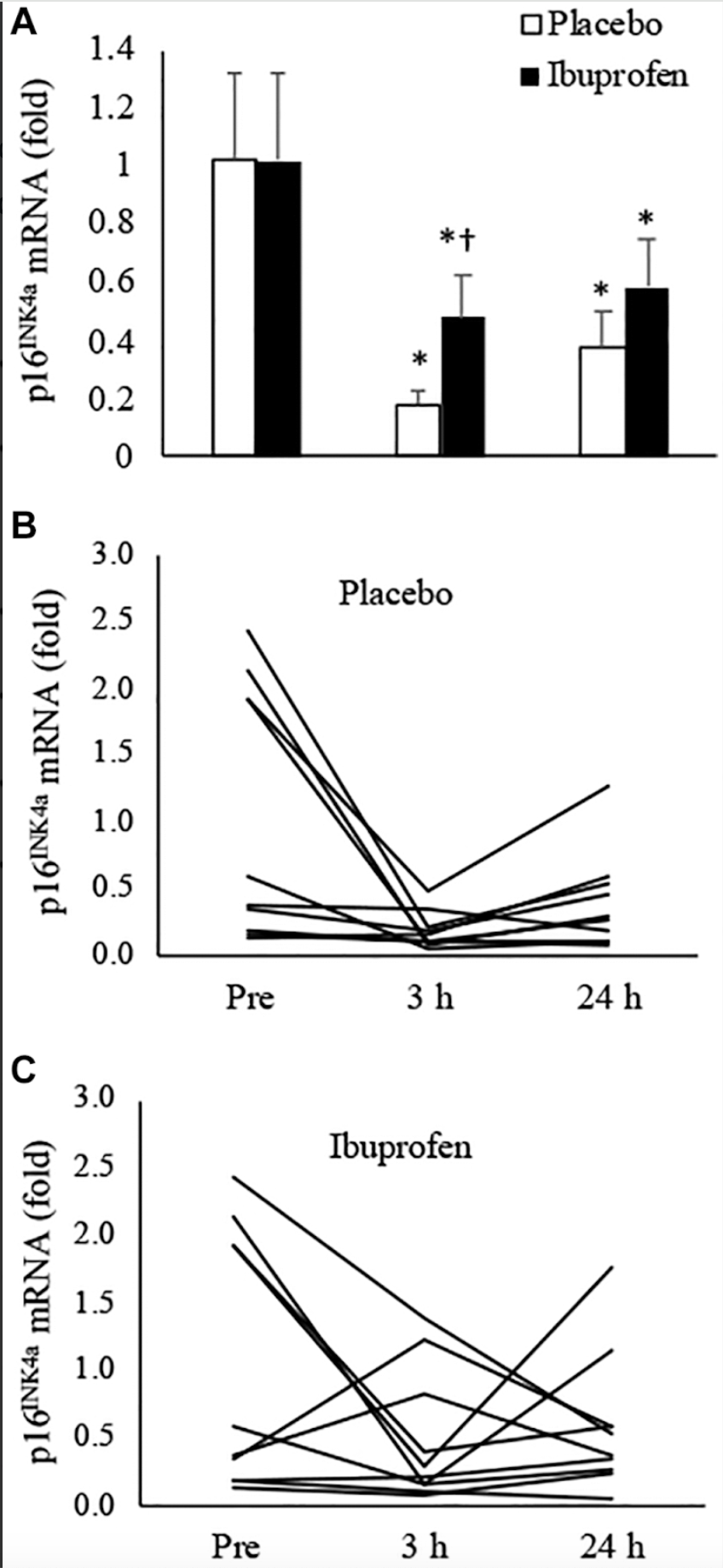
The significant reduction in p16^INK4a expression following high-intensity interval training (HIIT) highlights the potential of vigorous exercise as a non-pharmacological intervention to directly diminish cellular senescence—a key endpoint linked to aging and numerous age-related diseases.
By effectively lowering markers of senescent cells, HIIT may enhance tissue regeneration, improve muscle function, and reduce chronic inflammation, thereby contributing to healthier aging processes. The attenuated decrease in p16^INK4a observed in the ibuprofen condition suggests that suppressing the acute inflammatory response may impede the exercise-induced reduction of cellular senescence.
This raises clinical concerns regarding the routine use of nonsteroidal anti-inflammatory drugs (NSAIDs) around exercise sessions, as they may hinder the beneficial effects of exercise on senescent cell clearance. These findings underscore the importance of allowing natural inflammatory responses post-exercise to optimize the reduction of cellular senescence and promote overall health and longevity.
Next, the study's examination of individual participant data revealed intriguing nuances in how high-intensity exercise impacts cellular senescence and inflammation, particularly in relation to participants' baseline levels.
In the placebo condition—where participants engaged in high-intensity interval training (HIIT) without ibuprofen—those with elevated baseline levels of p16^INK4a experienced the most significant decreases post-exercise. Specifically, these individuals saw a major reduction in senescence markers, suggesting that HIIT may be particularly effective in reducing senescent cell burden in those starting with higher levels.
In the ibuprofen condition, a similar trend was observed: participants with high baseline senescence markers also showed decreases after exercise. However, an unexpected finding was that a few participants exhibited increases in p16^INK4a levels when ibuprofen was administered. This anomaly raises questions about the interplay between anti-inflammatory medications and the body's natural processes for managing senescent cells. Again, it suggests that inhibiting inflammation pharmacologically might, in some cases, impede the beneficial effects of exercise on reducing cellular senescence.
Inflammatory Markers
When assessing inflammation through the expression of CD11b—a marker indicating immune cell infiltration—the study found that both exercise conditions led to reductions in inflammation, but with notable differences:
At 3 Hours Post-Exercise:
- Exercise + Placebo: Participants showed an 87% decrease in inflammatory markers compared to baseline. This substantial reduction indicates that HIIT can rapidly diminish muscle inflammation shortly after activity.
- Exercise + Ibuprofen: There was a 66% decrease in inflammatory markers. While still significant, this reduction was less pronounced than in the placebo condition.
- Importantly, the decrease in inflammation in the ibuprofen condition was not statistically significant when compared to pre-exercise levels at this time point. This suggests that ibuprofen may blunt the immediate anti-inflammatory effects typically induced by exercise.
At 24 Hours Post-Exercise:
- Exercise + Placebo: Inflammatory markers remained reduced by 80% from baseline.
- Exercise + Ibuprofen: The reduction was 73%, indicating that over a longer recovery period, the anti-inflammatory benefits of exercise become more similar between the two conditions.
Consistent with the findings on cellular senescence, participants who began with higher baseline levels of inflammation experienced the most dramatic declines in CD11b expression after exercise. This pattern underscores the potential of HIIT to significantly reduce inflammation, particularly in individuals with elevated inflammatory states.
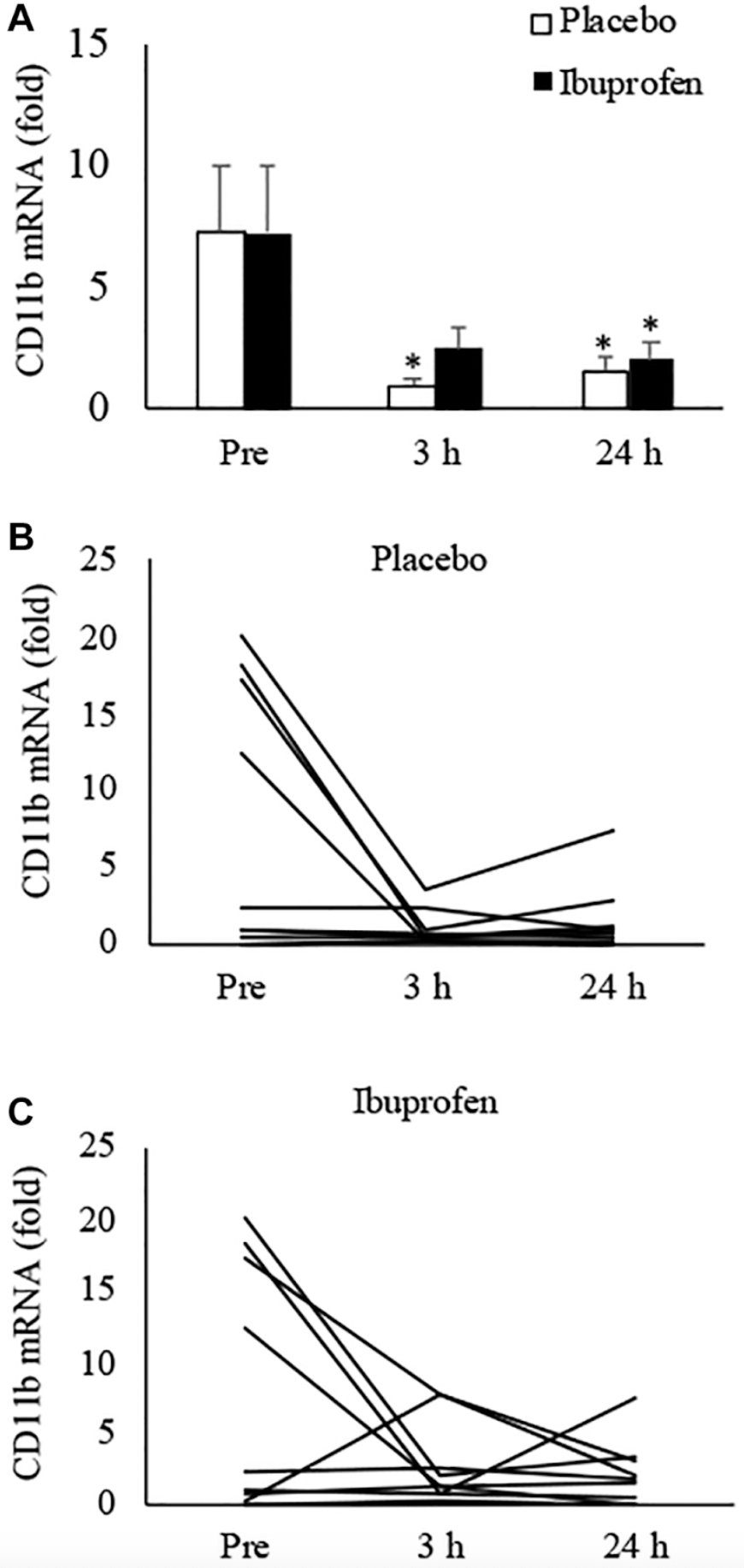
The differential responses among participants highlight several key insights into how high-intensity interval training (HIIT) affects cellular senescence and inflammation.
Baseline Levels Matter
Firstly, individuals with higher initial levels of senescence and inflammation markers benefited the most from HIIT. Those who began the study with elevated markers experienced more pronounced reductions in both p16^INK4a and CD11b expression after exercise. This suggests that people under greater physiological stress or exhibiting aging-related cellular changes may derive substantial improvements from high-intensity exercise. It indicates that HIIT could be particularly beneficial for populations with a higher burden of senescent cells or chronic inflammation, potentially mitigating some of the adverse effects associated with aging.
Inflammation's Dual Role
Secondly, the study underscores the dual role of inflammation in exercise-induced adaptations. Acute inflammation following exercise appears to be a catalyst for beneficial cellular changes. The attenuated reductions in senescence and inflammatory markers observed in participants who took ibuprofen imply that suppressing inflammation may hinder the body's ability to repair and rejuvenate at the cellular level. This finding emphasizes that acute inflammatory responses are not merely side effects to be mitigated but are integral to the adaptive processes that contribute to the anti-aging effects of exercise. Inflammation facilitates the removal of senescent cells and promotes tissue regeneration, highlighting its essential role in maintaining cellular health.
Potential Adverse Effects of NSAIDs
Lastly, the unexpected increases in senescence markers among some participants taking ibuprofen raise concerns about the routine use of nonsteroidal anti-inflammatory drugs (NSAIDs) around exercise sessions. These medications might interfere with the natural healing processes that contribute to the anti-aging benefits of exercise. By suppressing the acute inflammatory response, NSAIDs may impede the clearance of senescent cells and the subsequent anti-inflammatory phase that aids in recovery and adaptation. This observation suggests that habitual consumption of NSAIDs by athletes and physically active individuals could inadvertently diminish the cellular benefits of exercise, potentially affecting long-term health outcomes.
The study's findings reveal that allowing natural inflammatory responses to proceed post-exercise is crucial for optimizing the reduction of cellular senescence and promoting overall health and longevity. These insights call for a reevaluation of common practices involving anti-inflammatory interventions around exercise and support the development of exercise guidelines that maximize health benefits while minimizing unintended negative effects.
Embracing Inflammation: The Hidden Role of the Body's Natural Responses in Exercise Adaptation
For decades, athletes and fitness enthusiasts have sought ways to reduce inflammation after exercise, believing that minimizing soreness and swelling would expedite recovery and enhance performance. From popping anti-inflammatory pills to plunging into ice baths, these practices have been staples in training regimens. However, emerging research is challenging this conventional wisdom, revealing that these anti-inflammatory interventions may, paradoxically, hinder the very adaptations that improve fitness and health.
One striking study, published in the prestigious journal Science, highlighted the crucial role of histamines—compounds commonly associated with allergic reactions—in mediating the beneficial effects of exercise. Researchers discovered that when participants took antihistamines before exercising, their bodies exhibited a blunted response in vascular function, glucose metabolism, and overall exercise capacity. This suggests that histamines are essential messengers in the body's adaptation processes during and after physical activity, facilitating improved blood flow and metabolic efficiency [2].
Similarly, the long-favored practice of cold water immersion (CWI) post-exercise is now under scrutiny. While many athletes have embraced ice baths for their purported recovery benefits, studies have shown that immersing oneself in cold water immediately after resistance training can attenuate muscle hypertrophy and strength gains. The likely culprit is the anti-inflammatory effect of cold exposure, which suppresses the acute inflammatory response necessary for muscle repair and growth. By dampening inflammation, CWI may inadvertently hinder the very adaptations athletes aim to achieve through their training [3].
Antioxidant supplementation presents yet another paradox. Antioxidants like vitamins C and E are widely promoted for their health benefits, leading many to consume them in high doses around exercise sessions. However, excessive antioxidant intake can blunt training adaptations. Reactive oxygen species (ROS), often viewed solely as harmful byproducts of metabolism, actually serve as important signaling molecules that trigger adaptive responses in muscles. By neutralizing ROS through high-dose antioxidant supplements, we may interfere with these critical signaling pathways, reducing improvements in endurance, strength, and muscle function [4, 5].
Collectively, these findings highlight a critical insight: the acute inflammatory and oxidative stress responses induced by exercise are not merely collateral damage but integral components of the body's adaptation mechanisms. Suppressing these responses with anti-inflammatory agents—whether pharmaceuticals like ibuprofen, interventions like cold water immersion, antihistamines, or supplements like antioxidants—can diminish the effectiveness of exercise training.
Allowing the body's natural responses to proceed appears essential for maximizing adaptations to exercise—increasing mitochondrial function, promoting muscle hypertrophy, enhancing endurance, and reducing cellular senescence. Rather than seeking to eliminate inflammation and oxidative stress, embracing them as vital signals that drive adaptation and improvement may lead to better health and performance outcomes.
Intensity Matters
The discovery that exercise reduces markers of cellular senescence provides valuable insight into how physical activity may exert its "anti-aging" effects. By decreasing the number of senescent cells exercise helps mitigate chronic inflammation and tissue degradation associated with aging. This process contributes to healthier cellular environments and may delay the onset of age-related diseases.
A critical factor in this anti-aging effect appears to be the intensity of the exercise performed. The research team behind the current study previously demonstrated that high-intensity exercise, but not moderate-intensity exercise, significantly reduces senescence markers 24 hours post-exercise. This suggests that simply engaging in physical activity isn't enough; the exercise must reach a certain intensity threshold to elicit the desired cellular responses.
The key lies in the body's acute inflammatory response to high-intensity exercise. Such intense activity causes more significant muscle stress and microdamage compared to moderate exercise. This stress isn't detrimental; instead, it serves as a powerful signal that initiates a cascade of beneficial physiological processes.
This immediate reaction involves the release of pro-inflammatory cytokines and the attraction of immune cells, such as neutrophils and macrophages, to muscle tissue. Far from being detrimental, this acute inflammation acts as a catalyst for beneficial adaptations. It is essential for repairing damaged muscle fibers caused by the mechanical stress of intense activity and for stimulating the muscles to adapt to future demands. The influx of immune cells helps clear out debris and sets the stage for muscle regeneration and growth.
Following this initial inflammatory phase, the body undergoes a transition into an anti-inflammatory mode over the ensuing hours and days. During this period, the production of anti-inflammatory cytokines increases, and antioxidant enzymes like superoxide dismutase and glutathione peroxidase are upregulated. This shift helps resolve the initial inflammation, repair tissue, and restore homeostasis within the muscle environment. The anti-inflammatory response is crucial for attenuating excessive inflammation that could otherwise lead to tissue damage, ensuring that recovery proceeds efficiently.
Moreover, the heightened immune activity during these phases plays a significant role in clearing senescent cells—the so-called "zombie cells" that have ceased to divide but contribute to chronic inflammation through the senescence-associated secretory phenotype (SASP). Immune cells like macrophages can identify and remove these dysfunctional cells, reducing their accumulation within muscle tissue. By facilitating the clearance of senescent cells, the body not only mitigates chronic inflammation but also promotes healthier tissue function. This process may contribute to the anti-aging benefits associated with regular high-intensity exercise, as reducing senescent cell burden is linked to improved cellular health and longevity.
In essence, the interplay between the acute inflammatory response and the subsequent anti-inflammatory phase is vital for muscle adaptation and overall health. Embracing this natural sequence allows the body to repair, strengthen, and rejuvenate tissues effectively. Suppressing these responses—such as through the use of anti-inflammatory medications or interventions—may hinder these beneficial processes, underscoring the importance of allowing the body's innate mechanisms to operate unimpeded for optimal health outcomes.
Conclusion
Understanding that high-intensity exercise can reduce senescent cell markers emphasizes its potential role in promoting healthspan. The relationship between exercise intensity, inflammation, and cellular senescence offers a compelling narrative on how we might leverage physical activity for longevity. High-intensity exercise prompts the body to engage in processes that clear out senescent cells and reduce chronic inflammation, tackling two fundamental hallmarks of aging. By thoughtfully incorporating higher-intensity workouts into our routines, we can tap into these mechanisms, potentially extending our healthspan and improving our quality of life.
- Jean W, Lin Y, Ang P, Goto K, Lin C, Dewi L, Liao Y, Huang C, Kuo C. Senolytic effects of exercise in human muscles require acute inflammation. Aging (Albany NY). 2024 May 15; 16:8599-8610 . https://doi.org/10.18632/aging.205827
- Thibaux Van der Stede et al. ,Histamine H1 and H2 receptors are essential transducers of the integrative exercise training response in humans.Sci. Adv.7,eabf2856(2021).DOI:10.1126/sciadv.abf2856
- Piñero A, Burke R, Augustin F, et al. Throwing cold water on muscle growth: A systematic review with meta‐analysis of the effects of postexercise cold water immersion on resistance training‐induced hypertrophy. European Journal of Sport Science. Published online February 5, 2024:ejsc.12074.
- Dutra MT, Alex S, Silva AF, Brown LE, Bottaro M. Antioxidant Supplementation Impairs Changes in Body Composition Induced by Strength Training in Young Women. Int J Exerc Sci. 2019 Mar 1;12(2):287-296. PMID: 30899342; PMCID: PMC6413849.
- Merry TL, Ristow M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J Physiol. 2016 Sep 15;594(18):5135-47. doi: 10.1113/JP270654. Epub 2016 Jan 18. PMID: 26638792; PMCID: PMC5023714.