Mitochondrial and Metabolic Disparities Between Sedentary and Active Individuals: A Comparative Analysis at Rest and During Exercise
Prefer to listen? Hit play for a conversational, audio‑style summary of this article’s key points.
In a world increasingly characterized by sedentary behavior, the toll on public health is becoming alarmingly evident. While the benefits of regular physical activity are well-documented, the deeper, more insidious effects of inactivity on the body’s cellular and metabolic health have remained somewhat underexplored. As modern lifestyles drift further from the physically active routines of our ancestors, the need to understand the cellular consequences of inactivity has never been more pressing.
In a groundbreaking study, Dr. Inigo San-Millan from the Department of Physiology, Metabolism, Mitochondria, and Cancer at the Colorado School of Medicine released a pivotal paper exploring the differences in energy processing between sedentary and active individuals from a mitochondrial perspective. The results were dramatic and provide critical insights that quantify the underlying metabolic inefficiencies associated with inactivity.
San-Millan's study addresses this knowledge gap by focusing on the metabolic and cellular differences between sedentary and active individuals.
This paper goes beyond traditional markers of metabolic dysfunction, such as hyperglycemia and insulin resistance, by examining cellular bioenergetics—the detailed processes by which cells convert fuel into energy. It focuses on critical junctions in the metabolic process where inefficiencies arise, such as impaired pyruvate metabolism, reduced fatty acid oxidation, and lower electron transport system (ETS) capacity in sedentary individuals. These inefficiencies act as bottlenecks, limiting the cells' ability to produce energy efficiently and potentially setting the stage for the development of metabolic diseases.
The power of this study lies in its ability to paint a clear picture of what an efficient metabolic engine looks like versus a compromised one. By meticulously quantifying the bioenergetics of each component of the metabolic engine—ranging from pyruvate metabolism and fatty acid oxidation to the electron transport system (ETS)—Professor San-Millan’s research provides a detailed map of how energy is produced and where inefficiencies arise. This level of detail is what makes this paper so important.
San-Millan's work underscores the central role of mitochondria as regulators of metabolic health, illustrating how regular physical activity tunes the metabolic engine, enabling it to efficiently burn glucose and fatty acids for energy. In contrast, the metabolic engine of sedentary individuals sputters, plagued by inefficiencies like increased lactate production, heightened oxidative stress, and an inability to efficiently use an array of fuel sources, which compromise their ability to sustain energy production and manage metabolic stress.
In this paradigm, San-Millan presents compelling data suggesting that the roots of insulin resistance (IR) and type 2 diabetes (T2D) could begin at the mitochondrial level years, or even decades, before they manifest as overt metabolic conditions. Altogether, it paints a compelling picture of why enhancing mitochondrial health through exercise is a fundamental component of improving healthspan.
Background
We are all familiar with the impact of physical inactivity on public health. A public health report in 2018 likened being sedentary to being worse than smoking. To put things into perspective, inactivity is responsible for more than 5 million deaths globally each year. Among the various health metrics, low cardiorespiratory fitness (CRF) stands out as the leading contributor to all-cause mortality.
Physical activity is intrinsic to human nature, a defining characteristic of our species. Yet, in modern societies, the normalization of sedentary behavior has distorted this reality. What is now seen as an "intervention" — regular physical activity — is, in fact, the natural state embedded in our genetic makeup.
This reframing has had profound implications, even in the field of medical research. Many studies have used "healthy sedentary" individuals as control groups, treating the absence of physical activity as a baseline rather than recognizing it as a deviation from our evolutionary norm.
To understand the metabolic characteristics of these healthy sedentary individuals, Professor San-Millan sought to conduct one of the most expansive studies on the metabolic function and bioenergetic dysfunction of this population. By examining this population, the study seeks to uncover potential underlying metabolic disturbances that could, over time, contribute to the development of age-related chronic diseases.
The research centered on skeletal muscle, the largest and most metabolically active organ in the human body, making it an ideal site for studying cellular metabolism and bioenergetics. The team hypothesized that type 2 diabetes (T2D) likely originates in skeletal muscle, as approximately 80-85% of glucose is disposed of by this tissue during both rest and after a meal.
Under normal conditions, skeletal muscle mitochondria act like highly efficient engines, converting glucose into energy through a process known as oxidative phosphorylation (OXPHOS). Think of OXPHOS as the smooth, high-performance engine of your car. In this process, electrons from nutrients like glucose are passed along a series of steps, similar to how fuel moves through different parts of the engine. This electron transfer builds up pressure, much like building up torque in an engine, which then powers ATP synthase—the cell’s version of the car's crankshaft. The result is the production of ATP, the fuel that powers everything your body does.
OXPHOS is the most efficient way for cells to produce energy, especially during physical activity when the demand for power is high. Just like a well-tuned engine can maximize fuel efficiency and keep the car running smoothly over long distances, OXPHOS maximizes the energy extracted from glucose, allowing your muscles to keep going without breaking down.
However, when the mitochondria aren't functioning properly, the efficiency of OXPHOS drops. Imagine your car's engine starting to misfire or lose power—it can still run, but not as efficiently. In this scenario, glucose still enters the muscle cells, but it can’t be fully converted into energy through OXPHOS. Instead, the body shifts to a less efficient "backup generator" mode, called cytosolic fermentation. This is like switching from a powerful engine to a small, sputtering backup motor. Instead of getting the maximum energy out of glucose, the body now produces less energy and generates more waste—lactate, the cellular equivalent of exhaust fumes that can build up and cause muscle fatigue.
This shift from a powerful, efficient engine (OXPHOS) to a struggling backup motor (anaerobic glycolysis) is often seen in sedentary individuals, where mitochondrial dysfunction prevents the full use of glucose. The consequences of this "engine failure" can spread throughout the metabolic system, increasing the risk of developing chronic diseases like type 2 diabetes and heart disease. Maintaining efficient mitochondrial function through OXPHOS is like keeping your engine finely tuned, ensuring optimal performance and long-term health.
This is what we mean when we refer to bioenergetics. Bioenergetics involves the entire process of fuel conversion, from how cells take in nutrients, to how they process them in the mitochondria, and finally, how they produce and use ATP. How much energy can we output from those nutrient fuel sources?
It’s not just about efficiency, but also flexibility. A well-functioning cell, like a well-tuned engine, can switch between different fuel sources, like a hybrid engine, adapting to the body's needs in various situations, such as during exercise or fasting. When this system runs smoothly, the body can perform at its best. But when bioenergetics is disrupted, such as through impaired mitochondrial function, the body’s ability to generate and use energy effectively can break down, leading to fatigue, metabolic disorders, and other health problems.
In the context of this paper, we will examine glucose, fat, and lactate as fuel sources. How well can the cell switch fuel sources to oxidize them for energy? In the case of lactate, it’s important to note that it is both a byproduct and a fuel source. We will review how a poor metabolic engine produces more lactate as a byproduct but is also compromised in recycling it as a fuel source. Overall, active individuals can seamlessly switch between fuel sources—glucose, fats, or lactate—whereas sedentary individuals struggle with this metabolic flexibility. Let’s take a closer look.
How Do You Measure a Compromised Metabolic Engine? Key Indicators
Before diving into the results, it’s important to understand the key indicators that Professor San-Millan’s team used to assess the state of the metabolic engine. These metrics were carefully selected to quantify the efficiency and health of the mitochondria of these two populations. By focusing on these specific areas, the study aimed to reveal the underlying mechanisms that distinguish a well-functioning metabolic system from one that is compromised.
Mitochondrial Pyruvate Oxidation
Mitochondrial pyruvate oxidation was a crucial metric in this study because it serves as a pivotal point in the metabolic pathway where energy production can either proceed efficiently or become bottlenecked.
Pyruvate is the end product of glycolysis and must be transported into the mitochondria to be fully oxidized into acetyl-CoA. This oxidation fuels the TCA cycle, leading to ATP production through oxidative phosphorylation.
San-Millan focused on this process because, in sedentary individuals, it is believed that mitochondrial pyruvate oxidation becomes less efficient. This inefficiency can lead to a diversion of pyruvate towards less effective pathways, such as lactate production, reducing ATP output and contributing to fatigue and metabolic dysfunction.
Electron Transport System (ETS) Capacity
The electron transport system (ETS) is the final stage of oxidative phosphorylation, where the energy from nutrients is harnessed to produce ATP. Professor San-Millan prioritized ETS capacity as a key metric because it directly impacts how efficiently cells generate energy. A robust ETS ensures that electrons are transferred smoothly through a series of complexes in the mitochondria, culminating in the production of ATP.
However, the study hypothesized that sedentary individuals might have a reduced ETS capacity, leading to lower ATP production and increased electron leakage. This leakage can result in the formation of reactive oxygen species (ROS), harmful byproducts that cause cellular damage and contribute to metabolic dysfunction.
Fatty Acid Oxidation
Fatty acid oxidation was another critical metric in the study, selected for its role in determining metabolic flexibility and energy efficiency. In active individuals, fatty acids are effectively oxidized in the mitochondria through beta-oxidation, providing a sustained energy source, particularly during prolonged physical activity. This process helps preserve glucose stores, and supports continuous ATP production.
San-Millan hypothesized that sedentary individuals might experience impaired fatty acid oxidation, leading to a reduced mitochondrial capacity to utilize fat as an energy source.
This impairment could contribute to fat accumulation and metabolic disorders such as obesity and insulin resistance. By focusing on fatty acid oxidation, the study aimed to highlight how the reduced ability to burn fat in sedentary individuals could be a major factor in their overall metabolic inefficiency.
Increased Lactate Production and Reduced Lactate Clearance
Lactate production and clearance are direct indicators of how well the metabolic engine handles stress during exercise. Professor San-Millan recognized that an efficient metabolic system should manage lactate, a byproduct of anaerobic metabolism, by either utilizing it as a fuel or clearing it from the bloodstream.
In sedentary individuals, reduced mitochondrial function limits this capability, leading to a buildup of lactate, which causes early fatigue and diminished exercise performance. This bottleneck in lactate handling reflects the broader inefficiencies in the metabolic engine of sedentary individuals.
Increased Reactive Oxygen Species (ROS) Production
Lastly, Professor San-Millan measured reactive oxygen species (ROS) production as a key indicator of mitochondrial function. ROS are naturally occurring byproducts of mitochondrial activity, particularly during oxidative phosphorylation.
While a certain amount of ROS is normal, excessive production can lead to oxidative stress, which damages cells and accelerates aging. San-Millan focused on ROS because reduced mitochondrial efficiency in sedentary individuals may lead to higher ROS levels, potentially overwhelming the body’s antioxidant defenses.
This increase in ROS is not just a marker of mitochondrial stress but also a potential contributor to the development of chronic diseases such as cardiovascular disease and type 2 diabetes. By assessing ROS levels, the study aimed to quantify the extent of oxidative damage in sedentary individuals and its implications for long-term health.
Experiment Design
The study was conducted in two phases to assess the bioenergetics and muscle metabolism of both sedentary (SED) and moderately active (AC) individuals. A total of 19 participants volunteered—9 sedentary and 10 moderately active.
Phase 1: Resting Assessments
In the first phase, the researchers focused on muscle metabolism and mitochondrial function during rest. Muscle biopsies were taken from all participants to analyze various aspects of mitochondrial activity, including:
- Mitochondrial respiration: How effectively the mitochondria use carbohydrates, fatty acids, and amino acids to produce energy.
- Electron transport chain (ETC) efficiency: How well the mitochondria transfer electrons to generate ATP.
- Reactive oxygen species (ROS) production: Measuring the levels of potentially harmful molecules produced during energy generation.
- Fatty acid transporter activity: How efficiently the cells transport fatty acids into the mitochondria for energy use.
- Mitochondrial pyruvate carrier (MPC) function: Assessing the transport of pyruvate into the mitochondria for energy production. Additionally, advanced metabolic tracking methods, such as 13C-labeled lactate and pyruvate fluxomics, were used to gain deeper insights into cellular bioenergetics.
Phase 2: Exercise Assessments
The second phase of the study involved exercise testing to evaluate how well participants' muscles functioned during physical activity. The researchers conducted graded exercise testing (GXT), where participants exercised at gradually increasing intensities. During this phase, the team measured:
- Carbohydrate and fat oxidation: How efficiently the body burns carbohydrates and fats for energy during exercise.
- Lactate clearance: The body's ability to remove lactate, a byproduct of anaerobic metabolism, from the bloodstream, which reflects metabolic flexibility.
This exercise phase allowed the researchers to assess mitochondrial function in a non-invasive manner, correlating these findings with the data obtained from muscle biopsies. This two-phase approach helped the researchers validate their methods and provided a comprehensive view of metabolic health and mitochondrial function in both sedentary and active individuals.
We’ll begin by reviewing the muscle biopsy analysis from the rested phase. This will help us correlate the baseline mitochondrial behavior with the results observed during the exercise phase. First, we’ll analyze the mitochondrial function through the bioenergetics data on energy output. Next, we’ll examine the efficiency of enzymes responsible for transporting nutrients into the mitochondria. Finally, we’ll evaluate the study’s data on metabolites to assess the differences in fuel oxidation between active and sedentary individuals. This is the power of San-Milan’s study; it probably provides the most comprehensive view of mitochondrial function from many different vantage points.
Results: Resting Conditions
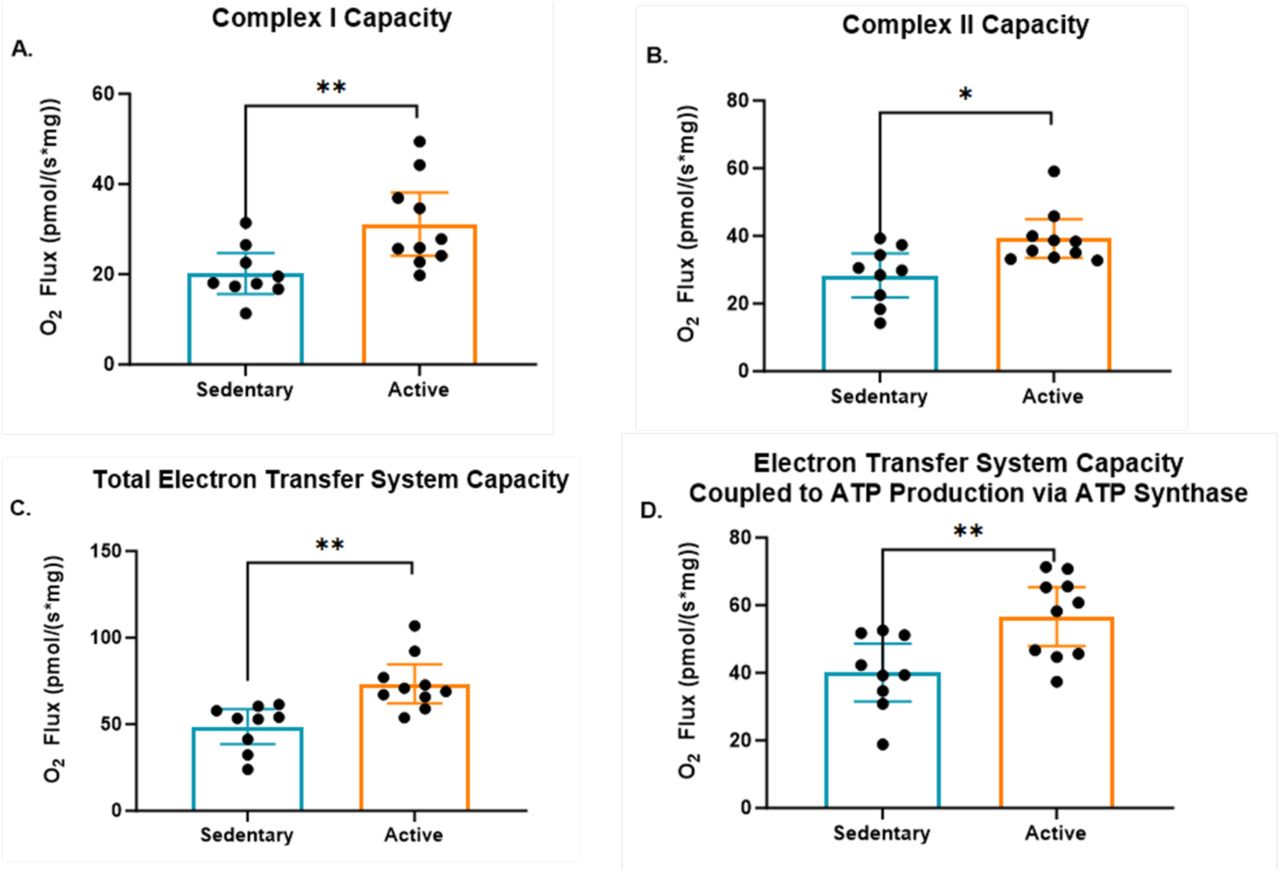
Mitochondrial Complex I and II Capacity
The study revealed a significant difference in the capacity of mitochondrial complexes between active individuals and sedentary individuals. Specifically, mitochondrial complex I capacity was 36% higher in active individuals compared to sedentary individuals, and complex II capacity was also elevated by 28% in active individuals. These findings suggest that active individuals have more powerful mitochondrial function at rest, which may contribute to greater energy efficiency and cellular resilience.
As a background, Complex I (NADH oxidoreductase) is the largest and first enzyme complex in the ETC, which is essential for cellular respiration. It plays a crucial role in the initial step of the ETC by accepting electrons from NADH, a product of the Krebs cycle. As these electrons are transferred through Complex I, protons are pumped across the inner mitochondrial membrane, creating a proton gradient that drives ATP synthesis. This step is critical because it sets the pace for the entire ETC, influencing how efficiently the cell can produce ATP, the energy currency of the cell.
Complex II (succinate dehydrogenase), on the other hand, is unique because it participates in both the Krebs cycle and the electron transport chain. It transfers electrons from FADH2, another key molecule produced in the Krebs cycle, directly to the ETC without pumping protons. While Complex II doesn't contribute directly to the proton gradient as Complex I does, it still plays a vital role in maintaining the flow of electrons through the ETC, ensuring that the process continues efficiently.
An increase in the capacities of both Complex I and Complex II in active individuals indicates enhanced electron flow through the ETC. This enhanced flow allows for more efficient ATP production, which is critical for meeting the energy demands of active muscles during exercise and recovery. The higher capacities of these complexes in active individuals suggest that their mitochondria are better equipped to handle the demands of energy production, leading to improved overall metabolic function, greater energy efficiency, and increased cellular resilience to stress. Simply put, active individuals produce more energy from the same amount of fuel.
At this junction in the metabolic pathway or metabolic engine, active individuals have a higher yield of energy production than sedentary individuals—they have higher-performing metabolic engines.
Total Electron Transfer System (ETS) Capacity
Building on the increased capacities of Complex I and Complex II, the study further revealed that the total capacity of the electron transfer system (ETS) was significantly higher (34%) in active individuals compared to their sedentary counterparts. This finding underscores the broader implications of having a more efficient electron transport chain, as it suggests that the mitochondria in active individuals are not only more robust at specific steps of the process but are also holistically better equipped to handle the entire flow of electrons necessary for ATP production.
ETS capacity is critical for ATP synthesis, as it represents the overall ability of mitochondria to efficiently manage and transfer electrons through the various complexes. A higher ETS capacity means that active individuals can sustain a more effective oxidative phosphorylation process, ensuring a steady supply of ATP even at rest.
This enhanced function provides them with greater metabolic flexibility, allowing their cells to seamlessly switch between fuel sources as needed and to respond more effectively to energy demands.
As a system, the electron transport chain of active individuals produces more energy in the form of ATP than that of sedentary individuals—reflecting a higher-performing metabolic engine.
ETS Capacity Coupled to ATP Production via ATP Synthase
The advantages of enhanced mitochondrial function in active individuals extend beyond just the total ETS capacity. The study also found that the capacity of the ETS specifically coupled to ATP production through ATP synthase was significantly higher (34%) in active individuals compared to sedentary individuals. This finding adds another layer to our understanding of how efficiently active individuals convert energy at the cellular level.
ATP synthase, the enzyme responsible for the final step of oxidative phosphorylation, plays a crucial role in synthesizing ATP from ADP and inorganic phosphate, using the proton gradient created by the electron transport chain. The fact that active individuals have a higher capacity for coupling ETS activity directly to ATP production suggests that their mitochondria are not only better at generating a proton gradient but also more effective at harnessing that gradient to produce ATP. This improved energy conversion process is likely a critical factor behind the enhanced physical performance and faster recovery times observed in active populations, as it ensures that their cells have a reliable and efficient energy supply to meet the demands of exercise and daily activities.
This underscores the idea that the metabolic engine in active individuals is functioning at a higher capacity, utilizing each element of the electron transport chain more efficiently than the sputtering metabolic engine of sedentary individuals.
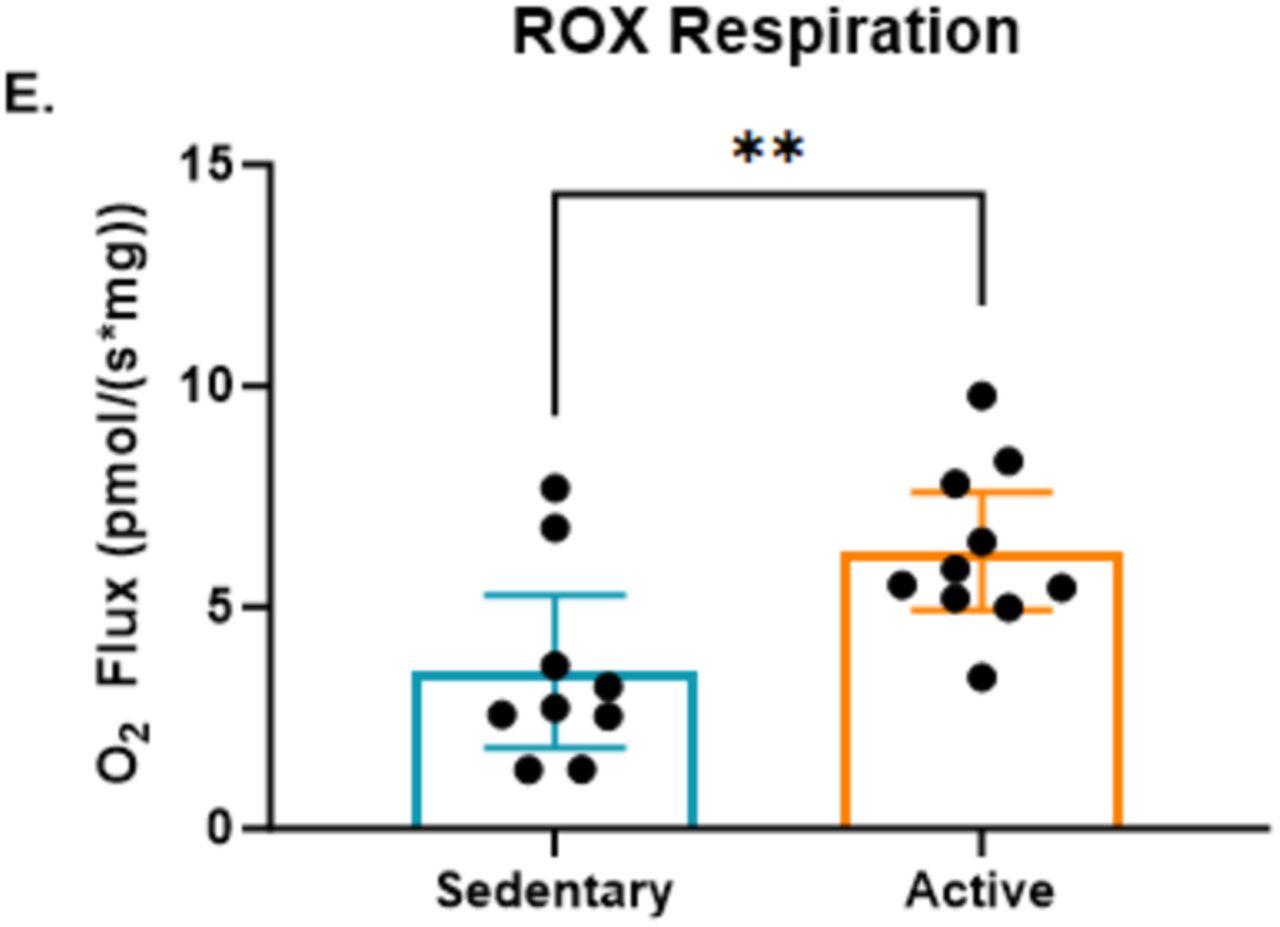
When we talk about metabolism, there is always going to be an element of metabolic exhaust. We have already introduced the idea of ROS and oxidative stress. The ETC leaks electrons that cause some amount of oxidative damage. However, here too, we see the benefits of having a high-functioning metabolic engine in terms of reducing that oxidative stress.
Beyond the enhanced capacity for ATP production, the study found that residual oxygen consumption (ROX)—the oxygen used after the ETC is inhibited—was significantly higher in active individuals compared to sedentary individuals. This observation adds a nuanced perspective to the metabolic efficiency seen in active individuals.
ROX reflects oxygen use in processes not directly related to energy production, such as the production of reactive oxygen species (ROS). The higher ROX in active individuals suggests that their bodies may be more adept at detoxifying ROS, possibly as a result of increased antioxidant activity stimulated by regular exercise. This enhanced detoxification serves as a critical protective mechanism, reducing oxidative damage to cells and supporting healthier mitochondria over time. In this way, the body’s ability to handle metabolic byproducts more effectively in active individuals not only contributes to improved immediate performance but also plays a role in long-term cellular health and resilience. We will explore more data on this point later in our analysis. For now, the important takeaway is that the study demonstrated that active individuals had less oxidative stress.
Now let’s dive into the data regarding nutrient flexibility in these respective populations. Let’s start with the analysis of fatty acid oxidation.
Fatty Acid Oxidation
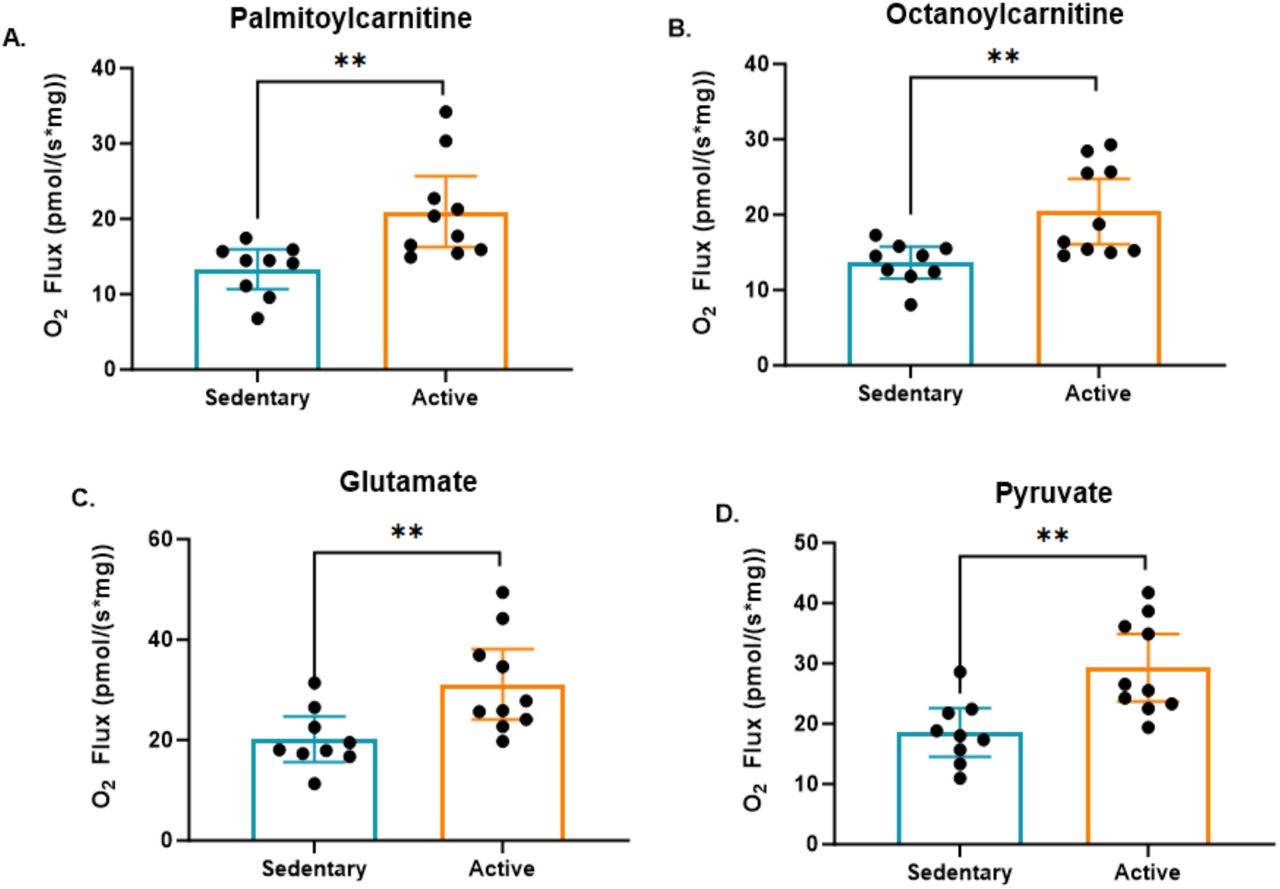
Another critical area where active individuals demonstrated superiority was in their capacity for fatty acid oxidation. The study showed that active individuals had significantly higher fatty acid oxidation capacity compared to sedentary individuals, using both palmitoylcarnitine (a long-chain fatty acid) and octanoylcarnitine (a medium-chain fatty acid) as fuel substrates.
This indicates that active individuals are more efficient at oxidizing fatty acids for energy. Fatty acid oxidation is especially important during fasting or prolonged exercise, when fats become the primary energy source. The enhanced ability to oxidize both long- and medium-chain fatty acids suggests that active individuals have greater metabolic flexibility, leading to better metabolic health and improved endurance.
The increased fatty acid oxidation capacity likely reflects adaptations in mitochondrial function, with regular physical activity boosting both the number and efficiency of mitochondria in skeletal muscle. These adaptations allow active individuals to manage energy balance and fat stores more effectively, reducing the risk of obesity and related metabolic disorders.
Returning to our analogy of the metabolic engine, active individuals function much like a hybrid engine, efficiently utilizing multiple fuel sources to optimize performance and maintain metabolic health. In contrast, sedentary individuals have marked impairment of fat oxidation.
Impaired fat oxidation in this group has been linked to the accumulation of intramuscular and intramyocellular fat. Intramyocellular fat refers to fat that builds up within muscle fibers; while a certain amount can serve as an energy reserve during exercise, excessive accumulation becomes problematic.
When sedentary individuals struggle to properly transport and oxidize fatty acids, the accumulation of fat in muscle cells disrupts insulin signaling. This buildup of intramyocellular fat may contribute to the development of insulin resistance—further disrupting metabolic function.
Pyruvate Oxidation
Building on the enhanced capacity for fatty acid oxidation, the study also highlighted a significant difference in another critical aspect of metabolism: pyruvate oxidation. As a biochemistry reminder, pyruvate is a key molecule in energy production, formed as the end product of glycolysis—the process by which glucose is broken down for energy. After glycolysis, pyruvate has two possible fates: under low-oxygen conditions, it can be converted into lactate, or, in the presence of oxygen, it is transported into the mitochondria where it enters the tricarboxylic acid (TCA) cycle. In the TCA cycle, pyruvate is further oxidized to produce ATP through oxidative phosphorylation, making it a vital link between anaerobic and aerobic metabolism.
The study found that pyruvate oxidation was significantly higher in active individuals compared to sedentary subjects. This increased pyruvate oxidation suggests that active individuals have a greater capacity for glucose utilization via aerobic pathways, leading to more efficient energy production. We’ll see this in the VO2 max analysis, where active individuals seem to be able to stay in an aerobic state longer than sedentary individuals.
The higher pyruvate oxidation capacity reflects the enhanced mitochondrial function observed in physically active individuals. This efficiency in managing both carbohydrate and fat metabolism further reinforces the overall metabolic flexibility that characterizes active individuals. By optimizing the use of glucose through aerobic metabolism, active individuals are better equipped to sustain energy production and maintain metabolic health, even under different physiological demands, without producing excessive lactate as a byproduct.
Amino Acid Oxidation
Continuing the exploration of metabolic flexibility, the study also revealed significant differences in amino acid oxidation between active and sedentary individuals. Specifically, the oxidation of amino acids, represented in the study by the substrate glutamate, was significantly higher in active individuals. Amino acids can serve as an alternative energy source, particularly during periods of fasting, prolonged exercise, or caloric restriction, when other energy reserves might be low.
The increased oxidation of glutamate in active individuals suggests a more adaptable metabolic profile, allowing them to efficiently tap into amino acids as a fuel source when needed. This ability reflects a broader metabolic flexibility, where the body can seamlessly switch between oxidizing carbohydrates, fats, and amino acids to meet its energy demands. Again, this flexibility is likely a key adaptation resulting from regular physical activity, enabling active individuals to maintain energy balance and resilience under varying conditions.
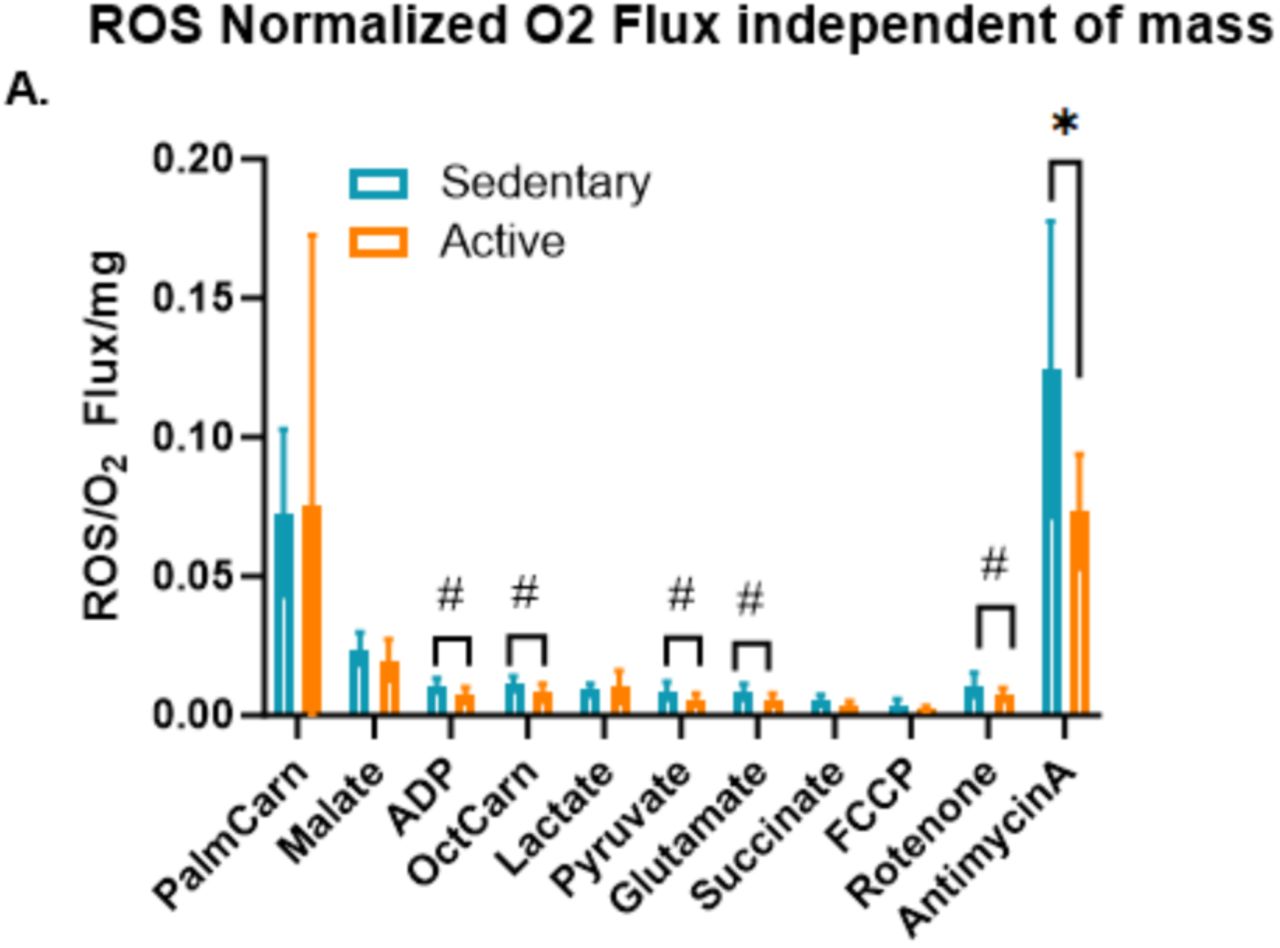
Reactive Oxygen Species (ROS) Production in Sedentary vs. Active Individuals
Beyond measuring the efficiency of metabolism and mitochondrial function in these distinct populations, the study showed how much more oxidative stress occurs in sedentary mitochondrial samples. Specifically, the study found that reactive oxygen species (ROS) production, normalized to oxygen flux, was higher in sedentary individuals compared to active individuals after the addition of various substrates and inhibitors, such as ADP, octanoylcarnitine, pyruvate, glutamate, and rotenone.
"Normalized to oxygen flux" means that the amount of ROS produced was adjusted relative to the total oxygen consumption in the mitochondria, providing insight into how efficiently the mitochondria are managing electrons. If ROS production is high relative to oxygen flux, it suggests that the mitochondria are leaking more electrons, resulting in the formation of harmful ROS rather than efficient ATP production.
The substrates used in the study, such as octanoylcarnitine (a medium-chain fatty acid) and pyruvate (a key product of glucose metabolism), were chosen to stimulate specific energy pathways, allowing researchers to observe mitochondrial responses under different conditions. In contrast, inhibitors like rotenone and antimycin A were used to block specific complexes in the electron transport chain (ETC), disrupting normal electron flow and increasing ROS production. By doing so, the study created a number of conditions and variables to see how they affected ROS production.
Notably, ROS production was significantly higher in sedentary individuals when exposed to antimycin A, an inhibitor of complex III in the ETC. This suggests that sedentary individuals are more susceptible to oxidative stress due to less efficient mitochondrial function.
The increased ROS production in sedentary individuals is likely an early indicator of mitochondrial dysfunction, which can lead to long-term cellular damage and contribute to the development of chronic diseases such as cardiovascular disease, diabetes, and neurodegenerative conditions.
ROS are natural byproducts of mitochondrial respiration, particularly during the electron transport process. Normally, mitochondria maintain a balance known as mitochondrial redox homeostasis, which keeps ROS levels in check through the cell's antioxidant defenses. While small amounts of ROS are essential for cellular signaling, excessive ROS production can overwhelm the system, leading to oxidative stress. This stress can damage lipids, proteins, and DNA, contributing to aging and the progression of chronic diseases. For this reason, oxidative stress is considered one of the 12 Hallmarks of Aging—a root cause of cellular dysfunction and systemic aging.
The study's observation that sedentary individuals produce more ROS in response to substrates like octanoylcarnitine, pyruvate, and glutamate suggests an imbalance in mitochondrial redox homeostasis. This imbalance indicates that the mitochondria in sedentary individuals produce more ROS than their cells can manage, leading to oxidative stress and cellular damage.
In contrast, active individuals seem to have better control over ROS production, likely due to enhanced mitochondrial efficiency. This efficiency allows mitochondria to manage electron flow more effectively, reducing excess ROS generation. Regular physical activity also boosts antioxidant defenses, providing added protection against oxidative stress. As a result, active individuals are better equipped to protect their cells from damage, supporting overall metabolic health and reducing the risk of chronic diseases.
These findings highlight a critical weakness in the mitochondrial function of sedentary individuals: even at rest, their mitochondria struggle to manage oxidative stress, increasing the risk of cellular damage and disease progression over time. The trend toward higher ROS production in sedentary individuals suggests that a sedentary lifestyle may contribute to elevated oxidative stress, even under normal resting conditions.
Over time, this chronic ROS elevation could lead to cumulative damage, promoting the development of metabolic disorders, cardiovascular diseases, and other age-related conditions. On the other hand, the lower ROS levels in active individuals may reflect the protective effects of physical activity, which is known to enhance antioxidant defenses and improve mitochondrial function.
Skeletal Muscle Glucose Transporter Cell Membrane Activity
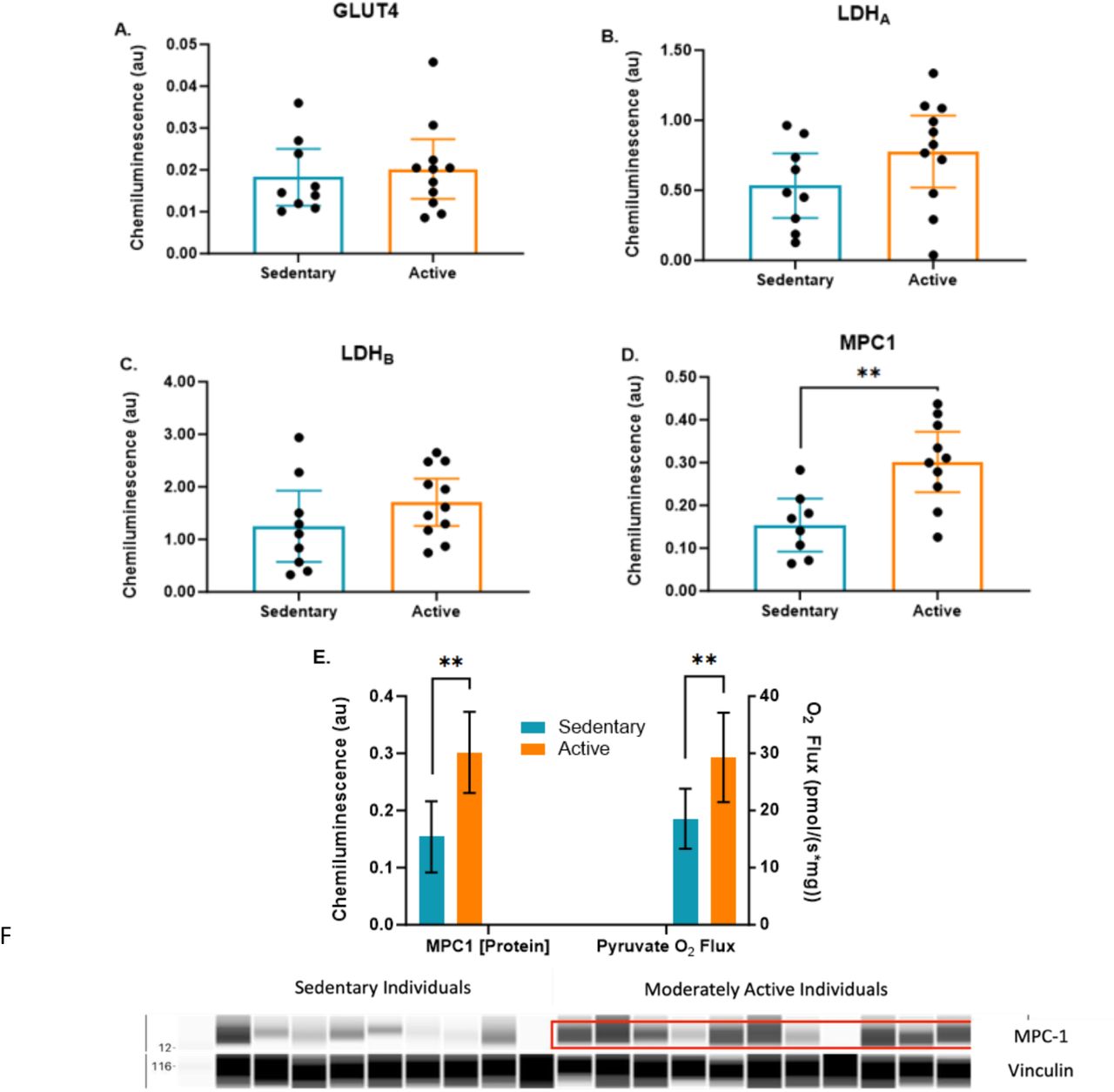
Next, the researchers looked beyond the mitochondria to evaluate if metabolic dysfunction occurs before glucose is processed by the mitochondria. Specifically, they evaluated whether sedentary individuals had a lower capacity to get glucose into the cell itself. To do so, they evaluated the levels of glucose transporters on the cell membrane. The study found that the quantity of skeletal muscle glucose transporter (GLUT4) and lactate dehydrogenase (LDH) isoforms A and B were similar between sedentary and active individuals.
GLUT4 is a critical protein that functions as the primary transporter responsible for glucose uptake in skeletal muscle and adipose tissue. When the body senses insulin or engages in physical exercise, GLUT4 transporters are activated, moving to the cell surface to facilitate the entry of glucose from the bloodstream into the muscle cells, where it can be used for energy. Given the importance of GLUT4 in regulating blood sugar levels and allowing glucose to enter the cell, the fact that both groups had similar levels of GLUT4 suggests that, at the level of protein expression, the capacity for glucose transport into muscle cells may not differ significantly between sedentary and active individuals under resting conditions.
The study also looked at LDH, an enzyme that plays a key role in the conversion of pyruvate to lactate and vice versa, depending on the oxygen availability in the muscle. LDH helps maintain the balance between anaerobic and aerobic metabolism by allowing cells to continue producing energy when oxygen is limited (such as during intense exercise). The similar levels of LDH in both groups indicate that the basic mechanisms for lactate production and utilization are comparable between sedentary and active individuals. This suggests that, at least in terms of LDH expression, the ability to manage lactate production and clearance is not significantly different between these two populations.
This finding is particularly significant because it suggests that mitochondrial dysfunction may occur before any issues with glucose transport at the cellular level, such as those involving the GLUT4 transporter. The observed decrease in pyruvate oxidation and ATP production in sedentary individuals was not due to an impaired ability of glucose to enter the cell—both the active and sedentary groups had similar levels of GLUT4 transporters.
Through their “inside-out” approach, the major finding of the study suggests that the downregulation of glucose metabolism might actually begin within the mitochondria, rather than at the cell membrane where GLUT4 and insulin receptors operate. The initial metabolic dysfunction seems to originate from the mitochondria's reduced ability to oxidize pyruvate, rather than from a failure of glucose to be transported into the cell. This mitochondrial dysfunction could potentially develop years before any noticeable issues with glucose uptake, insulin resistance, or GLUT4 dysregulation.
Mitochondrial Pyruvate Carrier I (MPC1) and Metabolic Engine Efficiency Building on this inside-out theory of metabolic dysfunction, another critical finding of the study was the significant difference in levels of mitochondrial pyruvate carrier I (MPC1) between sedentary and active individuals. Specifically, sedentary individuals displayed markedly lower MPC1 content.
MPC1 plays a pivotal role in the metabolic engine by transporting pyruvate into the mitochondria. Once inside the mitochondria, pyruvate is converted into acetyl-CoA and enters the tricarboxylic acid (TCA) cycle, driving ATP production through oxidative phosphorylation.
In sedentary individuals, the reduced levels of MPC1 create a bottleneck in this energy production process, much like a clogged fuel line in an engine. Without efficient transport of pyruvate into the mitochondria, the cells are forced to rely more heavily on the less efficient anaerobic glycolysis, leading to an accumulation of lactate. This buildup not only limits energy production but also contributes to muscle fatigue and overall metabolic inefficiency, akin to an engine sputtering and stalling due to inadequate fuel flow.
San-Milan’s study underscores the importance of MPC1 by highlighting the combined reduction in both MPC1 expression and pyruvate oxidation in sedentary individuals compared to their active counterparts. The implications for metabolic health are significant. The reduced MPC1 content and corresponding decrease in pyruvate oxidation reveal a critical malfunction in the metabolic engine of sedentary individuals, creating a stark contrast with the more efficient engine observed in those with active lifestyles.
While other metabolic markers, such as GLUT4 and lactate dehydrogenase (LDH), remained unchanged, the compromised mitochondrial handling of pyruvate in sedentary individuals signals downstream disruptions in glucose metabolism. This inefficiency in energy production could lead to the accumulation of metabolic byproducts like lactate, further clogging the engine and contributing to broader metabolic dysregulation. Over time, these disruptions may set the stage for the development of non-communicable diseases (NCDs) such as type 2 diabetes and cardiovascular disease.
Understanding the importance of maintaining an efficient metabolic engine underscores how even small disruptions in mitochondrial function can have cascading effects on overall metabolic health. As we see from the data on active individuals, regular physical activity plays a crucial role in keeping this engine running smoothly, optimizing energy production.
The study also explored the metabolic adaptation that distinguished active individuals from their sedentary counterparts in terms of specific adaptations to oxidize fatty acids for energy. Like pyruvate metabolism, which requires a carrier to transport pyruvate into the mitochondria, fatty acid metabolism requires its own set of transporters. San-Milan’s group focused on the activity levels of carnitine palmitoyl transferase I (CPT1) to measure fatty acid metabolism adaptations in active individuals relative to sedentary individuals.
The study revealed that active individuals had significantly higher activity of CPT1 compared to sedentary individuals. Going back to our metabolic engine analogy, think of CPT1 as the fuel injector in the metabolic engine, responsible for transporting long-chain fatty acids into the mitochondria, where they are broken down through beta-oxidation to produce energy.
This process is particularly important during prolonged physical activity or fasting when the body shifts to burning fat as its primary fuel source. In active individuals, the enhanced activity of CPT1 ensures that their metabolic engines run efficiently on fat, giving them the flexibility to switch between fuel sources and sustain energy production over longer periods.
The increased CPT1 activity in active individuals reflects their body’s adaptation to regular physical activity, which demands more efficient fat oxidation. This upregulation of CPT1 activity facilitates the smoother transfer of fatty acids into the mitochondria, allowing active individuals to tap into their fat reserves for energy during extended exercise sessions. This efficient fat utilization also helps to preserve glucose stores, reducing the risk of premature fatigue and supporting greater endurance. The ability to flexibly switch between fuel sources, like a hybrid engine switching between gas and electric power, is a key factor in the improved metabolic health and performance seen in active individuals.
Interestingly, the study found no significant difference in the activity of carnitine palmitoyl transferase II (CPT2) between active and sedentary individuals. CPT2 is responsible for the final step of fatty acid transport, moving the fuel molecules from the outer compartment of the mitochondria into the inner matrix, where beta-oxidation takes place. The similar CPT2 activity in both groups suggests that this step operates steadily regardless of physical activity levels. The real control point, or throttle, in the metabolic engine appears to be CPT1, which governs the rate at which fatty acids enter the mitochondria in response to the demands of physical activity.
This is critically important when we think about age-related chronic diseases and metabolic disorders. When sedentary individuals are unable to effectively transport and oxidize fatty acids, these fats can accumulate in and around muscle cells, especially near the mitochondria. Over time, this buildup of fatty acids may lead to detrimental effects, including the development of insulin resistance (IR). The inability to properly manage fat oxidation not only contributes to metabolic inefficiency but also sets the stage for broader metabolic disorders, highlighting the critical importance of maintaining robust mitochondrial function through regular physical activity.
Results: Cardiolipin Molecular Species
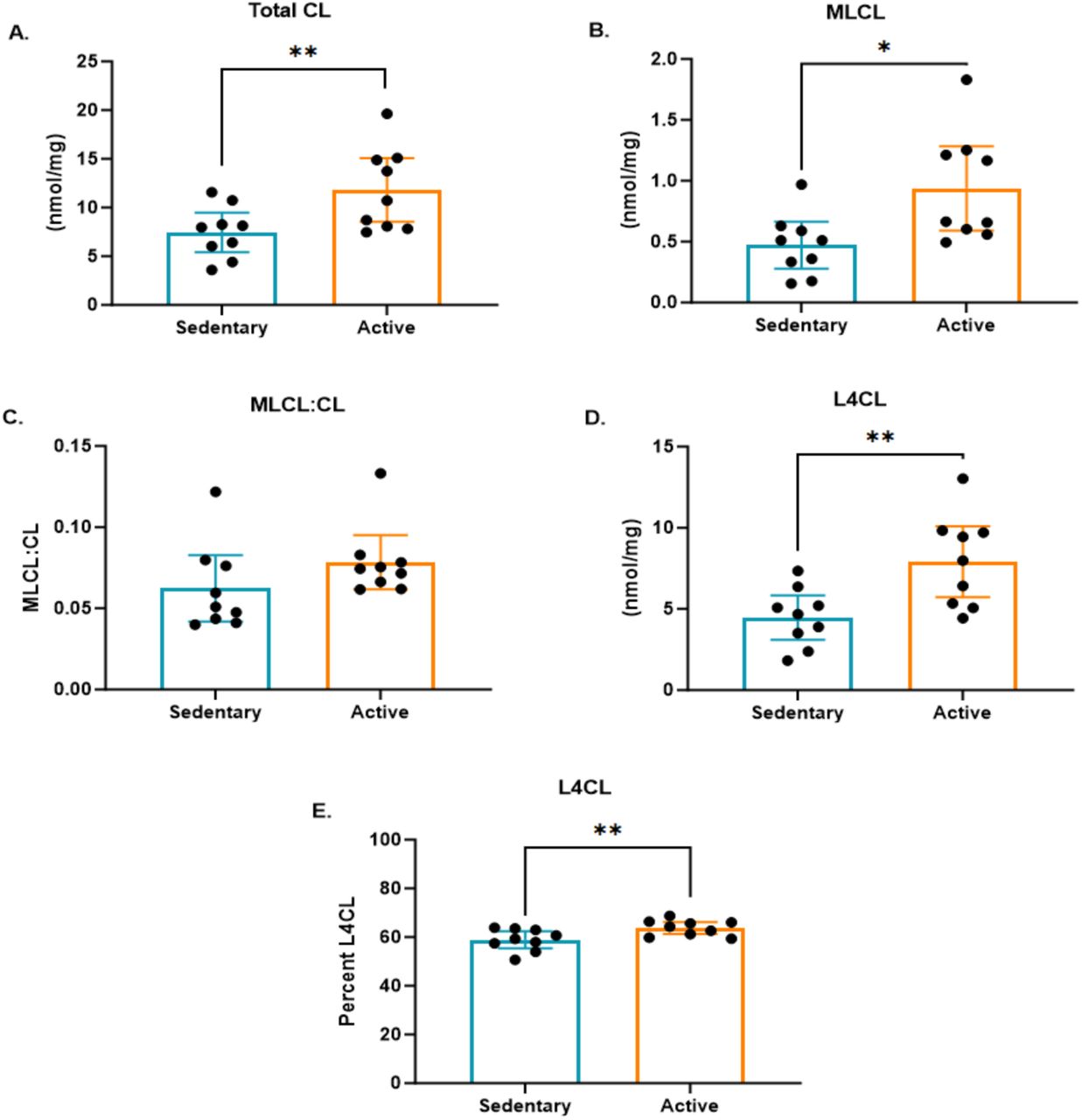
Next, San-Milan examined elements of the structure of the mitochondria in these two populations. In the study, significant differences were observed in cardiolipin (CL) content between sedentary and active individuals, with active participants showing higher total CL content.
Why does this matter? Cardiolipin is like the high-performance oil in the mitochondrial engine—it’s a specialized phospholipid found in the inner mitochondrial membrane, playing a vital role in maintaining the structure and function of the engine’s core components.
By supporting key proteins involved in oxidative phosphorylation and stabilizing the electron transport chain (ETC), cardiolipin helps the engine run smoothly and efficiently, ensuring maximum energy output. The increased CL content in active individuals suggests that their mitochondrial engines have stronger structural integrity and are better tuned for optimal performance, leading to improved energy production and overall metabolic efficiency.
Cardiolipin is particularly important for ensuring that the ETC operates efficiently, reducing the likelihood of electron leakage, which can produce harmful ROS activity. With higher CL content, the mitochondria of active individuals are better equipped to handle the demands of energy production, supporting more effective oxidative phosphorylation and keeping the metabolic engine running at peak performance.
The study also found an increase in monolysocardiolipin (MLCL) content in the active group. MLCL is a derivative of cardiolipin, formed when one of its four fatty acyl chains is removed. Elevated MLCL levels can indicate ongoing cardiolipin remodeling or turnover, processes that are essential for maintaining the optimal functionality of mitochondria, especially under conditions of increased metabolic demand, such as exercise.
However, the ratio of MLCL to CL was similar between the active and sedentary groups, suggesting that while active individuals may have higher levels of cardiolipin turnover, this is balanced by the regeneration of fully functional cardiolipin molecules. This balanced turnover may reflect a healthy adaptation to regular physical activity, where mitochondria constantly renew and optimize their membranes to support sustained energy production.
Additionally, a significant increase in tetralineolyl cardiolipin (L4CL) was observed in the active group, along with a higher percentage of L4CL. L4CL is the high-octane version of cardiolipin, containing four linoleic acid chains that make it the most functionally active form. It plays a critical role in ensuring the ETC operates at full capacity and maintains the integrity of the mitochondrial engine. The elevated levels of L4CL in active individuals suggest that their mitochondria are better optimized for handling the increased energy demands of regular exercise, much like how a performance car benefits from high-grade fuel to maximize power and efficiency.
These findings have a profound impact on understanding the effect of physical activity on the structure and function of the mitochondrial engine. The higher total cardiolipin and L4CL content in active individuals suggests that their mitochondria are better equipped to support efficient energy production and resist metabolic breakdowns. The increased MLCL content indicates ongoing maintenance, ensuring the engine remains in peak condition.
In contrast, the lower cardiolipin content in sedentary individuals suggests that their mitochondrial engines are not as well-maintained, leading to inefficiencies in energy production and a greater risk of breakdowns in the form of metabolic diseases. These adaptations in cardiolipin content and composition may be key mechanisms through which regular physical activity enhances mitochondrial function, metabolic flexibility, and overall cellular health. For sedentary individuals, the lack of these adaptations could contribute to a greater risk of developing chronic metabolic conditions over time.
Resting Mitochondria Results: Metabolomics Analysis of Skeletal Muscle Tissue
To explore the metabolic differences in skeletal muscle tissue between sedentary and active individuals at rest, the study employed high-throughput metabolomics using mass spectrometry. This advanced technique allowed the researchers to measure 292 different metabolites—small molecules produced or used during the body’s biochemical processes, such as energy production, fat metabolism, and muscle function.
By testing for these metabolites, the researchers could understand how the cells in sedentary and active individuals manage their energy resources. Metabolites serve as indicators of how efficiently various pathways are working. For example, high levels of glycolytic metabolites (involved in breaking down glucose) may indicate a greater reliance on carbohydrates for energy, while elevated levels of fatty acid oxidation intermediates suggest a higher capacity for fat metabolism.
By identifying which pathways are upregulated or downregulated in each group, the researchers could pinpoint specific areas of metabolic inefficiency in sedentary individuals—such as reduced fat metabolism or lower mitochondrial function—that might contribute to long-term health risks like obesity, insulin resistance, and type 2 diabetes.
Among the significantly different metabolites, several intermediates of glycolysis (the breakdown of glucose for energy) and fatty acid oxidation stood out. Active individuals had higher levels of fatty acid oxidation intermediates, such as acylcarnitines, indicating enhanced fat metabolism. This suggests that active individuals can rely more on fat as a fuel source, likely due to increased mitochondrial capacity for breaking down fatty acids.
Conversely, sedentary individuals showed higher levels of glycolytic intermediates, such as glucose and glyceraldehyde 3-phosphate, suggesting a greater reliance on carbohydrate metabolism. This could indicate lower metabolic flexibility, meaning that sedentary individuals may have a reduced ability to switch between burning carbohydrates and fats, which is a key aspect of metabolic health.
The metabolomics analysis confirmed that sedentary individuals generally exhibited downregulated carbohydrate and fat metabolism. The distinct metabolic profile of the sedentary group was marked by lower levels of fatty acid oxidation intermediates and a heavier reliance on glucose metabolism. This suggests that sedentary individuals may have reduced mitochondrial function, which limits their ability to efficiently switch between burning carbohydrates and fats as energy sources—a hallmark of reduced metabolic flexibility. These findings highlight the metabolic disadvantages of a sedentary lifestyle, as the body becomes less capable of adapting to different fuel sources as needed, potentially leading to long-term health consequences.
Exercise Data Analysis
VO2max Differences
In the second phase of the study, San-Milan’s team shifted focus to how well participants' muscles performed during physical activity. The researchers employed graded exercise testing (GXT), where participants exercised at progressively increasing intensities, to evaluate key aspects of metabolic and muscular function. The first major evaluation involved VO2max testing.
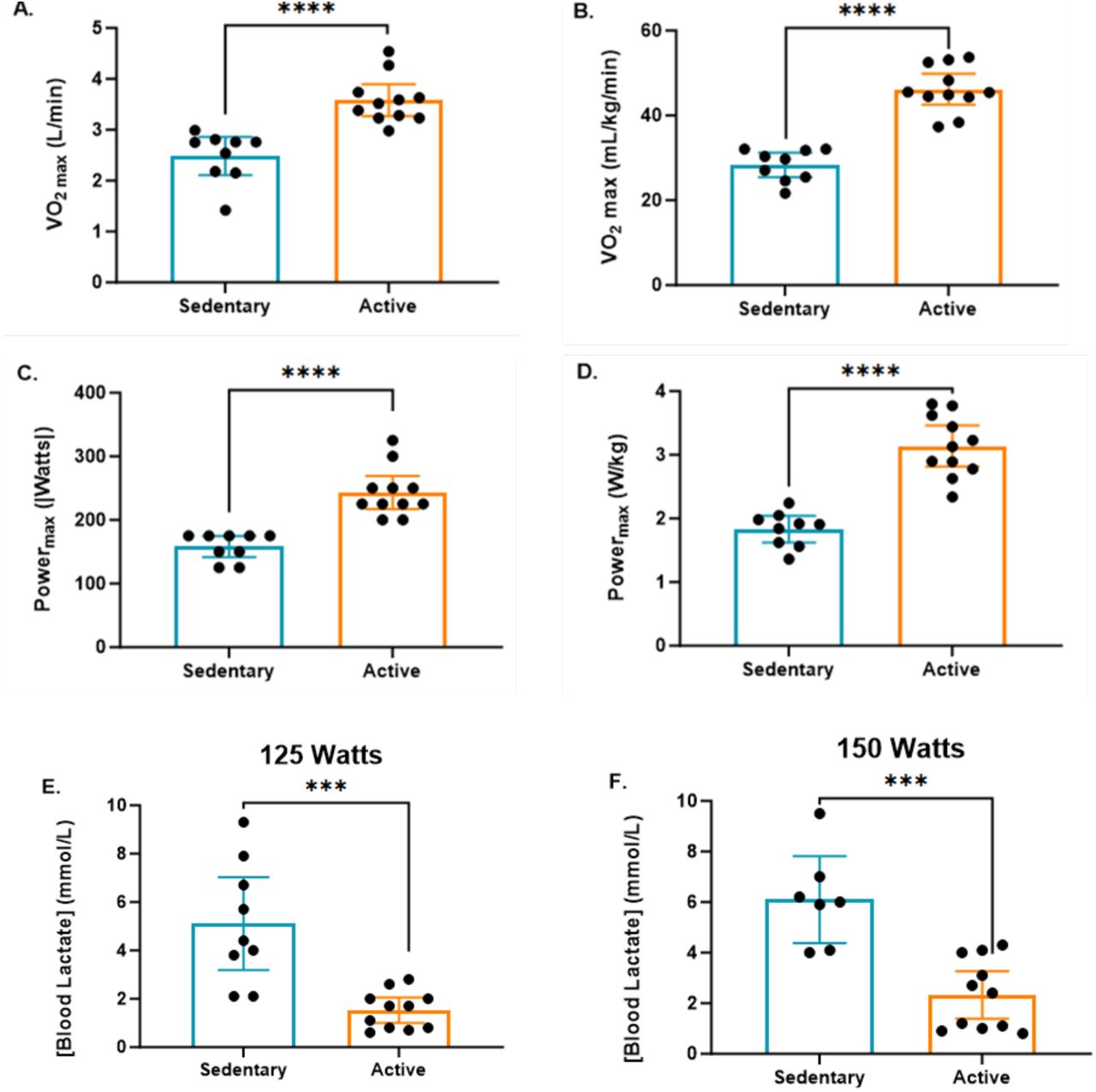
The study revealed that sedentary individuals exhibited significantly lower VO2max values, both in absolute and relative terms (31% and 38%, respectively), compared to active individuals. VO2max, the maximal oxygen uptake, is a critical indicator of cardiorespiratory fitness and reflects the body’s ability to deliver and utilize oxygen during sustained exercise. The significantly reduced VO2max in sedentary individuals indicates a diminished capacity for aerobic metabolism, which is a hallmark of lower cardiovascular and metabolic health. This lower VO2max suggests that sedentary individuals have a limited ability to perform sustained aerobic exercise, which predisposes them to fatigue earlier and reduces their overall exercise tolerance.
In contrast, the higher VO2max values in active individuals highlight their superior aerobic fitness, which is associated with more efficient oxygen delivery and utilization by the muscles. This enhanced aerobic capacity supports improved endurance performance, faster recovery, and better overall cardiovascular health.
Similarly, the study found that both absolute and relative maximal power output during the exercise test was significantly lower in sedentary individuals compared to active individuals (35% and 42%, respectively). Maximal power output represents the highest level of work or force that can be generated during exercise, and it is a key measure of muscular strength and endurance. The lower power output in sedentary individuals reflects weaker muscular performance and a reduced ability to sustain high-intensity exercise. This reduced power output is likely linked to the lower mitochondrial function and impaired energy metabolism observed in sedentary individuals, as well as their decreased muscle mass and strength.
On the other hand, the significantly higher power output in active individuals underscores their superior physical conditioning. Active individuals benefit from adaptations in muscle strength, mitochondrial density, and neuromuscular coordination, all of which contribute to greater power generation during exercise.
Lactate Clearance Capacity
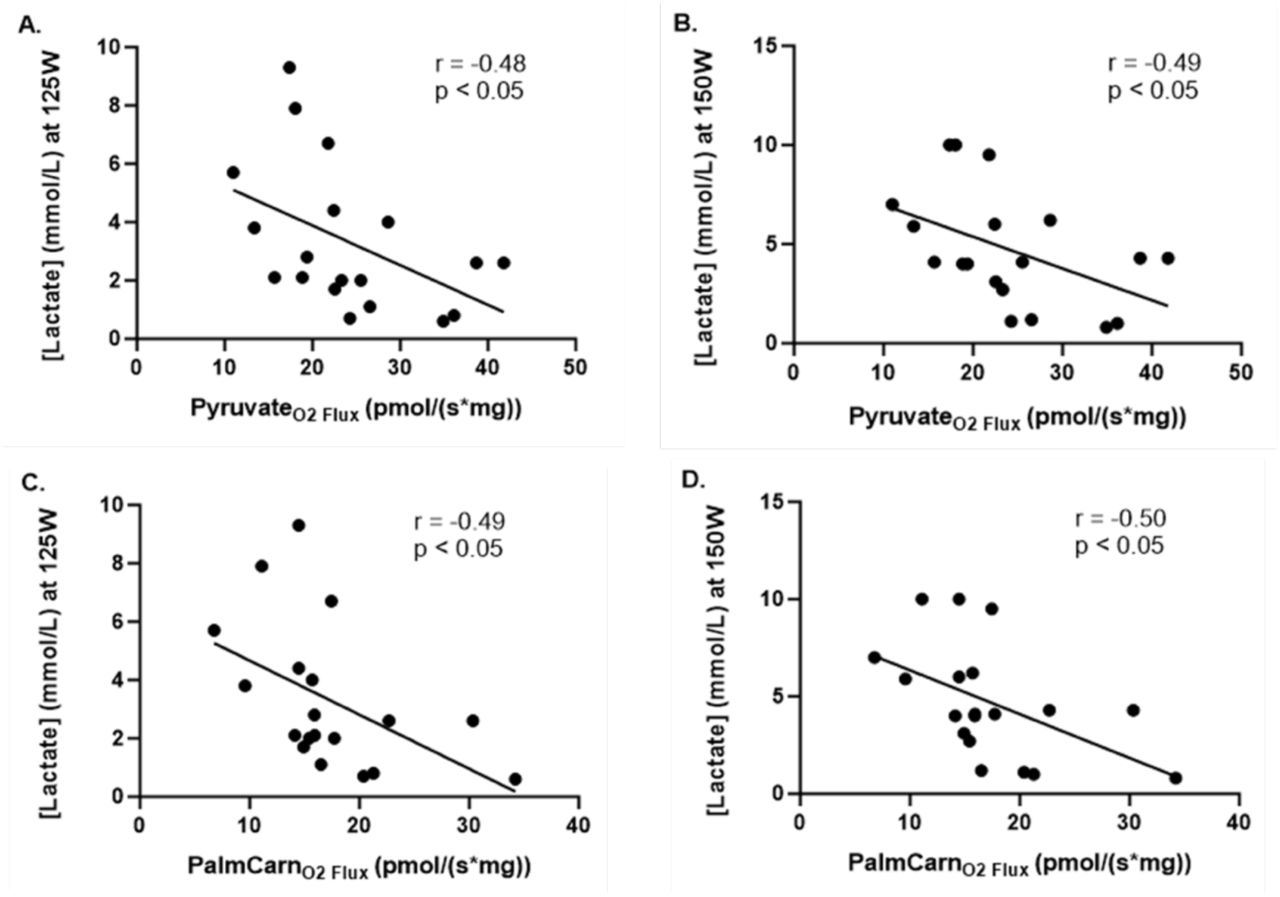
Building on the findings related to aerobic capacity and power output, the study also analyzed lactate clearance capacity—a measure of metabolic efficiency and the body’s ability to manage lactic acid buildup during exercise. The results showed that at both 125 Watts and 150 Watts, lactate clearance capacity was significantly superior in active individuals compared to sedentary individuals.
Lactate clearance refers to the body’s ability to remove lactate from the bloodstream, primarily through oxidation in the mitochondria, during and after exercise. During intense exercise, when the body’s demand for energy exceeds the oxygen available for aerobic metabolism, the body shifts to anaerobic metabolism, producing lactate as a byproduct. However, in active individuals, lactate isn’t merely a waste product; it’s recycled and used as an additional energy source through a process known as the lactate shuttle.
In this process, lactate produced in one muscle or tissue can be transported to other muscles, the heart, or the liver, where it is converted back into pyruvate. This pyruvate is then transformed into acetyl-CoA, which enters the TCA cycle (Krebs cycle) in the mitochondria, contributing to further ATP production. This recycling of lactate into usable energy is a key reason why active individuals have a higher lactate clearance capacity—they are not only removing lactate from the bloodstream but also repurposing it to fuel continued exercise.
The higher lactate clearance capacity in active individuals suggests that their metabolic systems are more efficient at processing lactate and preventing its accumulation, which can otherwise lead to fatigue and muscle soreness. In contrast, the lower lactate clearance capacity observed in sedentary individuals indicates a compromised ability to use lactate as a fuel source.
This impaired clearance could result in an earlier onset of fatigue during exercise and longer recovery times post-exercise. The superior lactate clearance in active individuals is likely due to several adaptations from regular physical activity, including enhanced mitochondrial function, increased capillary density, and improved oxidative capacity.
The significantly lower VO2max, maximal power output, and lactate clearance capacity in sedentary individuals reinforce the profound impact of physical inactivity on the performance and efficiency of the metabolic engine. These deficits suggest that sedentary individuals are less capable of sustaining both aerobic and anaerobic exercise, with their metabolic engines running underpowered and less efficiently. In contrast, active individuals demonstrate superior performance across these critical metrics, highlighting the importance of regular physical activity in improving metabolic performance and increasing bioenergetic capacity at every junction of the metabolic engine.
As the study further explored the relationships between energy metabolism and exercise performance, a series of important correlations emerged, providing insights on how different fuel sources are utilized during physical activity based on metabolic conditioning. San-Milan’s team saw similar trends as in the resting data, showing the metabolic flexibility among active individuals and a clogged and sputtering metabolic engine of sedentary individuals.
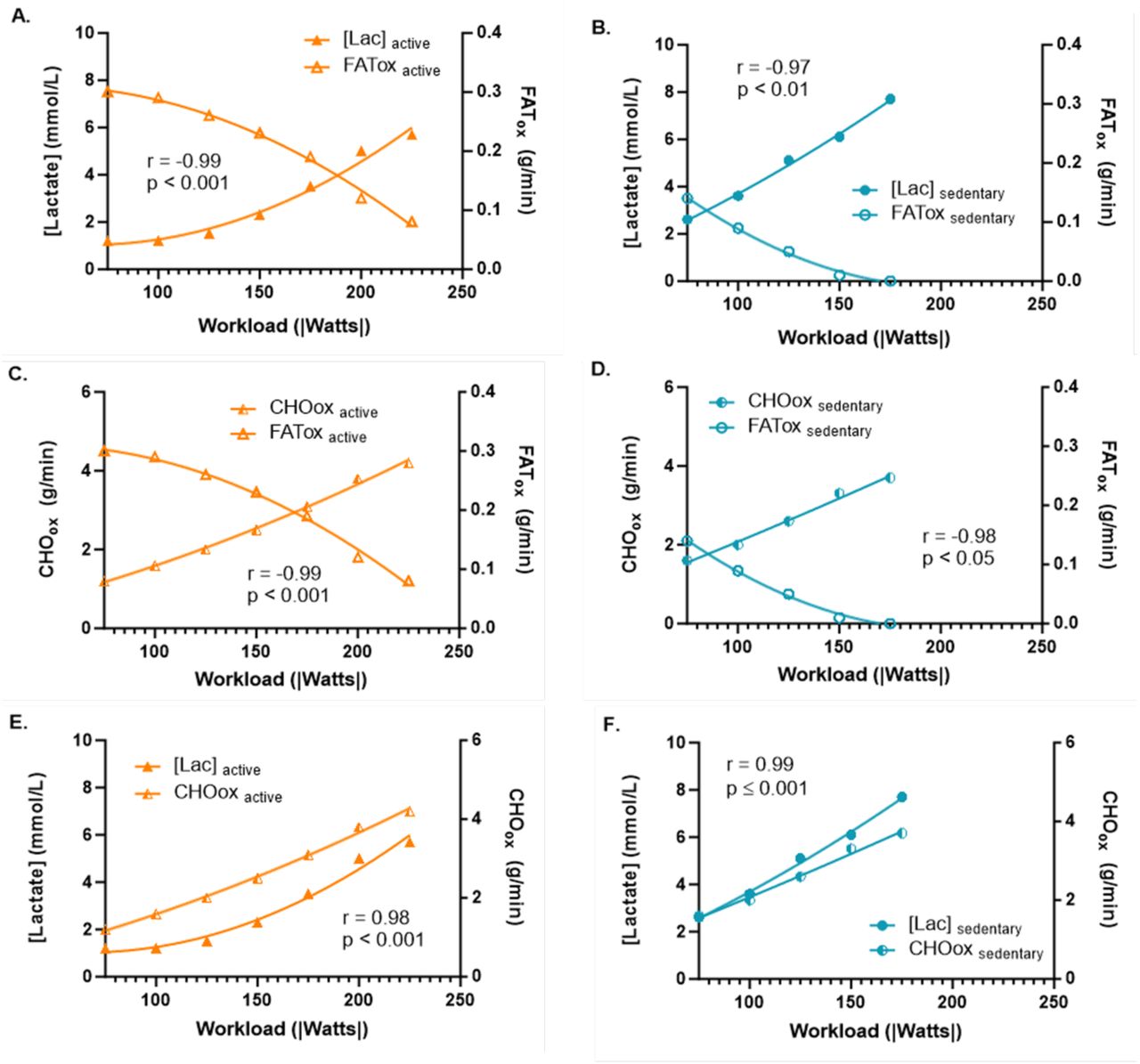
Inverse Correlation Between Blood Lactate Concentration and Fat Oxidation
The study demonstrated statistically significant inverse correlations between blood lactate concentration and global fat oxidation during exercise for both active and sedentary individuals—as lactate levels go up, fat oxidation goes down, and vice versa. This correlation was stronger in the active group compared to the sedentary group. The inverse relationship suggests that as blood lactate levels rise during exercise, the body’s ability to oxidize fats decreases.
Lactate accumulation typically signals a shift towards anaerobic metabolism, which relies more on carbohydrates than fats for energy. This finding indicates that both populations experience a reduction in fat oxidation as exercise intensity increases, but active individuals may be more efficient at managing this shift, potentially delaying the onset of lactate accumulation compared to their sedentary counterparts.
For active individuals, the stronger inverse correlation reflects their enhanced metabolic flexibility and ability to sustain fat oxidation at higher intensities of exercise. As emphasized in this analysis, regular physical activity leads to adaptations such as increased mitochondrial density and enhanced enzyme activity, which support the continued use of fats as a fuel source even during moderately intense exercise. In contrast, sedentary individuals may switch to carbohydrate metabolism more quickly as exercise intensity rises, reflecting a lower capacity for fat oxidation.
Inverse Correlation Between Carbohydrate Oxidation and Fat Oxidation
Similarly, the study found statistically significant inverse correlations between carbohydrate oxidation and fat oxidation for both groups. This correlation was stronger in the active group compared to the sedentary group. This inverse relationship is expected, as increasing reliance on carbohydrate oxidation typically occurs at the expense of fat oxidation, particularly as exercise intensity rises. Active individuals, with their greater metabolic flexibility, can maintain higher levels of fat oxidation for longer durations while gradually transitioning to carbohydrate metabolism as exercise becomes more intense.
In sedentary individuals, the weaker correlation suggests that their metabolic system may be less adaptable, resulting in a more rapid shift to carbohydrate oxidation and a more abrupt decline in fat utilization during exercise. Again, this limited metabolic flexibility could contribute to earlier fatigue and a lower overall capacity for prolonged exercise in sedentary individuals, as they are less able to efficiently balance fuel sources.
Correlation Between Blood Lactate Concentration and Carbohydrate Oxidation
The study also observed statistically significant correlations between blood lactate concentration and carbohydrate oxidation in both active and sedentary groups, with similar levels of significance. This positive correlation indicates that as lactate levels increase during exercise, carbohydrate oxidation also rises, reflecting the body's shift towards glycolysis (converting glucose to ATP) as a primary energy source. In both groups, rising lactate levels are associated with increased reliance on carbohydrates for energy, highlighting the interplay between lactate production and carbohydrate metabolism during higher-intensity exercise.
In active individuals, the correlation may reflect a more controlled and efficient transition to carbohydrate metabolism, allowing them to manage lactate accumulation and continue exercising at higher intensities without experiencing a dramatic decline in performance. Conversely, sedentary individuals may experience a more rapid accumulation of lactate and a corresponding spike in carbohydrate metabolism, leading to quicker exhaustion and reduced exercise tolerance.
The correlations between blood lactate, fat oxidation, and carbohydrate oxidation during exercise again reinforce the metabolic differences between active and sedentary individuals. This is another data set that shows active individuals display greater metabolic flexibility, allowing them to efficiently balance fat and carbohydrate utilization as exercise intensity increases. This flexibility supports better endurance performance and helps delay the onset of fatigue. Sedentary individuals, on the other hand, may experience a more rapid shift from fat to carbohydrate metabolism, coupled with earlier lactate accumulation, which can limit their exercise capacity and overall performance.
These findings emphasize the importance of regular physical activity in enhancing metabolic flexibility, improving lactate clearance, and optimizing energy utilization during exercise, all of which contribute to better long-term metabolic health and reduced risk of metabolic diseases.
Results: Ratios of Blood Lactate Concentration Relative to Mitochondrial Function
Ratios of Blood Lactate to Mitochondrial Pyruvate Oxidation Capacity
The study found that the ratios of blood lactate concentration during exercise at 125 Watts and 150 Watts, relative to resting mitochondrial pyruvate oxidation capacity, were significantly higher in sedentary individuals compared to active individuals. This finding suggests that sedentary individuals accumulate more lactate during exercise relative to their ability to oxidize pyruvate in the mitochondria at rest. The higher ratio in sedentary individuals indicates a mismatch between glycolytic flux and mitochondrial oxidative capacity, leading to greater reliance on anaerobic metabolism during exercise. Simply stated, sedentary individuals become anaerobic much faster than active individuals. This imbalance likely contributes to earlier lactate accumulation and reduced exercise tolerance in sedentary individuals, as their mitochondria may not be able to effectively keep up with the increased demand for aerobic ATP production during exercise.
Similarly, the ratios of blood lactate concentration during exercise, relative to total electron transfer system (ETS) capacity, were significantly higher in sedentary individuals compared to active individuals. As a reminder, the ETS is responsible for driving the production of ATP through oxidative phosphorylation by transferring electrons from electron donors, such as NADH and FADH2, to oxygen. A higher blood lactate to ETS capacity ratio in sedentary individuals suggests that their mitochondria have a reduced ability to efficiently handle the electrons generated from nutrient oxidation, leading to a greater reliance on anaerobic metabolism and lactate production during exercise.
This finding further highlights the impaired mitochondrial function in sedentary individuals, who may have fewer or less efficient mitochondria, reducing their capacity for oxidative phosphorylation and producing ATP in an aerobic manner.
The study also revealed that the ratios of blood lactate concentration during exercise, relative to ETS capacity specifically coupled to ATP production via ATP synthase, were significantly higher in sedentary individuals compared to active individuals.
ATP synthase is the enzyme that synthesizes ATP from ADP and inorganic phosphate, powered by the proton gradient generated by the ETC. A higher blood lactate to ETS-ATP synthase coupling ratio in sedentary individuals indicates that their mitochondria are less effective at converting the energy derived from electron transfer into usable ATP.
This inefficiency in ATP production forces sedentary individuals to rely more on anaerobic pathways, resulting in greater lactate accumulation during exercise. The higher ratio also suggests that sedentary individuals may experience a greater metabolic strain during exercise, as their mitochondria struggle to meet the energy demands of the working muscles.
The significantly higher ratios of blood lactate concentration relative to mitochondrial pyruvate oxidation capacity, ETS capacity, and ETS capacity coupled to ATP production in sedentary individuals underscore the limitations of their mitochondrial function. Sedentary individuals have a reduced ability to support sustained aerobic metabolism during exercise, leading to greater lactate production and earlier onset of fatigue.
In contrast, active individuals exhibit lower lactate to mitochondrial function ratios, reflecting their superior mitochondrial efficiency and greater capacity to meet energy demands through aerobic pathways. These adaptations, driven by regular physical activity, help to enhance exercise performance, delay fatigue, and promote better long-term metabolic health.
Results: Correlations Between Fat Oxidation (FATox) During Exercise and Mitochondrial Fatty Acid Oxidation at Rest
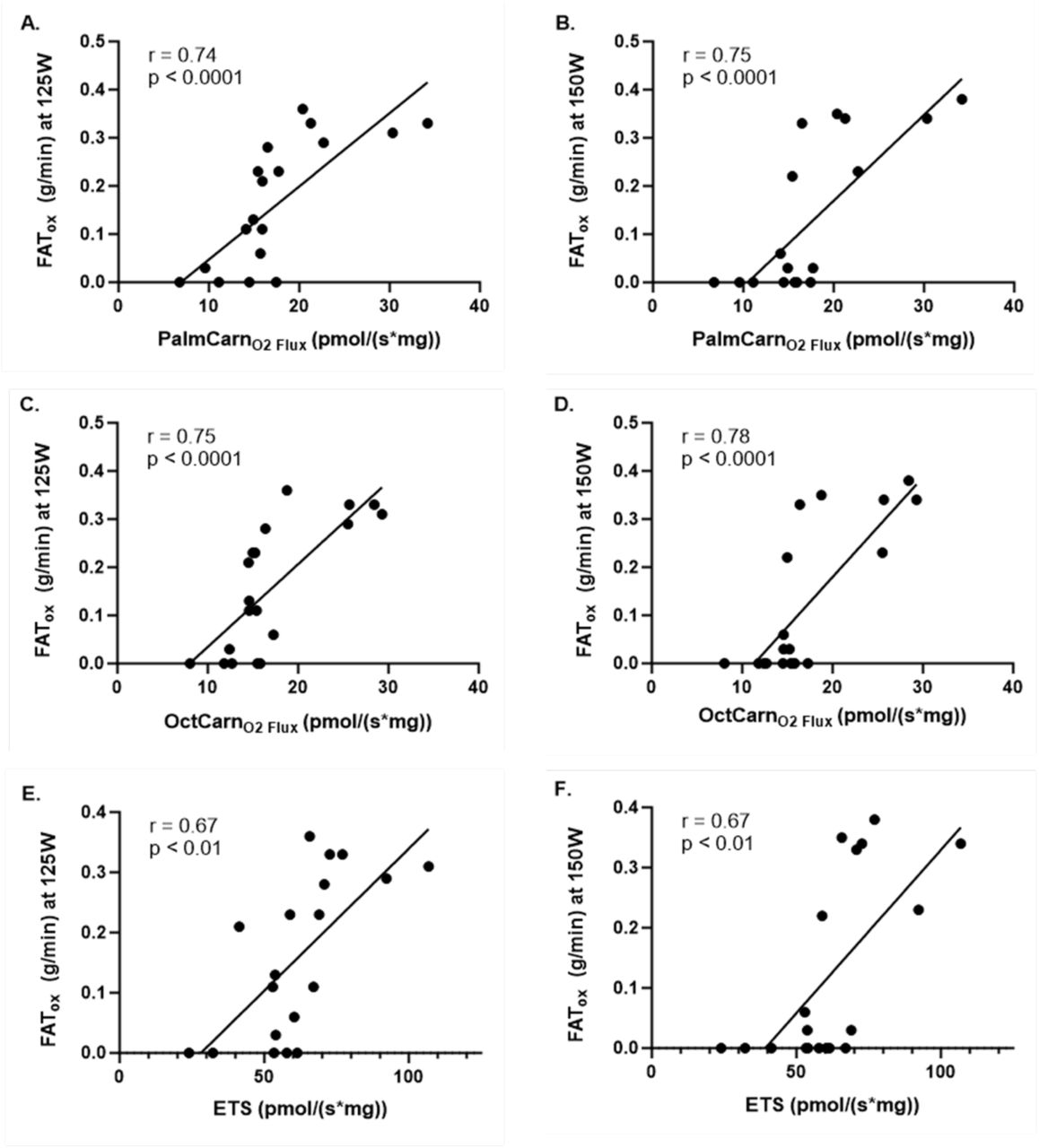
The study revealed strong correlations between fat oxidation during exercise at both 125 Watts and 150 Watts and mitochondrial oxidation of palmitoylcarnitine (a long-chain fatty acid (LCFA)) and octanoylcarnitine (a medium-chain fatty acid (MCFA)) at rest. These correlations were highly significant across the entire study group, encompassing both active and sedentary individuals. This finding suggests that an individual’s capacity for fat oxidation during exercise is closely linked to their mitochondrial ability to oxidize fatty acids at rest, regardless of their activity level.
The significant correlation also reflects the importance of mitochondrial adaptations to regular physical activity, which increase the capacity for long-chain fatty acid oxidation. In active individuals, higher levels of mitochondrial enzymes involved in beta-oxidation and improved fatty acid transport mechanisms support more efficient fat utilization during both rest and exercise. For sedentary individuals, reduced mitochondrial function and fatty acid oxidation capacity may limit their ability to rely on fat as a fuel source during exercise, leading to greater dependence on carbohydrate metabolism and earlier fatigue.
Correlation Between FATox During Exercise and Mitochondrial Resting ETS Capacity
The study found a strong correlation between fat oxidation during exercise at both 125 Watts and 150 Watts, and mitochondrial resting ETS capacity across both active and sedentary groups. This correlation indicates that an individual’s ability to oxidize fats during exercise is closely linked to the efficiency of their mitochondrial ETS at rest. The ETS is responsible for transferring electrons through a series of complexes in the inner mitochondrial membrane, ultimately driving ATP production through oxidative phosphorylation.
Increased ETS capacity at rest reflects a higher potential for ATP generation via oxidative pathways, which supports greater fat oxidation during exercise. Active individuals, who generally possess higher ETS capacity, are better equipped to efficiently utilize fats as a fuel source during exercise. Again, this correlation highlights the role of mitochondrial efficiency in determining the ability to sustain fat oxidation, which is essential for endurance performance and metabolic health. For sedentary individuals, lower ETS capacity contributes to reduced fat oxidation during exercise, leading to earlier reliance on carbohydrate metabolism and diminished exercise capacity.
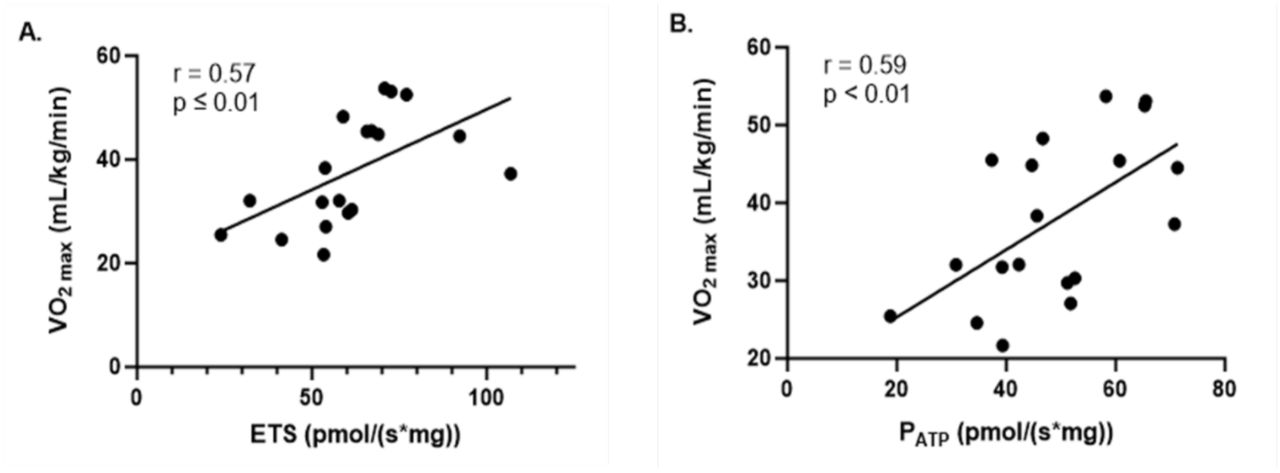
Correlation Between VO2max and ETS Capacity
The study also found strong correlations between VO2max, a key measure of cardiorespiratory fitness, and resting mitochondrial ETS capacity in both groups. As we have mentioned, VO2max reflects the maximal rate of oxygen uptake during intense exercise, which is directly related to the body’s ability to deliver and utilize oxygen for aerobic metabolism. The significant correlation between VO2max and ETS capacity suggests that individuals with higher VO2max values tend to have more efficient mitochondrial ETS function. This efficiency enables greater ATP production through oxidative phosphorylation, supporting the higher energy demands of aerobic exercise.
In active individuals, the correlation between VO2max and ETS capacity reinforces the importance of mitochondrial health in determining aerobic performance. Enhanced ETS function allows for better oxygen utilization and energy production, which contributes to the higher VO2max levels observed in active populations. For sedentary individuals, reduced ETS capacity may limit their oxygen uptake and utilization during exercise, leading to lower VO2max values and compromised aerobic fitness.
These strong correlations between fat oxidation during exercise, VO2max, and mitochondrial ETS capacity and ATP production (PATP) again reinforce the central role of mitochondrial function in determining exercise performance and aerobic fitness. Enhanced ETS and PATP capacity in active individuals support more efficient fat oxidation, better oxygen utilization, and greater ATP production, all of which contribute to superior exercise performance and endurance. These mitochondrial adaptations are critical for maintaining metabolic flexibility, delaying fatigue, and promoting long-term metabolic health.
For sedentary individuals, the reduced mitochondrial function reflected in lower ETS and PATP capacity is associated with diminished fat oxidation, lower VO2max, and compromised aerobic performance. These findings highlight the importance of regular physical activity in preserving and enhancing mitochondrial function to support better health outcomes and improved exercise capacity.
Inverse Correlations Between Blood Lactate Concentrations and Mitochondrial Pyruvate Oxidation
The study identified moderate inverse correlations between blood lactate concentrations during exercise at 125 Watts and 150 Watts and resting mitochondrial pyruvate oxidation for both active and sedentary groups combined. These inverse relationships indicate that individuals with higher resting pyruvate oxidation capacity tend to accumulate less lactate during exercise. Again, we are seeing that active individuals rely less on anaerobic metabolism, which is why we see less lactate buildup.
Efficient pyruvate oxidation reduces the need for anaerobic glycolysis, which produces lactate as a byproduct. Therefore, individuals with higher pyruvate oxidation capacity can better handle the metabolic demands of exercise through aerobic pathways, leading to lower lactate accumulation. The moderate inverse correlation suggests that mitochondrial function at rest is a significant determinant of lactate production during exercise across both active and sedentary populations.
The study also found moderate inverse correlations between blood lactate concentrations during exercise at 125 Watts and 150 Watts and resting mitochondrial fatty acid oxidation, measured using palmitoylcarnitine (a long-chain fatty acid (LCFA)), across both groups. This indicates that individuals with higher resting mitochondrial LCFA oxidation capacity are less likely to accumulate lactate during exercise.
Fatty acid oxidation in the mitochondria provides a significant source of energy during endurance exercise, particularly at moderate intensities. Efficient fatty acid oxidation reduces the reliance on glycolysis for energy, which in turn reduces lactate production. The observed inverse correlation suggests that individuals with better-developed mitochondrial fatty acid oxidation pathways are able to maintain more stable blood lactate levels during exercise, likely due to a more balanced and efficient energy metabolism that minimizes the shift toward anaerobic pathways.
These moderate inverse correlations between exercise blood lactate concentrations and resting mitochondrial pyruvate and fatty acid oxidation capacities highlight the critical role of mitochondrial function in managing lactate levels during exercise. Individuals with more efficient mitochondrial oxidative capacity—both in terms of carbohydrate (pyruvate) and fat (palmitoylcarnitine) metabolism—are better equipped to perform aerobic exercise without excessive lactate buildup.
In active individuals, these correlations likely reflect adaptations from regular physical activity, which enhances mitochondrial density and enzyme activity, allowing for more efficient energy production and reduced lactate production during exercise. In sedentary individuals, lower mitochondrial efficiency results in higher lactate accumulation during exercise, which is indicative of a greater reliance on anaerobic metabolism.
Closing Thoughts: Fine-Tuning the Metabolic Engine
The results of Professor San-Milan’s study reveal that even "healthy sedentary" individuals already exhibit significant cellular bioenergetic downregulations compared to their moderately active counterparts. These differences are profound, both at rest and during exercise, affecting multiple key components of the mitochondrial "engine." Active individuals consistently demonstrate superior mitochondrial performance, as evidenced by:
- Enhanced Electron Transport Chain (ETC) Function: Active individuals have a more efficient ETC, enabling better ATP production and energy utilization.
- Improved Substrate Transporter Activity: Active individuals show increased activity of transporters like the mitochondrial pyruvate carrier (MPC1) and carnitine palmitoyl transferase I (CPT1), facilitating more effective fuel utilization, whether from glucose or fatty acids.
- Superior Oxidation Processes: Active individuals exhibit higher rates of pyruvate and fatty acid oxidation, contributing to more efficient energy production and metabolic flexibility.
- Optimal Cardiolipin Configurations: Active individuals maintain better cardiolipin configurations, crucial for stabilizing the mitochondrial membrane and ensuring optimal ETC function.
- Reduced Reactive Oxygen Species (ROS) Production: Active individuals have lower ROS production, indicating less oxidative stress and better overall mitochondrial health.
- More Effective Lactate Clearance: Active individuals demonstrate superior lactate clearance, reflecting a more efficient metabolic engine that can manage and repurpose lactate as an energy source rather than letting it accumulate.
While some differences during exercise were expected, the presence of these disparities at rest was surprising, particularly since the sedentary group consisted of individuals without any known metabolic conditions.
One of the most critical findings from San-Milan’s study is the significant decrease in MPC1 activity and pyruvate oxidation in sedentary individuals, despite similar GLUT4 expression between the groups. MPC1 functions as a key valve in the metabolic engine, controlling the flow of pyruvate, a vital fuel derived from glucose, into the mitochondria where it can be fully "burned" for energy. In sedentary individuals, this valve appears to be malfunctioning, leading to a bottleneck in energy production. While their "fuel line" (GLUT4) is functioning properly, the inefficiency lies deeper within the engine—at the mitochondrial level. This suggests that the dysregulation of glucose metabolism may start within the mitochondria long before issues with GLUT4 and insulin function become apparent.
Furthermore, lactate, traditionally seen as merely a byproduct of anaerobic metabolism, has emerged as a crucial indicator of a clogged metabolic engine. In active individuals, lactate is efficiently cleared and even repurposed as a valuable fuel source, keeping their metabolic engine running smoothly. In contrast, sedentary individuals experience lactate accumulation, signaling that their engine is sputtering—unable to clear or utilize this byproduct effectively. This inefficiency forces a greater reliance on anaerobic pathways, leading to early fatigue and metabolic dysfunction.
For practical purposes, measuring your resting lactate levels through a simple serum chemistry lab test can provide valuable insight into the status of your metabolic engine. Elevated resting lactate levels would indicate compromised mitochondrial function, suggesting that your cells are struggling to efficiently produce energy, even at rest. This could be an early warning sign of metabolic inefficiency and a potential risk for developing metabolic disorders.
This insight is crucial because it indicates that the roots of insulin resistance (IR) and type 2 diabetes (T2D) could begin at the mitochondrial level years, or even decades, before they manifest as overt metabolic conditions. It underscores the importance of further research into pyruvate metabolism and lactate clearance as potential early targets for interventions that could prevent or delay the onset of these diseases.
Another key takeaway from San-Milan’s study is the strong correlation between exercise performance and mitochondrial function. The data suggests that metabolic testing and cardiopulmonary exercise testing (CPET) could serve as practical, non-invasive methods to assess mitochondrial health in millions of people. These tests can provide valuable insights into how well the metabolic engine is running, offering a window of opportunity to intervene before conditions like T2D and metabolic syndrome take hold.
Finally, San-Milan’s findings highlight a significant opportunity to intervene early through exercise and nutrition to fine-tune the metabolic engine of sedentary individuals. By doing so, it may be possible to avert the development of T2D and other metabolic disorders, ultimately improving the health and quality of life for millions.
Related studies