Sustained Postprandial Protein Anabolism: The body maintains elevated levels of protein anabolism (muscle building) during extended periods of high amino acid levels in the blood following meal consumption.
Minimal Impact on Amino Acid Oxidation: The ingestion of protein has a negligible effect on the oxidation (breakdown) of amino acids throughout the body.
No Modulation of Muscle Protein Autophagy: The process of protein ingestion does not alter the autophagy of muscle proteins, a cellular mechanism for clearing out damaged or unnecessary proteins.
Exogenous Amino Acids as Primary Precursors: In the postprandial phase (after eating), externally sourced amino acids (from ingested food) are the principal contributors to the accumulation of protein in the body.
Introduction: Debunking the Protein Myth – A New Perspective on Protein Utilization
For years, a pervasive myth in nutrition and fitness circles has been that the human body can only utilize up to 30 grams of protein in a single sitting. This belief has shaped dietary guidelines and influenced the eating habits of athletes and health-conscious individuals alike. However, new research is turning this notion on its head, suggesting that there may be no definitive upper limit to how our bodies respond to protein ingestion.
This latest study marks a significant shift in our understanding of protein metabolism, particularly in the context of post-exercise recovery. It reveals that consuming increasingly larger amounts of protein leads to a corresponding increase in protein absorption, muscle protein synthesis rates, and overall whole-body protein balance. These findings not only challenge the long-held belief in a strict protein utilization limit but also open up new avenues in nutritional science and dietary planning.
This article aims to delve into the nuances of this study, examining how it reshapes our understanding of protein metabolism and its practical implications for everyday nutrition and athletic performance.
The Dynamic Nature of Skeletal Muscle: Balancing Synthesis and Breakdown
Skeletal muscles are dynamic and adaptable, responding to various stimuli including physical exercise, dietary intake, and different disease states. Central to this adaptability is the continuous turnover of muscle proteins, which involves two key processes: synthesizing new proteins and breaking down old or damaged ones.
The interplay between muscle protein synthesis and breakdown is crucial not only for muscle mass changes but also for overall health and longevity. Muscle protein synthesis, highly responsive to external influences, is significantly boosted by activities like resistance training and the consumption of protein-rich foods, which supply the essential amino acids needed for muscle building.
On the other hand, muscle protein breakdown, though less immediately reactive, is equally important. It removes damaged or unneeded proteins, maintaining muscle function and adaptability.
A critical time for muscle health is the postprandial state after eating. During this period, dietary amino acids mainly drive muscle protein synthesis. As breakdown rates are more constant in the short term, the post-meal phase is key for achieving a net positive muscle protein balance, essential for muscle growth or maintenance. Adequate muscle mass brings several health benefits, including enhanced insulin sensitivity, increased basal metabolic rate, and greater resilience against illnesses and injuries.
This balance is particularly crucial in aging or disease conditions like sarcopenia, the age-related decline in muscle mass, and cachexia, the muscle wasting associated with chronic illness. These conditions highlight the importance of muscle protein turnover in developing strategies to maintain muscle health and combat muscle-related diseases.
Understanding Protein Metabolism Through Stable Isotope Methodology
Stable isotope methodology provides a comprehensive framework for assessing the kinetics of amino acids derived from dietary protein and their metabolic destiny. This technique involves the use of non-radioactive isotopes as tracers to track the metabolic pathways of amino acids. By incorporating these isotopes into proteins and monitoring their distribution and alteration in the body, researchers can gain detailed insights into protein digestion, absorption, and subsequent metabolic transformations.
By tracing proteins we see that the journey of protein metabolism begins when ingested proteins are enzymatically broken down into amino acids within the digestive system. While a fraction of these amino acids is metabolized by the liver, a significant portion enters the bloodstream, where they become available to other tissues.
Once in the bloodstream, these amino acids follow one of two primary routes. They either contribute to the formation of new tissue proteins through protein synthesis or undergo further breakdown in a process known as catabolism or oxidation.
Amino acids serve a dual function in the body. Beyond acting as metabolic precursors vital for protein synthesis, they also play a crucial role as signaling molecules. They regulate anabolic and catabolic pathways, thus influencing the body's metabolism and growth.
A key anabolic nutrient-sensing pathway, particularly relevant in the context of aging, is the mammalian target of rapamycin complex 1 (mTORC1) signaling cascade. This pathway is central to cell growth regulation and is highly responsive to the availability of essential amino acids, such as leucine, in the bloodstream. High leucine levels, for instance, activate mTORC1, promoting protein synthesis and cellular growth.
The responsiveness of mTORC1 to amino acid concentrations highlights the deep interplay between dietary intake and cellular processes, effectively linking nutrition with cellular function and health.
Exploring the Limits of Protein Utilization: Insights from Previous Dose-Response Studies
In the realm of protein metabolism research, dose-response studies offer vital insights into the optimal amount of dietary protein required to maximize muscle protein synthesis, particularly following exercise. These studies provide a fascinating glimpse into the body's capacity to utilize protein and challenge some long-held assumptions.
Dose-dependent studies have suggested that for healthy, young adults, consuming 20–25 grams of protein appears to be the sweet spot for maximizing muscle protein synthesis rates following exercise.
Intriguingly, consuming larger amounts of protein did not seem to yield further increases in these synthesis rates. Instead, the excess amino acids are suggested to be oxidized, not used in anabolic (muscle-building) processes. This finding has led to current dietary recommendations advising an even distribution of protein intake throughout the day, capping protein consumption at around 20–25 grams per meal.
However, this human-centric view of protein metabolism finds an interesting counterpoint in the natural world. For example, consider the feeding habits of many animal species, such as snakes. Snakes often consume large meals, sometimes exceeding 25% of their body mass. This results in a prolonged process of protein digestion and amino acid absorption, keeping their protein synthesis rates elevated for about ten days. Interestingly, only a small fraction of the ingested protein (approximately 5%) is directed toward oxidation.
This stark contrast raises questions about the transient nature of anabolic responses to feeding in humans and the presumed limited capacity of muscle tissue to incorporate dietary-derived amino acids. The prevailing concept, derived from metabolic tracer studies in humans, has primarily focused on the protein synthetic response following moderate protein intake (up to about 45 grams) over short durations (no more than six hours). These parameters may not adequately represent the body's ability to handle larger protein doses, as such short time frames might not allow for complete digestion and absorption of amino acids.
Therefore, these dose-response studies and comparative insights from the animal kingdom suggest that our understanding of protein metabolism, particularly in the context of large protein intakes, might still be evolving. This opens up to reconsider our current dietary guidelines and to explore the full potential of protein in human health and athletic performance.
No Upper Limit to Protein's Anabolic Impact in Humans: A New Perspective
The long-standing belief in nutrition science is that our bodies can only effectively use up to 30 grams of protein per meal. However, a recent study in Cell Reports Medicine challenges this notion, pushing us to reconsider what we know about protein metabolism.
In the study, thirty-six healthy men, aged between 18 and 40, participated in a randomized controlled trial. They were given three different doses of milk protein: 0 grams (serving as a control condition), 25 grams, or a substantial 100 grams. A unique aspect of this experiment was the use of a special isotope to label the proteins in the milk, enabling researchers to track the proteins' journey and fate within the body after ingestion.
Before consuming the protein drink, the participants underwent a 60-minute resistance training session. This included a 5-minute cycling warm-up, followed by four sets of 10 repetitions across a range of exercises like leg presses, leg extensions, lat pulldowns, and chest presses. These exercises were performed at 80% of the participants' one-repetition maximum, ensuring a significant level of muscular engagement.
To gain insights into protein handling kinetics—essentially, how the body processes and utilizes the ingested protein and amino acids—researchers collected blood samples and muscle biopsies from participants. These were taken both before the exercise and in the 12 hours following the combination of exercise and protein ingestion.
The Results
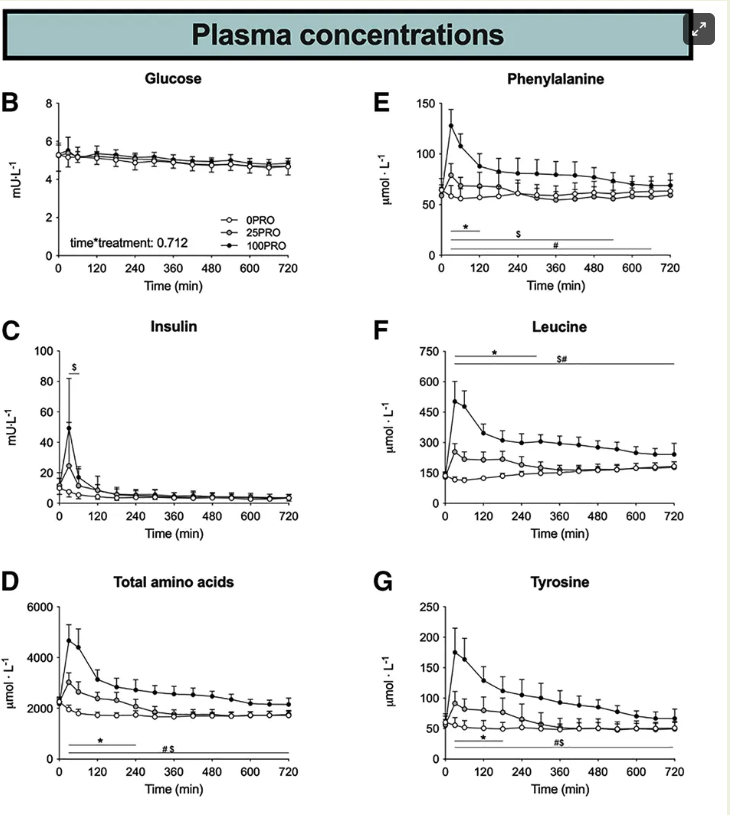
The study's findings were clear and compelling. Following the ingestion of protein, there was a rapid and dose-dependent increase in plasma amino acid levels—a critical indicator of how the body processes and utilizes the ingested protein.
When participants consumed 25 grams of protein, their amino acid levels in the plasma were significantly elevated compared to the control group, who ingested no protein. This elevation in amino acids was sustained for up to 5 hours post-ingestion, indicating a prolonged period of protein synthesis and utilization.
However, the most striking results emerged from the group that consumed 100 grams of protein. In this case, the concentration of amino acids in the plasma not only exceeded those observed in the 25-gram group but also remained elevated for an extended duration of up to 12 hours. This finding is particularly noteworthy as it implies that even at this later time point, the protein was still being digested and the amino acids were continuing to be absorbed and utilized by the body.
Analyzing Protein Metabolism: Synthesis Versus Oxidation
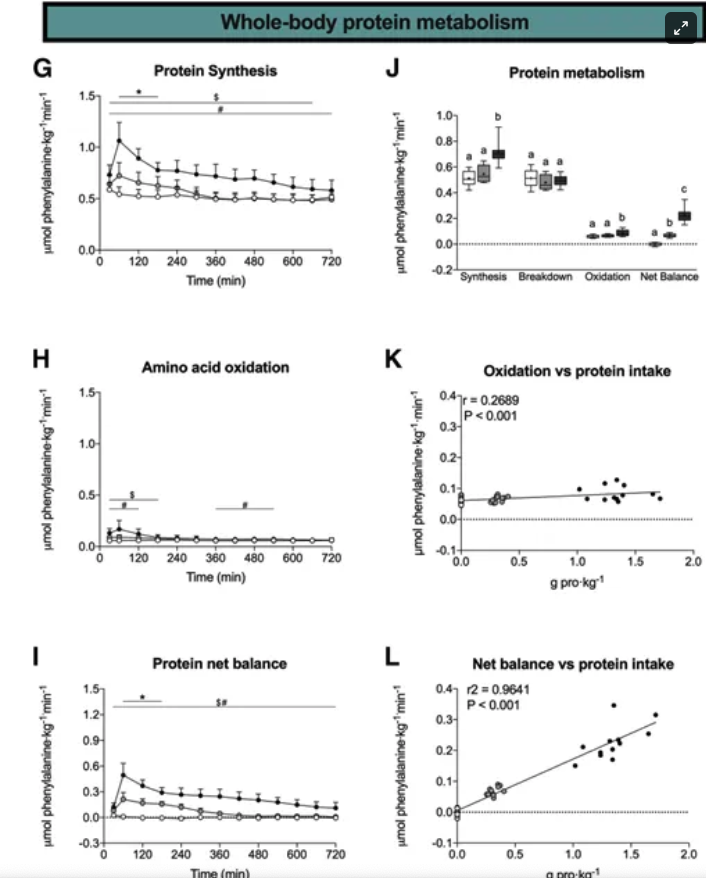
Next the researchers attempted to evaluate the relationship between protein intake dose and two key aspects of protein metabolism: whole-body protein synthesis and amino acid oxidation.
The correlation observed in the study is quite revealing. After consuming 25 grams of protein, participants showed an increase in whole-body protein synthesis compared to the control group, who ingested no protein. This increase was even more pronounced in the group that consumed 100 grams of protein, indicating a clear dose-response relationship between protein intake and the body's protein synthesis capabilities.
However, the study's findings regarding amino acid oxidation (the breakdown of amino acids) paint a different picture. Despite the increased protein intake, amino acid oxidation did not rise in proportion to the amount of protein consumed. This is a critical observation, as it suggests that the body does not simply ramp up the breakdown of amino acids as more protein is ingested.
These results collectively point to an intriguing conclusion: as protein intake increases, there is a corresponding increase in blood amino acid concentrations, their uptake into tissues, and the promotion of whole-body protein balance. This occurs in a dose-responsive manner, suggesting that the body is capable of effectively utilizing higher amounts of protein than previously thought.
What stands out, particularly, is the finding that amino acid oxidation remains relatively "negligible" even with high protein intake. This suggests that the body has a robust capacity for protein synthesis and utilization without resorting to a proportional increase in amino acid breakdown. These insights offer a fresh perspective on protein metabolism, challenging the traditional view that there is a limited window of protein utilization.
The Impact of Protein Intake on Muscle Protein Synthesis
As we delve further into the study's findings, a particularly intriguing aspect comes to light regarding muscle protein synthesis, specifically in the myofibrillar component, during the 12-hour postprandial (post-meal) period.
The results here mirror the earlier observations regarding whole-body protein synthesis but focus more narrowly on muscle protein. When participants consumed 100 grams of protein, the increase in muscle protein synthesis rates was notably higher than the increase observed with 25 grams of protein. In both cases, these rates were significantly greater than those seen in the control group, who did not consume any protein.
Myofibrillar proteins are crucial as they are the main components of muscle fibers, responsible for muscle contraction and overall muscle health. The enhanced synthesis of these proteins is vital for muscle growth, repair, and overall function.
This part of the study's results is particularly significant for understanding how dietary protein directly impacts muscle growth and repair, especially in the context of resistance training and athletic performance. The finding that 100 grams of protein leads to a greater increase in muscle protein synthesis than 25 grams challenges the conventional wisdom that there's a plateau effect in protein utilization for muscle synthesis.
The implication of these results is substantial: it suggests that consuming higher amounts of protein post-exercise can lead to more significant muscle protein synthesis, potentially benefiting muscle growth and recovery more than previously believed.
The Protein Synthesis Response: Unpacking the Details
The study's detailed analysis of muscle protein synthesis rates offers profound insights into how the body responds to varying protein doses, especially over an extended postprandial period.
In the first four hours following protein ingestion, the study observed a 20% higher rate of muscle protein synthesis in participants who consumed 100 grams of protein compared to those who ingested 25 grams. This difference became even more pronounced during the 4–12 hour postprandial period, where the muscle protein synthesis rates were 40% higher in the 100-gram group. Overall, this equated to a 30% larger synthetic response after consuming 100 grams of protein, as opposed to 25 grams.
A key factor in explaining these higher synthesis rates was the sustained presence of protein-derived amino acids in the bloodstream. In the 100-gram condition, 26% of the amino acids appeared after 4 hours, 44% after 8 hours, and 53% after 12 hours. This pattern indicates that a larger dose of protein can indeed be fully utilized by the body, albeit over a longer period for complete digestion and absorption.
These findings challenge the previously held belief that there is a rapid plateau in the body's ability to utilize amino acids from ingested protein. Instead, the body appears capable of sustaining muscle protein synthesis over a much longer duration when provided with a higher dose of protein.
Furthermore, an interesting aspect of the study was the percentage of protein-derived amino acids incorporated into skeletal muscle tissue. Approximately 13–18% of these amino acids were used in muscle synthesis, a figure higher than previously reported. This enhanced incorporation can likely be attributed to the prior exercise bout, which acted as a potent anabolic stimulus, priming the muscles for increased protein uptake and synthesis.
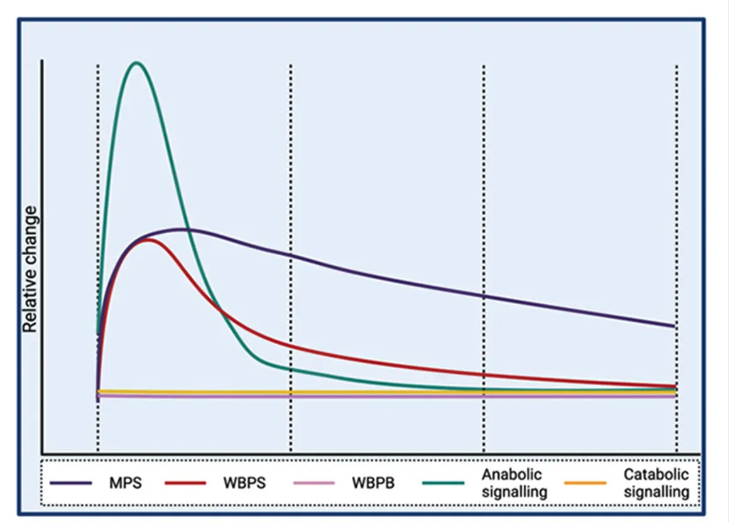
This comprehensive time course analysis of muscle protein synthetic response, alongside whole-body protein synthesis and breakdown, and muscle anabolic and catabolic signaling responses to protein ingestion, paints a detailed picture of the body's remarkable capacity to handle and utilize dietary protein.
Molecular and Cellular Insights: Understanding the Protein Synthetic Response
As we delve into the molecular and cellular dynamics behind the protein synthetic response, a key focus is the role of the mTOR, an important regulator of protein synthesis and muscle growth.
As mentioned earlier, mTOR is particularly responsive to amino acids, especially leucine, which is known for its potent ability to stimulate this pathway. The study's findings revealed a fascinating aspect of mTOR activation: while it was significantly elevated for up to four hours following protein ingestion, this increase was not sustained throughout the entire 12-hour period during which anabolic signaling remained elevated.
This observation suggests that a transient spike in mTOR activation is both necessary and sufficient to boost postprandial muscle protein synthesis. This challenges the traditional view that continuous, prolonged mTOR activation is required for effective protein synthesis.
The study's data compellingly dispel the myth that the body can only utilize 20–30 grams of protein in a single sitting. The consumption of up to four times this amount led to a more pronounced anabolic response. This finding is important as it shows that higher protein intake doesn't result in wastage, as previously thought. Instead, the extra amino acids are efficiently absorbed into the circulation and eventually incorporated into tissues, such as muscles.
In terms of utilization efficiency, the study found that over 85% of the available amino acids were used for protein synthesis, with only about 15% undergoing oxidation. This high rate of utilization underscores the body's remarkable capacity to use larger amounts of protein for muscle building and repair, rather than merely breaking them down for energy.
These insights into the molecular and cellular mechanisms of protein synthesis not only challenge longstanding nutritional beliefs but also provide a more nuanced understanding of how our bodies process and utilize dietary protein.
Protein Type and Anabolic Response
Would the results observed with milk protein hold true for other types of protein? This question is essential in understanding whether the findings of the study can be generalized across different dietary sources of protein.
Milk protein, which was used in the study, presents a unique blend of whey (20%) and casein (80%) proteins. Whey is known for its rapid digestibility, while casein digests more slowly. This combination in milk protein potentially contributes to its ability to prolong anabolic responses following ingestion. It leads us to ponder whether a similar effect would be observed with other protein sources like whey alone, pea protein, or soy protein, which have different digestion rates and amino acid profiles.
The authors of the study, however, believe that the type of protein may not significantly alter the observed effects. This assumption is grounded in previous literature, which suggests that while the rate of digestion and amino acid composition can influence the initial response to protein ingestion, the overall capacity of the body to utilize protein for synthesis over an extended period may not be drastically different across various protein types.
This perspective is important as it implies that the enhanced ability of the body to utilize higher amounts of protein, as demonstrated in the study, might be applicable to a range of protein sources.
Rethinking Protein Consumption Patterns
The findings of this study carry significant practical implications, particularly in how we approach protein consumption patterns for optimizing muscle protein synthesis and overall health.
Traditionally, the advice has been to evenly distribute protein intake throughout the day to maximize protein synthesis. This approach is based on the belief that there is a limit to how much protein can be effectively utilized in one sitting, with excess being wasted or used for energy rather than muscle synthesis.
However, the results of this study challenge this notion. They suggest that consuming larger amounts of protein in fewer meals may be just as effective, if not more so, for promoting muscle protein synthesis. For instance, if someone prefers to have two meals per day with 50+ grams of protein each, or even a single meal with 100+ grams of protein, this study indicates that such a feeding pattern could be a viable strategy for muscle growth and repair.
This flexibility in protein consumption patterns could be particularly beneficial for various lifestyles and dietary preferences. People with busy schedules, those who practice intermittent fasting, or even athletes with specific nutritional strategies might find it more practical to consume larger amounts of protein in fewer sittings.
It's important to note, however, that while this study opens up new possibilities in dietary planning, individual responses to protein intake can vary based on factors like age, activity level, and overall health.
Assessing the Duration of the Anabolic Response and Rethinking Protein Intake Guidelines
A pivotal question arising from the study is whether the anabolic response to high protein intake extends beyond the 12-hour period that was observed. While this specific question remains unanswered due to the study's time constraints, the findings offer some intriguing clues.
Notably, many of the metabolic responses measured in the study had not returned to baseline levels even after 12 hours. This observation led the authors to suggest that the cumulative metabolic responses to consuming 100 grams of protein might be even more significant than what was captured in their data. They propose that their assessment should be viewed as a conservative, minimal estimate of the body's capacity to handle and utilize such a high protein intake.
This perspective challenges the longstanding dietary guideline that recommends limiting protein intake to 20–30 grams per meal to optimize muscle protein synthesis. The study's findings suggest that this limit may have been overly restrictive and not reflective of the body's true capabilities.
It's important to highlight that the emphasis of these findings is not on encouraging the consumption of 100 grams of protein in a single sitting. Instead, the study aims to broaden our understanding of protein metabolism, illustrating that the body can efficiently utilize larger quantities of protein than previously believed. This has implications for the flexibility and personalization of dietary planning.
For many people, it may seem intuitive to consume more than 20–30 grams of protein in a meal, especially given common dietary habits. However, having scientific evidence to support this practice helps to dispel myths and outdated notions about protein consumption. It opens the door for more tailored nutritional strategies that align with individual dietary preferences, lifestyles, and health goals.