Autophagy, Cellular Senescence, and the Promise of Topical Rapamycin in Hair Regeneration
Hair Follicle Regeneration Cycle: Hair follicles, dynamic mini-organs, undergo cycles of growth, regression, rest, and shedding, which are essential for maintaining hair density and texture. The transition from rest to growth phase is critical for hair regeneration, facilitated by the interaction between hair follicle stem cells (HFSCs) and dermal papilla cells (DPCs).
Role of HFSCs and DPCs: HFSCs, capable of self-renewal and differentiation, are pivotal for hair growth. They remain quiescent until receiving signals to regenerate hair. DPCs, situated at the follicle base, act as a command center, secreting growth factors that influence HFSCs' activity. This interaction is vital for initiating the anagen phase of hair growth.
Impairment of Communication: When the signaling between HFSCs and DPCs is disrupted, it impairs the hair follicle's regenerative capacity, leading to hair growth issues. The communication involves a complex interplay of signaling molecules, including growth factors and components of the extracellular matrix, crucial for hair follicle structure and function.
Growth Factors and Extracellular Matrix Components: DPCs release growth factors like VEGF and IGF-1, which are essential for blood vessel formation and stimulating hair growth. They also contribute to the extracellular matrix, providing structural and biochemical support.
Cellular Senescence in Hair Growth Impairment: Cellular senescence, a state where cells stop dividing but don't die, serves as a barrier against cancer but also contributes to aging and age-related diseases. Senescent cells release the senescence-associated secretory phenotype (SASP), a mix of molecules that deteriorate tissue function, including in hair follicles.
Adverse Effects of SASP: The SASP can induce chronic inflammation, potentially leading to autoimmune-like responses and damaging healthy cells, which exacerbates tissue dysfunction. It can also aberrantly stimulate the proliferation of nearby cells, disrupting the hair growth cycle and leading to hair loss.
Dysregulation of Autophagy and Senescence: The dysfunction in autophagy and the impairment of cellular senescence in both HFSCs and DPCs are crucial to understanding how the hair growth process becomes dysregulated, leading to impaired hair growth.
Evidence of Senescence in DPCs: Studies comparing DPCs from balding and non-balding scalps reveal molecular differences, with balding DPCs exhibiting signs of premature senescence. This is characterized by a loss of proliferative capacity, changes in cell morphology indicative of aging, and positive staining for senescence-associated β-galactosidase (SA-β-Gal), confirming the presence of senescence.
Role of Androgens in AGA: Androgens, particularly dihydrotestosterone (DHT), play a significant role in the pathophysiology of AGA by affecting hair follicle dynamics. Elevated levels of androgen receptors (ARs) in DPCs from balding scalps amplify the effects of DHT, contributing to follicular miniaturization and hair loss. Senescent DPCs exhibit heightened sensitivity to androgens due to overexpression of ARs, exacerbating the aging and dysfunction of hair follicles.
Research on Senescence-Resistant Mice: Studies using p53 knockout mice, which lack a key gene involved in the cellular response to DNA damage, provide insights into the aging mechanisms of DPCs and their implications for hair growth. These cells avoid traditional senescence markers and maintain hair-regenerating capabilities, highlighting the role of the p53 pathway in hair follicle health.
Oxidative Stress and Follicle Senescence: Oxidative stress, characterized by an imbalance between reactive oxygen species (ROS) production and antioxidant defense, accelerates DPC senescence. This imbalance is linked to the miniaturization of hair follicles in AGA, underscoring the importance of oxidative stress in hair loss pathologies.
Aged HFSCs and Alopecia: Aging in HFSCs compromises their regenerative potential, contributing to common signs of aging hair, such as greying, thinning, and loss. The cyclic removal of aged HFSCs through terminal epidermal differentiation reduces the pool of cells available for hair regeneration.
Autophagy is a cellular self-cleaning process that maintains cell integrity by degrading damaged organelles, misfolded proteins, and cellular debris. This process is crucial for adapting to stress and nutrient scarcity, ensuring cell survival and function. Impaired autophagy is linked to various diseases, including neurodegenerative disorders, highlighting its importance in cellular health.
Autophagy in Hair Follicle Function: Autophagy supports the health of hair follicle stem cells, crucial for hair growth and regeneration. A decline in autophagic activity with age contributes to cellular damage accumulation, affecting hair follicle stem cell health and leading to diminished hair regeneration capabilities.
Preserving Stem Cell "Stemness": Autophagy is essential in maintaining the stemness of various stem cell types, including hematopoietic and muscle stem cells, by ensuring their capacity for self-renewal and differentiation. This underscores autophagy's broad importance in stem cell health across different tissues.
Impact of Autophagy on Hair Follicle Stem Cells (HFSCs): Proper autophagic activity is critical for HFSCs to maintain their regenerative capabilities, especially as they age. Autophagy helps in recycling cellular components, preventing damage, and dysfunction, thus preserving the health and vitality of HFSCs.
Experimental Evidence Linking Autophagy to Hair Growth: Studies involving small molecules like α-ketoglutarate (α-KG) and drugs like rapamycin and metformin have shown that stimulating autophagy can promote the anagen hair growth phase and contribute to hair regeneration. These findings suggest that activating autophagic pathways could be a promising strategy for hair loss treatment.
Pharmacological Manipulation of Autophagy for Hair Growth: Pharmacological agents that activate or inhibit autophagy have demonstrated significant effects on hair follicle stem cell activation and the hair follicle cycle. For example, rapamycin enhances HFSC proliferation and accelerates the transition to the anagen phase, while inhibitors of autophagy impede this process.
Conservation of Autophagy's Effects Across Species: The regulatory effects of autophagy on HFSC activation and the hair growth cycle observed in mice are consistent in human models. Induction of autophagy promotes HFSC proliferation and prolongs the anagen phase in human hair follicle organ cultures, suggesting a conserved mechanism across species.
Potential for Autophagy-Modulating Treatments: The ability of autophagy to support HFSC health and stimulate hair growth presents a compelling avenue for developing new treatments for hair loss conditions. Modulating autophagy could offer innovative strategies for enhancing hair follicle regeneration and combating alopecia.
Hair loss, often perceived merely as a superficial sign of aging, actually signifies a deeper, systemic aging process within the body. The health of hair follicles, essential for hair growth, deteriorates due to two main cellular phenomena: impaired autophagy and increased cellular senescence. The decline in hair follicle health not only results in visible signs of aging but also reveals the underlying, widespread degradation of healthy tissues, indicative of the body's overall aging process.
This research narrative review delves into the intricate relationship between cellular senescence and autophagy dysfunction and their roles in hair follicle degeneration. By examining the molecular dynamics of autophagy, we aim to underscore its critical role in maintaining hair follicle integrity. Importantly, we explore how the dysfunctions of senescence and autophagy impact hair follicle stem cells (HFSCs), which are vital for hair growth and regeneration. Understanding these interactions opens the door to innovative therapeutic strategies designed to rejuvenate these cellular pathways, potentially reversing the effects of aging on hair health.
Central to our analysis is the mechanistic target of rapamycin (mTOR) pathway, a central regulator of autophagy. We will explore a range of studies that utilize rapamycin, an mTOR inhibitor, to stimulate autophagy, ultimately leading to the regeneration of follicles. By enhancing autophagy, rapamycin could counteract the negative effects of cellular aging and dysfunction, offering a novel approach to hair loss treatment. This comprehensive review aims to broaden the understanding of the current landscape of hair loss treatments, highlighting the potential of mTOR modulation to improve hair follicle health and regeneration.
In doing so, we not only bring to light pathways to combat hair loss but also contribute to the broader field of aging research. By understanding and manipulating the cellular mechanisms underlying hair follicle aging, we can gain insights into systemic aging processes, potentially uncovering new strategies to promote healthspan and combat age-related diseases. This integrative approach underscores the importance of cellular maintenance mechanisms like autophagy in preserving tissue health and function, offering a window into the complex biology of aging and regeneration.
A Closer Look at Hair Follicle Dynamics
To understand the role of autophagy and senescence in hair regeneration, we first need to understand the natural regenerative capacity of a hair follicle and the important role of hair follicle stem cells (HFSCs).
Hair follicles are dynamic mini-organs that undergo cycles of growth (anagen), regression (catagen), rest (telogen), and shedding (exogen) throughout an individual's life. This cyclical nature is crucial for maintaining hair density and texture [1, 2]. The transition from the rest phase (telogen) to the growth phase (anagen) is imperative for normal hair growth. When a hair follicle cannot transition to the anagen growth phase, it ceases to function.
At the core of this regenerative process are the hair follicle stem cells (HFSCs) and dermal papilla cells (DPCs), whose symbiotic communication is fundamental for kick-starting the anagen growth phase, and ultimately promoting hair growth. HFSCs, residing in a specialized niche within the follicle, boast the ability to both self-renew and generate the diverse cell types constituting the hair follicle. DPCs, located at the follicle's base, act as the command center for growth signals, influencing HFSCs' activity through a complex interplay of signaling molecules [3, 4].
When the communication between these two cell types is impaired we start to lose the regenerative capacity of the hair follicle—this is particularly important for HFSCs.
These stem cells are the engine of hair growth, capable of springing into action to regenerate hair during the hair growth cycle's anagen phase, or growth phase. Their ability to remain quiescent and inactive, yet poised for activation, underscores their remarkable adaptability, which is essential for hair growth. However, they need a signal to initiate this active phase.
This is where DPCs come into play. DPCs are located at the base of the hair follicle and secrete growth factors that signal HFSCs to begin the hair production process.
The interaction between DPCs and HFSCs is a dance of biochemical signals, with DPCs releasing growth factors that signal HFSCs to transition from their resting state into active proliferation and differentiation [3, 4]. When these signals get disrupted, the ability of hair to regenerate is significantly impaired.
The types of DPC-secreted molecules include:
Growth Factors: These are proteins that promote cell growth and differentiation. DPCs release growth factors like vascular endothelial growth factor (VEGF) and insulin-like growth factor 1 (IGF-1). VEGF plays a key role in the formation of blood vessels, ensuring that hair follicles get enough blood supply, while IGF-1 is known to stimulate hair growth [5, 6].
Extracellular Matrix Components: These are the network of proteins that provide structural and biochemical support to surrounding cells. DPCs contribute to this matrix, which is essential for maintaining the hair follicle's structure and function [5, 6].
In order for there to be proper hair regeneration we need the proper amount of growth factor to be secreted in order to have the HFSCs spring into action. As we’ll review, dysfunction in the form of senescence and the impairment of autophagy in both HFSCs and DPCs are going to be critical to our understanding of how this process becomes dysregulated and ultimately impair hair growth.
The role of cellular senescence in hair growth impairment
Cellular senescence refers to the phenomenon where cells cease to divide but do not die. It occurs in response to cellular damage in order to prevent cellular damage from propagating and eventually becoming tumors. While senescence serves as a critical barrier against cancer by preventing the proliferation of damaged cells, it also contributes to aging and age-related diseases when senescent cells accumulate over time. [7]
Senescent cells secrete a variety of pro-inflammatory molecules, growth factors, and proteases, known collectively as the senescence-associated secretory phenotype (SASP). The SASP is a witch's brew of proinflammatory and pro-growth stimulating molecules, which drives the deterioration of healthy tissue function [7].
The adverse effects of the SASP on tissues, including hair follicles, manifest through several mechanisms:
- Induction of Inflammatory Responses: The release of pro-inflammatory cytokines by SASP can induce chronic inflammation, attracting immune cells and potentially triggering an autoimmune-like response. This inflammatory milieu not only damages adjacent healthy cells but also promotes their senescence, leading to a vicious cycle of cellular aging. Over time, the increased presence of senescent cells within tissues, including the scalp's hair follicles, exacerbates tissue dysfunction and contributes to the decline in tissue regenerative capacity, manifesting in aging phenotypes such as hair thinning and loss.
- Promotion of Unhealthy Tissue Growth: SASP components, including various growth factors, can aberrantly stimulate the proliferation of nearby cells. This uncontrolled growth stimulation contributes to the expansion of both senescent cell populations and potentially pre-cancerous cells, further compromising tissue integrity and function. In the context of hair follicles, such dysregulated cell proliferation can disrupt the hair growth cycle, leading to altered hair structure, reduced hair regeneration, and eventually, hair loss.
The SASP is an accelerant of overall tissue decline. This same process occurs in hair follicles.
Why do we think senescence plays a role in the disruption of hair growth regeneration?
When we look at DPC cell samples of balding scalps and non-balding scalps there are molecular signatures that make them distinguishable. [8, 9, 10]. When DPCs from balding scalps are grown in the lab (a process known as in vitro culture), they show early signs of aging, a condition termed 'premature senescence'. This means that these cells appear to age faster than expected. They exhibit characteristics typical of old cells, such as changes in their appearance and function.
In a study that evaluated the role of senescence in a common form of balding called androgenic alopecia (AGA), nearly all the DPCs from the balding scalp had lost their proliferative capacity [10]. Moreover, these cells change their shape, transitioning from their typical elongated fibroblastic shape to a larger form with pronounced stress filaments—a hallmark of senescence in fibroblasts.
To establish further whether this loss of proliferative capacity was a consequence of premature senescence the researchers used a marker of senescence, a marker called senescence-associated β-galactosidase (SA-β-Gal).
In the balding DPC, the sample showed strong positive SA-β-Gal staining in the majority of cells, whereas the non-balding DPCs were all negative for SA-β-Gal although occasional positive cells were observed. Through the increase of SA-β-Gal positive cells, it's clear that the balding DPCs had entered into a state of senescence. [10]
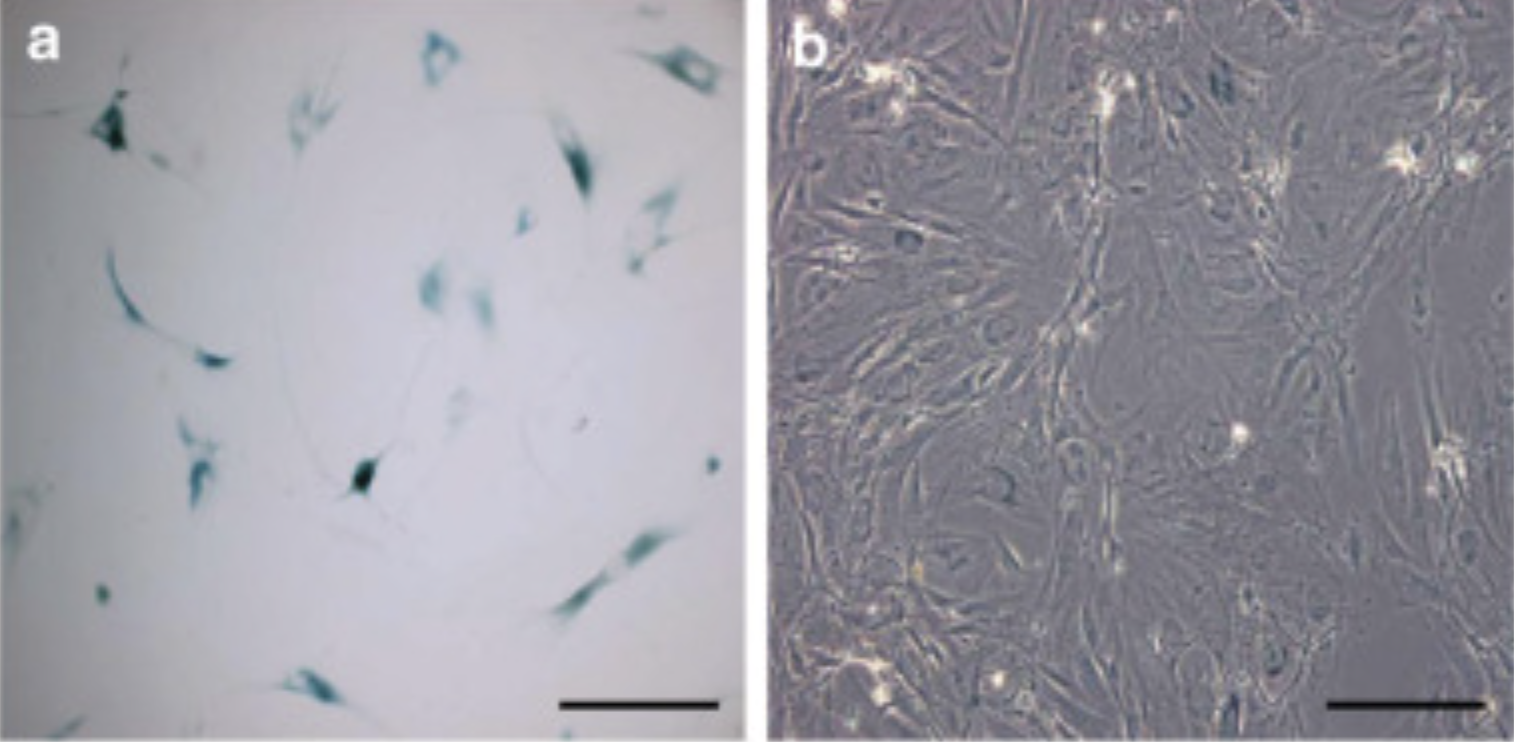
Together these results suggest that the loss of proliferative capacity in the balding DPC was associated with premature senescence [10]. While senescence is clearly a signature in the balding DPC samples, what is actually happening on a molecular level that is preventing senescent cells from regenerating and entering into the anagen growth phase?
The Role of Cellular Senescence in Disrupting Hair Follicle Communication and Contributing to Androgenetic Alopecia
The detrimental impact of cellular senescence on hair growth transcends a mere reduction in the proliferative capacity of hair follicle cells. It extends to a profound disruption in the essential communication between dermal papilla cells (DPCs) and hair follicle stem cells (HFSCs), critical for the hair follicle's ability to undergo successful regeneration.
This disruption leads to a hair follicle progressively less capable of maintaining the anagen (growth) phase of the hair cycle, culminating in the progressive miniaturization of hair follicles. Such miniaturization is a defining feature of androgenetic alopecia (AGA), manifesting clinically as hair thinning and, ultimately, hair loss [3, 11, 12]. This phenomenon is driven by the altered behavior and secretory profiles of senescent cells within the hair follicle environment:
Senescent Dermal Papilla Cells (DPCs): Upon entering a senescent state, DPCs shift their secretory patterns from promoting to hindering hair follicle activity. They begin releasing factors that not only fail to activate HFSCs but actively inhibit their activation and proliferation. This secretion of inhibitory and pro-inflammatory signals disrupts the follicle's entry into the anagen phase, effectively stalling the hair growth cycle at a critical juncture of regeneration. The transition from growth-promoting to inhibitory signaling by senescent DPCs represents a significant barrier to hair follicle regeneration and growth [6, 11, 12].
Senescent Hair Follicle Stem Cells (HFSCs): Aging HFSCs exhibit a diminished responsiveness to growth-promoting cues, a consequence of both intrinsic senescence and the altered microenvironment created by senescent cells. This reduced sensitivity to activation signals compromises the regenerative potential of HFSCs, further impeding the natural hair renewal process. The combined effect of senescent HFSCs and DPCs creates a cellular milieu that is not conducive to hair growth, exacerbating the cycle of hair follicle miniaturization and loss [3, 11, 12].
In essence, the accumulation of senescent cells within the hair follicle introduces a barrier to the normal cyclical rhythm of hair growth and regeneration. By altering the cellular landscape and communication networks within the follicle, senescence effectively suppresses the hair's proliferative potential, paving the way for the characteristic signs of hair aging and loss. [12]
The Impact of Androgens and Cellular Senescence on Hair Follicle Dynamics and Androgenetic Alopecia Development
Androgens, notably testosterone and its more potent metabolite dihydrotestosterone (DHT), play a central role in hair follicle behavior. These hormones regulate hair growth by influencing the transformation of different hair types. For instance, they can turn thin, short vellus hair (the type of fine hair found on most of the body) into thicker, longer terminal hair (the type of hair found on the scalp, among other areas). This dual role of androgens is particularly pronounced in the context of androgenetic alopecia (AGA), where their influence on hair follicle dynamics is both central and paradoxical [12, 13].
In AGA, while testosterone is a significant androgen, DHT takes a more central role. Its potency surpasses that of testosterone, primarily due to its higher affinity for androgen receptors (ARs) within the hair follicle. Upon binding to ARs, DHT-AR complexes translocate into the nucleus, acting as transcription factors that modulate gene expression patterns critical for hair growth and follicle development. The process not only alters the hair growth cycle but also triggers the miniaturization of hair follicles, a defining feature of AGA, leading to progressive hair thinning and loss [13].
The sensitivity and distribution of ARs across different body regions underlie the diverse effects of androgens on hair growth. While certain areas experience an androgen-induced enhancement of hair growth, the scalp, especially its vertex, undergoes androgen-driven hair loss in AGA. This phenomenon can be attributed to the differential expression and localization of ARs, which render the scalp follicles particularly susceptible to androgenic signaling.
Emerging research indicates elevated levels of both ARs and DHT within the DPCs of follicles from balding areas of the scalp. This elevation, coupled with the increased activity of AR-DHT complexes, is instrumental in the follicular miniaturization characteristic of AGA. It is postulated that the senescence of DPCs, exacerbated by their overexpression of androgen receptors, precipitates this process. The heightened AR expression in senescent DPCs amplifies their responsiveness to androgens, catalyzing a cascade of events that accelerate cellular aging and compromise the regenerative capacity of hair follicles [8, 10].
This enhanced sensitivity to androgens, mediated by the overexpression of ARs in aged, senescent DPCs, serves as a critical mechanism underlying the premature aging and dysfunction of hair follicles in AGA. By elucidating the molecular interactions between androgens, ARs, and DPCs, we gain valuable insights into the pathophysiology of AGA and the role of cellular senescence in driving this common form of hair loss.
What Senescent-Resistant, Genetically Engineered Mice Teach Us About Senescence's Impact
An intriguing aspect of research into dermal papilla cell (DPC) senescence involves the study of cells from p53 knockout mice. The p53 gene, often hailed as the "guardian of the genome," plays a pivotal role in safeguarding cellular integrity by orchestrating responses to DNA damage. It does so by halting the proliferation of damaged cells, either through inducing apoptosis—a process of programmed cell death—or by enforcing a state of senescence to prevent their replication [14].
Investigations into DPCs from p53 knockout mice, which lack this crucial gene, have shed light on the aging mechanisms of these cells and their implications for hair growth. DPCs devoid of p53 bypass the traditional hallmarks of cellular senescence, such as the reduction in cell division rate and the accumulation of senescence-associated markers, even after numerous cell divisions. This resistance to senescence appears to preserve the DPCs' hair-regenerating capabilities, suggesting that the p53 pathway is intimately involved in the regulation of DPC aging and, by extension, hair follicle health [14].
In contrast, while senescent DPCs continue to express key genes associated with the dermal papilla, their ability to induce hair follicle growth diminishes. Essentially, these cells still 'look' like dermal papilla cells at the genetic level, but they can't perform their normal role in promoting hair growth [14]. This functional discrepancy underscores the profound impact of senescence on the cellular mechanisms underlying hair growth.
As DPCs undergo senescence, they begin to favor the proliferation of epidermal cells over that of follicular cells, effecting a shift in the tissue microenvironment that is disadvantageous for hair growth. This transition reflects a reprogramming of senescent DPCs, diverting resources away from hair follicle maintenance toward the expansion of the skin's epidermal layer [14].
A critical mediator in this process is the enhanced secretion of interleukin-6 (IL-6) by senescent DPCs. IL-6, an immune-modulating cytokine, emerges as a key inhibitory factor in hair follicle biology. It discourages the proliferation of follicular keratinocytes—the primary cellular constituents of the hair shaft—and hinders the colony-forming efficiency of hair follicle stem cells (HFSCs). Additionally, IL-6 impedes the hair cycle's progression from the resting phase (telogen) to the growth phase (anagen), further obstructing hair regeneration. [15].
This observation aligns with prior findings, wherein the conditioned medium from balding DPCs, enriched with senescence signals like IL-6, delayed hair cycle progression in experimental models. Mice engineered to overexpress IL-6 also exhibited decelerated hair growth, reinforcing the notion that IL-6 acts as a significant barrier to hair follicle vitality and growth [16].
The Role of Oxidative Stress in Accelerating Follicle Senescence
As we understand more about the consequences of DPC impairment, it is important to understand some of the causes of senescence transformation of these cell types. One of the causes, which is true across nearly all cell types, seems to be an increase in oxidative stress.
Oxidative stress describes the cellular strain induced by an imbalance between the production of reactive oxygen species (ROS) and the body’s ability to counteract their harmful effects with antioxidants. ROS, while being chemically reactive molecules crucial for energy production and immune responses in controlled quantities, can precipitate cellular damage when their levels surpass the body's antioxidative defense mechanisms [17, 18]. This imbalance is a central factor in the aging process and the pathogenesis of various diseases, including AGA.
In the context of hair follicle biology, ROS are produced as a natural by-product of cellular metabolism, particularly within the mitochondria, the cell's energy powerhouses. These molecules fulfill a dual role, facilitating essential cellular signaling pathways for normal hair follicle operations, and paradoxically, driving hair follicle aging and dysfunction upon excessive accumulation [19]. Maintaining a harmonious balance between ROS production and antioxidant defense is thus important for preserving hair follicle integrity.
In the scenario of AGA, this equilibrium is skewed towards an overproduction of ROS, alongside an elevation in malondialdehyde (MDA) levels, which serves as a marker of oxidative damage to lipid constituents of cellular membranes. Such an increase in oxidative stress is linked to precipitating various adverse effects on hair follicles, most notably inducing a premature transition from the growth phase (anagen) to the regression phase (catagen), thereby abbreviating the hair growth cycle [10, 20].
Additionally, the study on balding DPCs, revealed that DPCs from balding scalp regions exhibit elevated markers of oxidative stress and DNA damage. This increased vulnerability of balding DPCs to oxidative stress correlates with a rise in the secretion of hair growth inhibitors and a hastened progression into DPC senescence [10].
Furthermore, oxidative stress instigates a domino effect, wherein senescent cells transition into a senescence-associated secretory phenotype (SASP), discharging elevated levels of pro-inflammatory and tissue remodeling agents, including interleukins, matrix metalloproteinases, and growth factors. This altered secretory profile not only kindles inflammation but also incites senescence in adjacent cells, perpetuating a cycle of oxidative stress and cellular aging within the hair follicle ecosystem [21, 22].
This vicious cycle of ROS production and accumulation, DPC senescence, and SASP activation disrupts the normal signaling required for hair growth, leading to the hallmark miniaturization of hair follicles observed in AGA.
The elucidation of DPC senescence as a disruptive force in hair growth signaling raises a pertinent question: What ramifications arise as hair follicle stem cells (HFSCs) begin to exhibit signs of senescence? The investigation into HFSC senescence and its impact on hair regeneration and the broader implications for hair follicle health underscores the complexity of cellular aging within the hair follicle microenvironment and its contributions to hair loss pathologies.
The Role of Aged Hair Follicle Stem Cells in the Progression of Alopecia
Central to the process of hair regeneration are the hair follicle stem cells (HFSCs), known for their remarkable plasticity—the ability to differentiate into diverse cell types necessary for hair growth. Under physiological conditions, HFSCs are pivotal to sustaining the hair growth cycle, remaining mostly quiescent until prompted by specific cues to enter the anagen phase, or active growth phase of the hair cycle. During this phase, HFSCs differentiate into transient amplifying cells, which are characterized by their rapid proliferation and ability to differentiate into various cell types crucial for the formation of new hair follicles.
However, as we age, HFSCs start to lose their ability to kickstart the hair growth phase effectively [23]. This decline in function can be seen in common signs of aging hair, such as greying, thinning, and hair loss [24]. One important process that contributes to these aging characteristics is the cyclic removal of aged HFSCs through a process called terminal epidermal differentiation. In simpler terms, instead of contributing to new hair growth, these aged cells are gradually transformed into skin cells and are shed. This phenomenon not only reduces the pool of cells available for hair regeneration but also contributes to the visible aging of hair.
The understanding of senescence as a key factor in diminishing the regenerative potential of hair follicles has been well-established, alongside insights into the mechanisms by which senescence impacts HFSC functionality.
In this next section, we are going to review some of the studies exploring interventions targeting senescence as a driver of hair follicle regenerative decline. Notably, studies investigating the role of autophagy in rejuvenating HFSCs have unveiled potential strategies for mitigating the effects of aging on hair growth. These innovative approaches, which aim to reverse the decline in hair regeneration, offer promising avenues for developing treatments that address the root causes of hair loss associated with aging.
The Role of Autophagy in Reducing Hair Follicle Senescence
What is Autophagy?
Autophagy is a fundamental cellular process crucial for maintaining the health and functionality of various cell types, including stem cells in hair follicles. The word itself is derived from the Greek words “self-eating.” In the case of the cell, autophagy is a self-cleaning process that is important to maintain the integrity of the cell. By breaking down and recycling cellular debris, damaged organelles, and misfolded proteins, autophagy ensures that cells can manage stress and adapt to changing environmental conditions, such as nutrient deprivation.
The degradation of these components is not just a cleanup process; it also provides raw materials for the cell to use in building new components or generating energy, especially important under conditions of stress or nutrient scarcity. This function of autophagy is especially critical under conditions of stress or nutrient scarcity, where the cell must efficiently allocate its resources to survive and maintain function. Cells can recycle this cellular debris to use as cellular energy when there is no external source of energy.
When autophagy becomes impaired we see numerous serious consequences. The significance of autophagy in maintaining this cellular cleanup process and the breakdown of proteins has profound implications for understanding and treating neurodegenerative diseases [25].
Conditions such as Alzheimer's, Parkinson's, and Huntington's disease are characterized by the accumulation of protein aggregates, which are toxic to neuronal cells. Impairments in the autophagic process can exacerbate these conditions by allowing the buildup of harmful proteins. For instance, in Alzheimer's disease, the buildup of tau proteins can trigger neuroinflammatory responses leading to neuronal degradation. Hence, enhancing autophagic pathways has been identified as a promising therapeutic strategy to improve cellular clearance and slow the progression of neurodegenerative disorders [25].
Autophagy’s Essential Role in Hair Follicle Function
In the context of hair follicles, autophagy supports the stem cells that are fundamental to hair growth and regeneration, ensuring these cells remain healthy and capable of responding to the signals that regulate the hair growth cycle. Similar to the neurodegenerative disorders we mentioned, when autophagy is impaired in cells involved in hair growth we see a corresponding impairment in the ability to regenerate hair. When we look at stem cells in particular, the impairment of autophagy has significant implications for our ability to regenerate stem cells.
As cells age, there is a natural decline in autophagic activity, as shown by research [26, 27]. This decline is believed to contribute to the buildup of cellular damage over time, leading to age-related diseases like cancer and neurodegenerative disorders. In the context of hair follicles, this decline in autophagy with aging could have direct implications for the health of hair follicle stem cells. Since these stem cells are responsible for hair regeneration, a decrease in autophagic activity leads to diminished hair growth, increased hair thinning, and other signs of aging hair.
Autophagy's Crucial Role in Preserving Stem Cell "Stemness"
Autophagy is instrumental in preserving the 'stemness'—the ability of stem cells to self-renew and differentiate—across a variety of stem cell types. This process is essential for the maintenance of stem cell pools in various tissues, ensuring their capacity for regeneration and repair.
Broad Impact of Autophagy on Diverse Stem Cell Types
Research across different stem cell populations illustrates the universal importance of autophagy in sustaining stem cell health and functionality:
Hematopoietic Stem Cells (HSCs): Fundamental to the body’s hematopoietic system, HSCs give rise to the various cells that comprise the blood and immune systems. A pioneering study that appeared in the journal Nature, titled Autophagy maintains the metabolism and function of young and old stem cells underscores the role of autophagy, and specifically mitophagy—the selective autophagic degradation of mitochondria—in maintaining HSCs [28]. By preferentially eliminating healthy, active mitochondria, autophagy ensures HSCs remain in a quiescent state, crucial for preserving their 'stemness' and preventing premature exhaustion. This process is pivotal for the long-term integrity and viability of the HSC pool, safeguarding the body’s ability to regenerate blood cells.
Muscle Stem Cells (Satellite Cells): Essential for muscle repair and growth, muscle stem cells benefit from autophagy's supportive role in transitioning from quiescence to active participation in tissue regeneration. The work of Tang and Rando (2014) demonstrates how autophagy meets the heightened metabolic demands of these cells upon activation, facilitating their proliferation and differentiation. This autophagic function is vital not just for cell survival but for priming stem cells for effective regeneration, highlighting its significance in muscle health and repair [29].
These studies collectively spotlight the indispensable roles of autophagy in a range of stem cell types, indicating its critical contribution to cellular longevity and resilience. Autophagy's ability to maintain stem cell health and functionality carries broad implications, from regenerative medicine to the management of age-related conditions, emphasizing its fundamental role in cellular maintenance and repair mechanisms.
In the context of hair follicle biology, these insights suggest that autophagy could be equally critical in maintaining hair follicle stem cells. Proper autophagic activity might be essential for these cells to retain their regenerative abilities, especially as they age. This could include the turnover and recycling of cellular components to prevent damage and dysfunction.
Given the pivotal roles of autophagy in stem cell maintenance across various tissues, it's plausible to infer that autophagy holds similar importance in the context of hair follicle stem cells (HFSCs). Ensuring proper autophagic activity is likely crucial for HFSCs to maintain their regenerative capabilities, particularly as they age. This may involve the efficient turnover and recycling of cellular constituents to avert damage and dysfunction, thereby preserving the health and vitality of HFSCs.
These insights into autophagy's role in stem cell maintenance offer promising perspectives for hair loss treatments and the management of hair-related aging symptoms. Understanding and leveraging autophagy's protective and regenerative mechanisms could pave the way for developing novel therapeutic strategies aimed at enhancing hair follicle health and promoting hair regeneration. As we’ll review in the next section, numerous labs have tested this hypothesis to see if stimulation of autophagy could lead to a corresponding stimulation of hair growth.
What the studies tell us about autophagy and hair growth
To test whether autophagy can stimulate the anagen hair growth phase and be used to regrow hair, a research group at UCLA tested a series of small molecules that activate autophagy, including the metabolites α-ketoglutarate (α-KG) and α-ketobutyrate (α-KB), and the prescription drugs rapamycin and metformin, which are thought to increase autophagy by increasing mTOR and AMPK signaling.
The impetus for this investigation was rooted in the extensive body of research linking autophagy to longevity, particularly through the lens of dietary restriction. Dietary restriction has long been recognized for its potential to extend lifespan, with various regulators of cellular energy metabolism playing a crucial role in mediating this effect. Notably, α-KG, a metabolite whose concentrations increase under conditions of dietary restriction, has been documented to extend lifespan in model organisms like C. elegans by inducing autophagy. Inspired by these findings, the UCLA team probed whether α-KG could similarly promote hair regeneration by activating autophagic pathways [30].
Empirical Assessment and Findings
To establish a comparative framework, the study incorporated minoxidil, a widely recognized vasodilator used in the treatment of pattern hair loss, serving as a benchmark due to its common application as a positive control in hair growth research. In the experimental design, male mice at 6.5 weeks of age and in the telogen phase of the hair cycle were topically administered α-KG or the minoxidil control on an alternate-day basis. This methodological choice was motivated by the objective of identifying treatments that are readily adaptable for human application.
The results were striking: α-KG treatment markedly expedited hair regeneration, evidenced by the early emergence of skin pigmentation—a hallmark of the anagen phase attributed to the activity of follicular melanocytes—by the 12th day following treatment commencement.
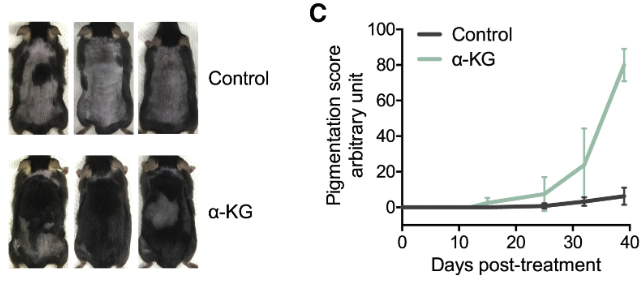
This finding sharply contrasted with the control group, where pigmentation was either negligible or entirely absent until day 39, the conclusion of the study period for histological and biochemical analyses. Notably, hair growth in the α-KG-treated mice initiated within 5–7 days post-treatment from the pigmented areas, culminating in extensive hair coverage by day 39. Conversely, the control group displayed minimal to no hair growth throughout the study duration.
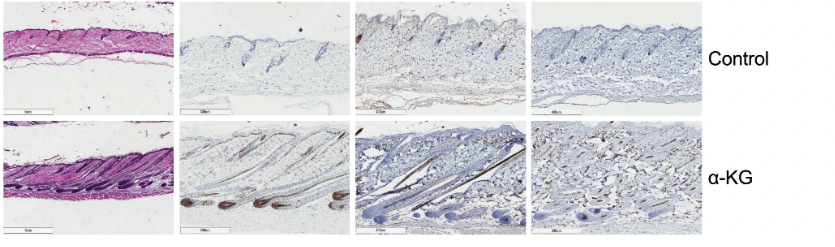
Further, the effectiveness of α-KG in stimulating hair growth was not confined to a specific age or gender. When treatment was initiated later in the telogen phase, at 8 weeks of age, the hair growth-promoting effects of α-KG were even more pronounced. Additionally, similar stimulatory effects were observed in female mice, underscoring the gender-independent action of α-KG on hair regeneration.
These findings bolster the hypothesis that stimulating autophagy, via agents like α-KG, holds promise for hair regeneration. To substantiate this premise further, the researchers embarked on replicating these results using rapamycin, aiming to solidify the link between autophagy activation and hair follicle rejuvenation.
Assessing the Role of Rapamycin in Promoting Hair Regeneration through TOR Inhibition and Autophagy Activation
Following the promising outcomes associated with metabolic activators of autophagy, the UCLA research team turned its attention to rapamycin, a compound known for its potent autophagy-enhancing properties, to evaluate its efficacy in hair regeneration.
Rapamycin exerts its effects through the inhibition of the mechanistic Target of Rapamycin (mTOR), an enzyme pivotal to regulating cellular growth, protein synthesis, and metabolism. mTOR serves as a critical sensor and effector in cellular responses to nutrient availability, toggling between promoting cellular growth in nutrient-rich conditions and activating autophagy to recycle cellular components under nutrient scarcity.
As we get older, mTOR activity gets heightened. This means there is more cellular activity and growth and less autophagic clearance activity. Such changes in mTOR dynamics contribute to the aging process, including the decline in regenerative capacities observed in various tissues. By inhibiting mTOR, rapamycin recalibrates mTOR activity to more healthy levels and stimulates autophagy.
Evaluating Rapamycin's Efficacy in Hair Regeneration
In their investigation, the UCLA researchers applied rapamycin topically to the skin of mice during the telogen phase, the resting stage of the hair cycle, to ascertain its potential to expedite the transition to the anagen phase, characterized by active hair growth. The rapamycin treatment resulted in a noticeable acceleration of hair regeneration. This effect was evident both visually, with a quicker onset of hair growth, and histologically, through analyses confirming the transition of hair follicles into the anagen growth phase.
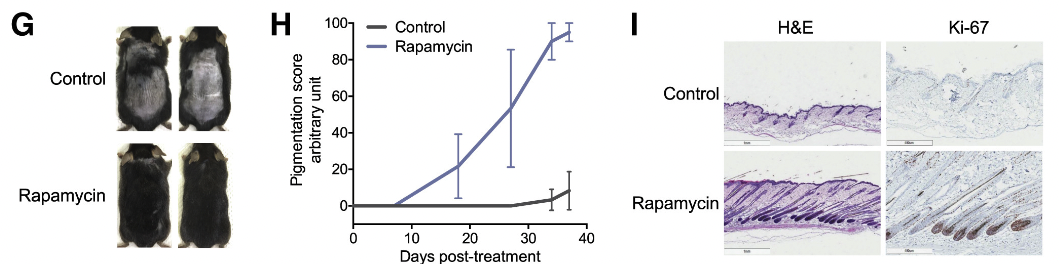
Moreover, the analysis revealed a significant upsurge in autophagy markers in the skin tissue of mice treated with rapamycin, underscoring the enhanced autophagic activity induced by mTOR inhibition. These findings not only corroborate the critical role of autophagy in hair follicle health and regeneration but also highlight the therapeutic potential of rapamycin as an autophagy activator in promoting hair growth.
Metformin and Hair Regeneration: Study Insights
Lastly, the experimenters also found a similar response to the topical use of metformin, a widely prescribed antidiabetic medication known for its capacity to inhibit the mTOR and activate autophagy. Metformin exerts its cellular effects partially by increasing the levels of AMP-activated protein kinase (AMPK), a crucial sensor of cellular energy status, which is activated under conditions of reduced cellular energy availability, such as during glucose deprivation.
Metformin's action as a fasting mimetic creates cellular conditions akin to those experienced during fasting, leading to elevated AMPK levels. Elevated AMPK activity, in turn, engages with mTOR, curtailing cellular growth and protein synthesis in the absence of sufficient nutrients. This regulatory mechanism ensures the conservation of cellular resources, promoting survival under conditions of metabolic stress by shifting the cellular focus towards maintenance and repair processes, including autophagy The study's findings revealed that topical application of metformin triggered responses similar to those observed with rapamycin and α-KG treatments, inducing autophagy and facilitating hair regeneration. This evidence points to a potentially significant role for autophagy induction in promoting hair growth, supported by metformin's ability to modulate key metabolic pathways involved in cellular energy sensing and resource allocation.
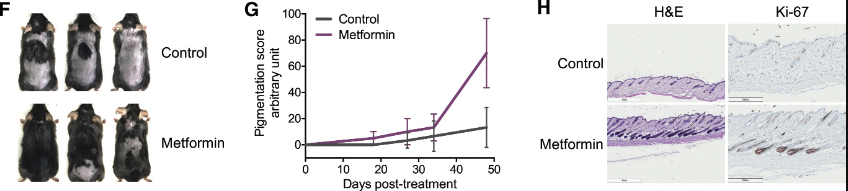
The topical use of metformin, by modulating the AMPK/mTOR axis and enhancing autophagic activity, showed promising results in accelerating the transition of hair follicles into the anagen phase, characterized by active hair growth. These outcomes align with the broader hypothesis that stimulating autophagy, through various molecular pathways, contributes to the revitalization of hair follicles and the promotion of hair regeneration.
Verifying Autophagy-Induced Hair Regeneration with SMER28
To verify that these results were indeed autophagy-induced and not caused by other consequences of mTOR inhibition, the experimenters utilized a molecule called SMERT 28 to test their hypothesis that autophagy induction alone would elicit hair regeneration. SMERT 28 is a molecule that induces autophagy in a way that is independent of mTOR inhibition.
Upon the topical application of SMER28 on the dorsal skin of mice, a pronounced upsurge in autophagy markers was observed, signifying the engagement of autophagic processes. Crucially, this enhancement in autophagic activity occurred without any associated increase in the molecular signals typically indicative of mTOR pathway inhibition. This distinction underscored SMER28’s capacity to activate autophagy via an mTOR-independent mechanism, providing a clear delineation of the specific role of autophagy in promoting hair regeneration.
The administration of SMER28 not only augmented autophagic activity but also significantly fostered hair regeneration, evident from both visual assessments and further analytical evaluations. These observations bolster the notion that the autophagic process itself, rather than any ancillary effects of mTOR inhibition, is instrumental in facilitating hair growth.
To further verify the necessity of autophagy in the hair-regenerative effects of SMER28, the researchers employed autophinib, an inhibitor of autophagy. Autophinib prevents the formation of the enzyme responsible for autophagy—also known as an autophagosom. The concurrent application of autophinib with SMER28 effectively neutralized the hair regrowth effects prompted by SMER28, underscoring the pivotal role of autophagy in this regenerative phenomenon. This dual-phase experimentation provided compelling evidence that autophagy stands as the driving force behind the hair regeneration observed in the study.
Autophagy in Hair Follicle Stem Cells: Exploring Cellular Mechanisms and Pharmacological Modulation for Hair Regeneration
The UCLA study highlighted the potential of certain metabolic intermediates and prescription drugs in enhancing hair follicle health through the modulation of autophagy. It identified compounds such as α-ketoglutarate (α-KG) and α-ketobutyrate (α-KB), alongside well-known medications like rapamycin and metformin, have revealed their capacity to activate follicular autophagy. This activation is achieved through the modulation of critical signaling pathways, namely mTOR and AMPK, facilitating the premature initiation of the anagen phase—the active growth phase of hair follicles.
While the UCLA study provided data on the association between the stimulation of autophagy and overall hair growth and regeneration, a gap remained in our understanding of how autophagy influences functionality of HFSCs specifically throughout the different stages of the hair cycle. As we’ve discussed HFSCs are pivotal for hair regeneration, and their failure to activate properly can lead to hair loss. Thus, dissecting the role of autophagy in the metabolism and activation of HFSCs is critical for developing targeted treatments for hair loss conditions.
Motivated by this knowledge gap, a new study that appeared in the Journal of Cell and Bioscience aimed to delve deeper into the role of autophagy within HFSCs during the various stages of the hair follicle cycle.
This study sought to understand the autophagic activity within HFSCs across the various phases of the hair cycle, with a particular emphasis on understanding how autophagy affects the metabolic and functional state of these cells. The researchers employed co-localization assays for autophagy markers and the HFSC-specific marker K15 on mouse dorsal skin, meticulously tracking the presence and fluctuation of autophagy markers from the early telogen rest phase through to the anagen growth phase.
Their observations spanned from the early telogen rest phase(50 days post-birth) through to the anagen growth phase (84 days post-birth of the follicle). They noted a sparse presence of autophagy markers in HFSCs during the early and middle/late telogen rest phases. However, a spike in these markers was evident during the transition from telogen to anagen, followed by a decrease upon entering the anagen phase. Concurrently, levels of P62, a protein that accumulates when autophagy is inhibited, diminished during the telogen-anagen transition, further corroborating the uptick in autophagic activity.
These findings indicate that autophagy is naturally ramped up during the ramp-up to the anagen growth phase. There is a notable induction during the critical transition phase between telogen and anagen. This pattern underscores the essential function of autophagy in activating HFSCs and initiating the hair follicle cycle, positioning autophagy not merely as a supportive process but as a critical driver of hair follicle renewal and stem cell activation.
The study represents a significant advance in our comprehension of autophagy's involvement in hair biology, establishing a direct link between autophagic activity and the regulation of HFSC functionality. This connection between autophagy and HFSC activation during the telogen-anagen transition opens new avenues for therapeutic strategies aimed at enhancing hair growth and addressing hair loss. By targeting autophagy modulation, future treatments could leverage this intrinsic cellular mechanism to stimulate HFSCs, potentially unlocking novel approaches for combating alopecia and other hair growth disorders.
Next, the researchers attempted to pharmacologically manipulate hair growth by introducing molecules to stimulate autophagy in the HFSCs. The study employed two pharmacological agents: rapamycin, known for its autophagy-activating properties, and 3-methyladenine (3-MA), an inhibitor of autophagy. This approach was designed to offer insights into how manipulating autophagy levels could impact HFSC behavior and the broader hair follicle cycle.
Their experiments treated both mouse and human hair follicles with rapamycin and 3-MA under controlled conditions. The inhibition of autophagy through 3-MA treatment resulted in a discernible impediment to the hair follicle cycle's progression. This reinforces what we saw in the UCLA study with the application of autophinib and its impairment of autophagy leading to the impairment of hair regeneration. This was characterized by a delay in the transition of hair follicles into the anagen phase, suggesting that adequate autophagic activity is essential for the normal cycling of hair follicles.
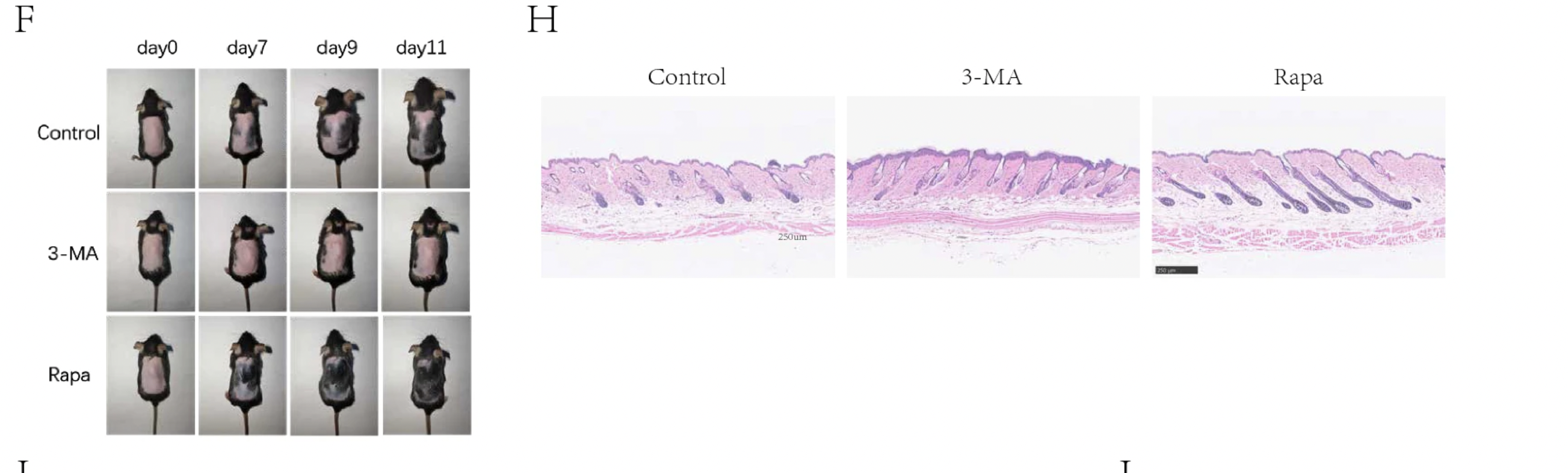
Conversely, stimulating autophagy through rapamycin administration produced beneficial outcomes on HFSC activation. Remarkably, rapamycin not only succeeded in activating HFSCs but also significantly enhanced their proliferation. This surge in HFSC activity, attributed to heightened autophagic processes, played a pivotal role in propelling the hair follicle into the anagen phase with greater ease.
Human Data: Exploring Autophagy's Effects on Human HFSC Activation and Hair Follicle Cycle Through Organ Culture Models
Building on the insights gained from mouse studies, the study expanded to examine whether the autophagy-mediated regulatory effects on hair follicle stem cell (HFSC) activation and the hair growth cycle observed in mice are consistent in human biology. For this purpose, the researchers employed a human hair follicle organ culture model, a sophisticated in vitro system that preserves the complex microenvironment and interactions essential for follicle function.
Their findings in the human model aligned closely with those observed in mice. The induction of autophagy was found to significantly promote the proliferation of human HFSCs, similar to its effects in the mouse model. Beyond merely promoting HFSC proliferation, autophagic activation extended the anagen phase within the hair follicles, proposing a mechanistic insight into how autophagy may underpin prolonged hair growth phases.
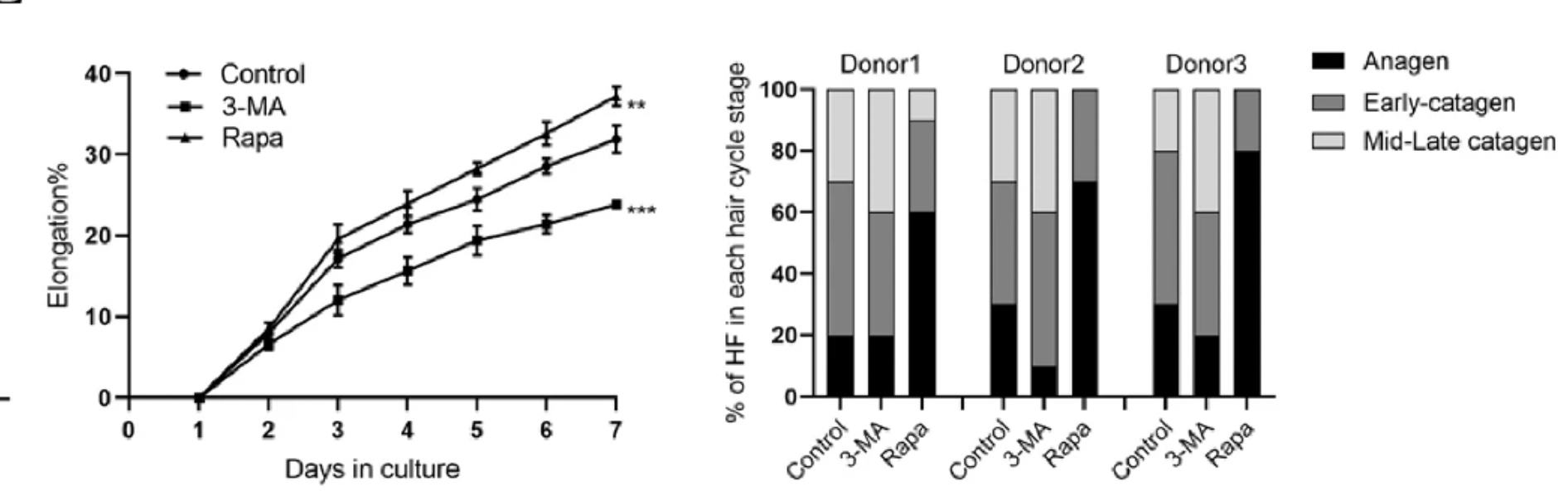
A particularly striking discovery from the human model was autophagy's capability to rejuvenate stem cell properties in human HFSCs. This revelation suggests that autophagy transcends its immediate regulatory role in hair growth and follicle cycling to potentially reinvigorate the intrinsic qualities and functional potential of HFSCs themselves.
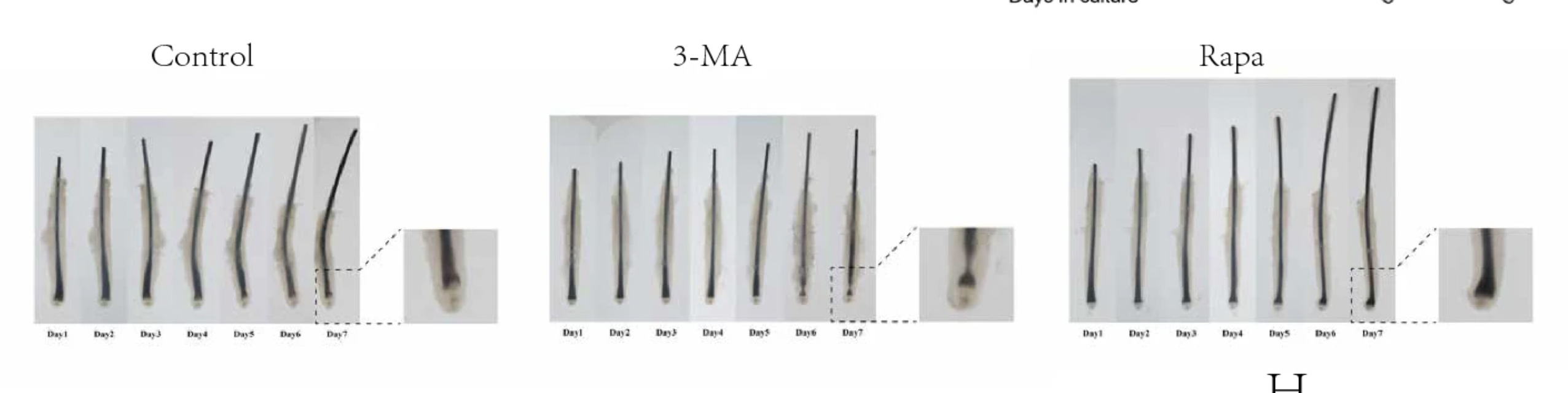
These results from human hair follicle organ cultures provide compelling evidence that autophagy's regulatory influence on HFSC activation and the hair growth cycle is indeed conserved across species. Furthermore, the capacity of autophagy to restore stem cell properties in HFSCs opens new avenues for understanding hair follicle biology and developing treatments for hair loss conditions, highlighting the therapeutic potential of modulating autophagy in hair regeneration strategies.
Conclusion
In wrapping up this extensive review of the roles of cellular senescence and autophagy within the context of hair follicle biology, we've unearthed the complex biological underpinnings that contribute to hair loss and its potential regeneration. This exploration has not only highlighted the deleterious effects of cellular aging on hair health but also brought to light the promising capabilities of autophagy to mitigate these impacts. Throughout this narrative, novel therapeutic targets have been identified, warranting further scientific inquiry.
Our deep dive into the interconnection between diminished autophagy, escalated cellular senescence, and their collective impact on hair follicle stem cells (HFSCs) has unveiled a pivotal revelation: hair loss transcends cosmetic concerns, reflecting intricate biological phenomena. The mechanistic target of rapamycin (mTOR) pathway, a linchpin in autophagy regulation, stands out as a critical focal point for future interventions. Here, rapamycin and similar autophagy-modulating compounds emerge as promising avenues for revitalizing hair follicle vitality and functionality.
Additionally, empirical evidence derived from both animal and human organ culture studies underscores autophagy's crucial role in bolstering the regenerative prowess of HFSCs. This underpins a strong case for the centrality of autophagy in pioneering advanced hair regeneration treatments. The discovery that autophagy not only facilitates HFSC activation but may also rejuvenate their inherent characteristics marks a significant stride toward developing efficacious hair loss remedies.
Beyond its contribution to decoding the mechanisms behind hair loss, this review serves as a pivotal bridge linking hair follicle aging to the expansive domain of aging research. By illuminating the cellular mechanisms driving hair follicle aging, we glean valuable insights into systemic aging, charting a course toward innovative approaches for enhancing healthspan and confronting age-related conditions.
Looking ahead, the prospect of targeting autophagy and cellular senescence in the battle against hair loss—and potentially in influencing systemic aging—represents an exhilarating frontier in biomedical exploration. The opportunity to leverage these biological mechanisms in formulating groundbreaking, efficacious treatments carries the dual promise of addressing hair loss and broader aging and regenerative medicine challenges. This pursuit, albeit daunting, underscores the imperative for ongoing research and interdisciplinary collaboration to unravel the complexities of aging and regeneration, aiming ultimately to enrich human health and extend longevity.
- Müller-Röver, S., Foitzik, K., Paus, R., Handjiski, B., van der Veen, C., Eichmüller, S., McKay, I.A. and Stenn, K.S., 2001. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. Journal of investigative dermatology, 117(1), pp.3-15. https://doi.org/10.1046/j.0022-202x.2001.01377.x
- Schneider, M. R., Schmidt-Ullrich, R., & Paus, R. (2009). The hair follicle as a dynamic miniorgan. Current biology, 19(3), R132-R142. https://www.sciencedirect.com/science/article/pii/S0960982208016266
- Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002 Feb;118(2):216-25. doi: 10.1046/j.0022-202x.2001.01670.x. PMID: 11841536. https://pubmed.ncbi.nlm.nih.gov/11841536/
- Wilson C, Cotsarelis G, Wei ZG, Fryer E, Margolis-Fryer J, Ostead M, Tokarek R, Sun TT, Lavker RM. Cells within the bulge region of mouse hair follicle transiently proliferate during early anagen: heterogeneity and functional differences of various hair cycles. Differentiation. 1994 Jan;55(2):127-36. doi: 10.1046/j.1432-0436.1994.5520127.x. PMID: 8143930. https://www.sciencedirect.com/science/article/pii/S0301468111603320?via%3Dihub
- Botchkarev VA, Kishimoto J. Molecular control of epithelial-mesenchymal interactions during hair follicle cycling. J Investig Dermatol Symp Proc. 2003;8(1):46–55. http://www.ncbi.nlm.nih.gov/pubmed/12894994
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118(5):635–48. http://www.ncbi.nlm.nih.gov/pubmed/15339667
- McHugh D, Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol. 2018;217(1):65–77. https://pubmed.ncbi.nlm.nih.gov/29114066/
- Randall VA, Hibberts NA, Hamada K. A comparison of the culture and growth of dermal papilla cells from hair follicles from non-balding and balding (androgenetic alopecia) scalp. Br J Dermatol. 1996;134(3):437–44. http://www.ncbi.nlm.nih.gov/pubmed/8731666
- Lin E, Zhu N, Cai B, Huang K, Lin C. The role of LncRNAs and miRNAs in hair papilla cell senescence and hair follicle regeneration (in Chinese). Chin J Aesth Plast Surg. 2018;29(07):4357+3.
- Bahta AW, Farjo N, Farjo B, Philpott MP. Premature senescence of balding dermal papilla cells in vitro is associated with p16(INK4a) expression. J Invest Dermatol. 2008 May;128(5):1088-94. doi: 10.1038/sj.jid.5701147. Epub 2007 Nov 8. PMID: 17989730. https://www.jidonline.org/article/S0022-202X(15)33852-5/fulltext
- Sennett R, Rendl M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin Cell Dev Biol. 2012 Oct;23(8):917-27. doi: 10.1016/j.semcdb.2012.08.011. Epub 2012 Aug 31. PMID: 22960356; PMCID: PMC3496047. https://pubmed.ncbi.nlm.nih.gov/22960356/
- Yongqiong Deng, Mengxue Wang, Yuxin He, Fuming Liu, Lingna Chen, Xia Xiong; Cellular Senescence: Ageing and Androgenetic Alopecia. Dermatology 3 August 2023; 239 (4): 533–541. https://doi.org/10.1159/000530681
- Inui S, Itami S. Molecular basis of androgenetic alopecia: from androgen to paracrine mediators through dermal papilla. J Dermatol Sci. 2011;61(1):1–6. https://pubmed.ncbi.nlm.nih.gov/21167691/
- Lin E, Zhu N, Cai B, Huang K, Lin C. The role of LncRNAs and miRNAs in hair papilla cell senescence and hair follicle regeneration (in Chinese). Chin J Aesth Plast Surg. 2018;29(07):4357+3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9368379/
- Hamada K, Randall VA. Inhibitory autocrine factors produced by the mesenchyme-derived hair follicle dermal papilla may be a key to male pattern baldness. Br J Dermatol. 2006;154(4):609–18. https://pubmed.ncbi.nlm.nih.gov/16536801/
- Turksen K, Kupper T, Degenstein L, Williams I, Fuchs E. Interleukin 6: insights to its function in skin by overexpression in transgenic mice. Proc Natl Acad Sci U S A. 1992;89(11):5068–72. https://pubmed.ncbi.nlm.nih.gov/1375756/
- Liu D, Xu Y. p53, oxidative stress, and aging. Antioxid Redox Signal. 2011;15(6):1669–78. https://pubmed.ncbi.nlm.nih.gov/21050134/
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 20072921R1836. https://pubmed.ncbi.nlm.nih.gov/16917020/
- Kloepper JE, Baris OR, Reuter K, Kobayashi K, Weiland D, Vidali S. Mitochondrial function in murine skin epithelium is crucial for hair follicle morphogenesis and epithelial-mesenchymal interactions. J Invest Dermatol. 2015;135(3):679–89. https://pubmed.ncbi.nlm.nih.gov/25371971/
- Zhu H, Wei Y, Min Z, Gao Y, Yang J. Effects of pine massoniana needle extract on androgenetic alopecia model rats via Nrf2-ARE mediated signaling pathway. J Nanjing Univ Tradit Chin Med. 2022;38(2):129–35. https://karger.com/drm/article/239/4/533/836627/Cellular-Senescence-Ageing-and-Androgenetic
- Wu M, Luo Q, Nie R, Yang X, Tang Z, Chen H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit Rev Food Sci Nutr. 2021;61(13):2175–93. https://doi.org/10.1080/10408398.2020.1773390
- Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–72. Published 2018 Apr 26. https://doi.org/10.2147/cia.s158513
- Messenger AG. Hair through the female life cycle. Br J Dermatol. 2011165Suppl 326. http://www.ncbi.nlm.nih.gov/pubmed/22171678
- Matsumura H, Mohri Y, Binh NT, Morinaga H, Fukuda M, Ito M. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science. 20163516273aad4395. http://www.ncbi.nlm.nih.gov/pubmed/26912707
- Mizushima, Noboru, Beth Levine, Ana Maria Cuervo, and Daniel J. Klionsky. "Autophagy fights disease through cellular self-digestion." nature 451, no. 7182 (2008): 1069-1075. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2670399/
- Cuervo, Ana Maria, and J. Fred Dice. "Age-related decline in chaperone-mediated autophagy." Journal of Biological Chemistry 275, no. 40 (2000): 31505-31513. https://www.jbc.org/article/S0021-9258(20)89436-8/abstract
- Levine, Beth, and Guido Kroemer. "Autophagy in the pathogenesis of disease." Cell 132, no. 1 (2008): 27-42. https://www.cell.com/fulltext/S0092-8674(07)01685-6
- Ho, Theodore T., Matthew R. Warr, Emmalee R. Adelman, Olivia M. Lansinger, Johanna Flach, Evgenia V. Verovskaya, Maria E. Figueroa, and Emmanuelle Passegué. "Autophagy maintains the metabolism and function of young and old stem cells." Nature 543, no. 7644 (2017): 205-210. https://www.nature.com/articles/nature21388
- Tang, Ann H., and Thomas A. Rando. "Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation." The EMBO journal 33, no. 23 (2014): 2782-2797. https://www.embopress.org/doi/abs/10.15252/EMBJ.201488278
- Chai M, Jiang M, Vergnes L, Fu X, de Barros SC, Doan NB, Huang W, Chu J, Jiao J, Herschman H, Crooks GM, Reue K, Huang J. Stimulation of Hair Growth by Small Molecules that Activate Autophagy. Cell Rep. 2019 Jun 18;27(12):3413-3421.e3. doi: 10.1016/j.celrep.2019.05.070. PMID: 31216464. https://www.sciencedirect.com/science/article/pii/S2211124719306990#bib37
- Parodi, Chiara, Jonathan A. Hardman, Giulia Allavena, Roberto Marotta, Tiziano Catelani, Marta Bertolini, Ralf Paus, and Benedetto Grimaldi. "Autophagy is essential for maintaining the growth of a human (mini-) organ: Evidence from scalp hair follicle organ culture." PLoS biology 16, no. 3 (2018): e2002864.
- Sun P, Wang Z, Li S, Yin J, Gan Y, Liu S, Lin Z, Wang H, Fan Z, Qu Q, Hu Z, Li K, Miao Y. Autophagy induces hair follicle stem cell activation and hair follicle regeneration by regulating glycolysis. Cell Biosci. 2024 Jan 5;14(1):6. doi: 10.1186/s13578-023-01177-2. PMID: 38183147; PMCID: PMC10770887.