Rapamycin
The most powerful tool to stop the acceleration of aging caused by mTOR dysfunction and cellular senescence.
As the scientific community grapples with neurodegenerative disorders, the study of mTOR signaling provides a glimmer of optimism. But what's the connection between mTOR and debilitating conditions like Alzheimer's or Parkinson's? And how can we leverage this knowledge to stave off cognitive decline? In this article, we dive deep into the molecular intricacies of mTOR pathways and their potentially profound implications in neurodegenerative diseases. Moreover, we delve into the therapeutic promise of rapamycin, a molecule that may herald a paradigm shift in neurodegenerative disease management and prophylaxis.
rapamycin
14 mins
By: Daniel Tawfik
The mammalian target of rapamycin (mTOR) pathway is an integral component of cellular function. Central to processes ranging from cell growth to protein synthesis, mTOR's significance isn't limited to cell biology.
Recent research is highlighting the potential implications of mTOR in the realm of neurology, specifically in aging and neurodegeneration.
Given the prominent role of mTOR in aging, a topic of intense scientific curiosity is whether mTOR can be harnessed as a target to combat aging-related neurodegeneration. Let's break down the reasons behind this hypothesis.
Role in Lifespan: The discovery that inhibiting mTOR can extend the lifespan in a variety of models, from yeast to mammals, has sparked intrigue. Such a consistent effect across diverse organisms suggests that mTOR plays a foundational role in aging.
Neurodegeneration and Aging: Aging remains the most substantial risk factor for neurodegenerative diseases like Alzheimer's and Parkinson's. Therefore, pathways implicated in aging are logical targets for research into these diseases.
Memory Formation: Memory formation is a sophisticated process, with multiple cellular pathways playing a part. mTOR's involvement in this process, particularly in late-phase long-term potentiation (LTP), underscores its importance in neurological function. Moreover, the process of memory formation often becomes compromised in many neurodegenerative diseases.
Digging deeper into memory function, studies have shown that mTORC1 is crucial for long-term potentiation pathway [1-4], a process by which synaptic connections strengthen, facilitating memory storage. Rapamycin, a drug known to inhibit mTOR, has been shown to impact rodent memory, especially in scenarios involving fear and spatial navigation. This underlines mTOR's pivotal role in memory formation across multiple brain regions [3,4].
Moreover, mTOR is known to boost the synthesis of proteins within dendrites, the branching structures of neurons in the hippocampus [5]. This region is pivotal for memory and learning. But while some mTOR activity is beneficial, too much can be detrimental. For example, in the context of a genetic condition like tuberous sclerosis, a rare genetic disorder that causes non-cancerous (benign) tumors to grow in many parts of the body, overactive mTOR leads to memory impairment. Fascinatingly, treatment with rapamycin, which inhibits mTOR, can normalize this [6]. Humans with mutations in genes related to tuberous sclerosis also show increased mTOR activity and memory dysfunction [6].
A link between mTOR and memory is further demonstrated in the context of cannabinoids, substances known to influence memory. Studies show that mTOR activity spikes when cannabinoids are ingested, leading to impaired memory. However, when rapamycin is introduced during this process, the disruption is reduced, suggesting mTOR's activity as a key modulator [47].
All these findings indicate a balancing act: there seems to be an optimal range of mTOR activity conducive to memory formation. This makes the precise dosing of inhibitors like rapamycin crucial for therapeutic purposes [8-11].
The mTOR signaling pathway plays an intricate role in various cellular functions, and unsurprisingly, its dysregulation has been associated with several diseases, particularly neurodegenerative disorders. Let's unpack this complex interplay in the context of some of the most debilitating neurological ailments.
It's been observed that mTOR signaling can go awry in multiple neurodegenerative disorders [11]. Alzheimer's disease (AD), characterized by memory loss and cognitive decline, stands out prominently in this context. Research has shown mTOR to be unusually active, or hyperactive, in both laboratory models (in vitro) and living organisms (in vivo) designed to mimic AD [9]. Furthermore, in post-mortem examinations of brain tissues from Alzheimer's patients, similar heightened mTOR activity has been detected [12].
Beta-amyloid (Aβ) is a protein fragment that accumulates abnormally in the brains of AD patients, forming clumps or plaques. These plaques are a hallmark of Alzheimer's pathology. What's intriguing is that this very beta-amyloid seems to have a role in the hyperactivity of mTOR observed in AD models.
Experiments on a specific mouse model of Alzheimer's (3×Tg-AD) demonstrated that reducing Aβ levels—either through direct injection of an Aβ-neutralizing antibody into the hippocampus or by cross-breeding transgenic mice with non-transgenic ones (thus reducing inherited amyloid burden)—was enough to normalize mTOR activity [9]. This suggests a direct link between beta-amyloid accumulation and mTOR dysregulation.
Another significant player in AD is the tau protein. In healthy neurons, tau helps stabilize microtubules, crucial for nutrient transportation. However, in AD, tau becomes hyperphosphorylated and forms twisted fibers known as neurofibrillary tangles (NFTs) inside neurons, leading to their dysfunction.
It's been observed that active mTORC1, a complex within the mTOR pathway, can boost the production of tau [12, 13]. In fruit flies (drosophila), overproduction of tau was linked to heightened mTORC1 activity [14]. This paints a scenario where mTOR dysregulation could kickstart a vicious cycle: hyperactive mTOR boosts tau production, which in turn might further activate mTOR, thereby promoting the formation of NFTs in AD.
Given these connections, interventions targeting mTOR have been explored. For instance, when specific mouse models of Alzheimer's (PDAPP and 3×Tg-AD) were treated with rapamycin, an mTOR inhibitor, there was a notable reduction in the brain levels and aggregation of both beta-amyloid and tau. This was accompanied by the restoration of typical mTOR activity levels and a notable recovery from cognitive impairments [8, 15].
Lastly, the process of autophagy, where cells "clean up" damaged components, has emerged as a crucial aspect of this discussion. Beta-amyloid is known to be broken down through autophagy [16, 17]. However, this degradation process appears to be compromised in AD patients and their corresponding mouse models [18], underscoring another avenue where mTOR's regulation might play a pivotal role.
At its core, the structure of a protein dictates its function. When proteins fold correctly, they perform their tasks effectively within cells. However, neurodegenerative diseases often emerge from a malfunction in this precise ballet of protein folding.
In these conditions, proteins do not assume their intended shapes, leading them to cluster together, forming aggregates and inclusions. Think of these aggregates as molecular traffic jams, obstructing the regular flow of cellular activity. While the identity of the misfolded proteins varies from one neurodegenerative disease to another, the mechanisms cells use to dispose of these problematic proteins remain consistent.
The cell possesses remarkable housekeeping systems that routinely identify and break down damaged or unnecessary proteins. Here's a snapshot of these processes:
Ubiquitin-Proteasome Pathway: Imagine a molecular 'tag' being placed on unwanted proteins. This tag, known as ubiquitin, marks proteins for rapid degradation. Once tagged, the protein is sent to a cellular machine called the proteasome, which can be thought of as a 'protein shredder', tearing the unwanted protein into its constituent parts for recycling.
Lysosomal Pathway: Another cleanup crew in the cell involves lysosomes, tiny sac-like structures filled with digestive enzymes. When proteins are identified for disposal, they are surrounded and sealed off by a lysosome. Inside, they're broken down into their basic components.
When the cell is under nutritional stress or 'starvation', it activates an enhanced form of the lysosomal pathway called autophagy. Picture this as the cell's version of 'spring cleaning'. Cellular components, including misfolded proteins, are rounded up and delivered to lysosomes for breakdown, allowing their components to be reused to craft new proteins.
Now, circling back to the mTOR pathway, it wears many hats, one of which is acting as a brake pedal for autophagy [19]. When mTOR is active, it suppresses this cleaning process. This means that any disruption to mTOR activity can have profound implications for how effectively cells clear out protein aggregates.
Alarming observations from post-mortem brain samples of individuals with various neurodegenerative diseases have consistently highlighted hiccups in autophagy [20-23]. The cellular equivalents of recycling bins, called autophagosomes, seem to be inadequately emptied [23-25]. These findings have drawn researchers to a compelling hypothesis: By increasing autophagy, could we improve the cell's ability to clear misfolded proteins? And by doing so, might we find new ways to treat a range of neurodegenerative diseases?
Recent evidence supports the view that rapamycin doesn't just inhibit mTOR; it actively promotes the cell's self-eating mechanism – autophagy [16, 22]. Specifically, rapamycin appears to facilitate autophagy in two steps:
Kick-starting Autophagosome Formation: The initial phase of autophagy involves the formation of double-membraned vesicles called autophagosomes, which encapsulate cellular waste. Rapamycin aids in the production of these cellular 'recycling bins'.
Boosting Lysosomal Production: For autophagy to conclude effectively, autophagosomes fuse with lysosomes, which carry the enzymes needed to digest and recycle the waste. Rapamycin seems to enhance the creation of these critical digestive sacs [16, 22].
Parkinson's Disease (PD) is characterized by the death of dopamine-producing neurons in the substantia nigra, a region of the midbrain, leading to motor impairments among other symptoms. A chemical model of PD uses a neurotoxin called MPTP to induce PD-like symptoms in animal studies.
When animals are exposed to MPTP, there's a build-up of autophagosomes in the midbrain, suggesting a bottleneck in the autophagy process. Interestingly, rapamycin treatment seemed to 'unclog' this bottleneck by promoting the efficient clearance of these autophagosomes, reducing the loss of those crucial dopamine-producing neurons [22].
Further cementing the connection between autophagy and PD is the protein alpha-synuclein. Abnormal clumps of this protein form the infamous Lewy bodies, another hallmark of PD. This protein doesn't just aggregate randomly; its degradation is linked, in part, to autophagy.
When researchers tinkered with cell cultures to produce mutated forms of alpha-synuclein, they noticed an alarming increase in aggregation if they blocked autophagy [26]. This suggests that ramping up autophagy, possibly with agents like rapamycin, might offer therapeutic benefits to PD patients by targeting alpha-synuclein aggregates.
While Parkinson's disease has garnered much attention in the context of rapamycin and autophagy, it's vital to recognize that this mechanism of action extends to other neurodegenerative disorders.
Huntington's disease is a genetic disorder characterized by the progressive death of brain cells, leading to motor, cognitive, and psychiatric symptoms. At the molecular level, the disease is caused by an abnormal expansion of a trinucleotide repeat within the huntingtin gene, leading to the production of a faulty protein. This mutant protein, burdened with an abnormally long stretch of glutamine residues, forms clumps within neurons.
Excitingly, research indicates that this problematic mutant huntingtin protein might be eliminated through autophagy [16, 27]. A particularly intriguing study utilized a derivative of rapamycin, temsirolimus, on mice mimicking Huntington's disease. Not only did these mice show a curbing of mTOR activity (a marker of increased autophagy), but they also exhibited improved motor coordination and a significant reduction in brain huntingtin aggregates [16].
Autophagy isn't just implicated in disease. It plays a substantial role in the natural aging process. Generally, as organisms age, the efficiency of autophagy declines.
A more specialized form of autophagy, chaperone-mediated autophagy (CMA), seems to be especially significant in maintaining protein balance within cells. When CMA falters with age, it causes disturbances in protein equilibrium and adversely impacts metabolic function in aging mice. Fascinatingly, tweaking the genetics of these mice to prevent this age-related CMA drop enhanced the function and equilibrium of liver cells [28]. This underscores the importance of autophagy not just in disease states, but in normal aging as well.
Simple organisms, such as C. elegans (a type of nematode), provide further insights. In these organisms, the efficiency of autophagy plummets after their reproductive age. Intriguingly, there's a strong link between reproductive span and overall lifespan in these nematodes [29].
While caution should be exercised when extrapolating results from model organisms to humans, these findings present an interesting hypothesis. Could the decline in autophagy following menopause in women be a contributing factor to the higher incidence of Alzheimer's disease observed in women compared to men [30]? This remains speculative, but it emphasizes the overarching importance of autophagy in various life processes and diseases.
Autophagy, a cellular process critical for clearing out damaged proteins, is certainly front and center in neurodegenerative research. However, the brain's health hinges on multiple mechanisms, and a holistic understanding requires venturing beyond just autophagy.
Central to understanding the broader landscape of Alzheimer's disease (AD) is a condition known as Cerebral Amyloid Angiopathy (CAA). CAA is essentially the deposition of beta-amyloid, a hallmark protein of AD, within the walls of brain blood vessels. Over time, this deposition weakens the vessels, predisposing the brain to hemorrhages. The ubiquity of CAA in Alzheimer's patients—it is present in over 80% of cases [31]—underscores its significance.
Beyond just structural damage, CAA compromises one of the brain's most essential needs: blood flow. Cerebral blood flow (CBF) brings oxygen and vital nutrients to the brain while helping to clear away waste. As CAA progresses, blood flow to various brain regions diminishes, exacerbating the symptoms of AD [32].
Given the damaging impact of CAA on blood flow, strategies aimed at restoring CBF are paramount. Here again, rapamycin shines with potential. Beyond its role in promoting autophagy, rapamycin might counteract the vascular effects of CAA in two pivotal ways.
First, by directly combating beta-amyloid deposition within the vessels. As the beta-amyloid burden decreases, vessel integrity could potentially be preserved, reducing the risk of hemorrhages.
The second avenue is even more direct: by enhancing blood flow. A study on J20 AD mice—a commonly used animal model for Alzheimer's—revealed that rapamycin administration boosted cerebral blood flow. This wasn't just a random effect. The mechanism behind it was traced back to an increase in the production of nitric oxide, a molecule famous for its vessel-relaxing properties. The enzyme eNOS, stimulated by rapamycin, is behind this surge in nitric oxide, leading to more relaxed and dilated vessels [33].
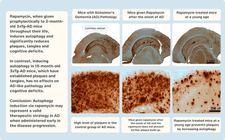
Recent studies have thrust the J20 AD mouse model into the limelight. These animals, genetically engineered to exhibit AD-like symptoms, are pivotal in our understanding of Alzheimer's progression. In groundbreaking experiments, J20 AD mice treated with rapamycin showed a retention of their cognitive abilities, contrasting sharply with their untreated counterparts who manifested cognitive decline [15]. Even more encouraging was the timing: rapamycin treatment was initiated post-symptom onset, implying that it could potentially halt, if not reverse, the progression of AD-like symptoms.
Rapamycin and Plaques
However, biology is rarely straightforward. Other studies using the 3×Tg mouse model painted a more complex picture. When treatment began post-symptom onset, around 15 months of age, rapamycin's effects were far less pronounced [34]. However, starting the treatment early in life showed marked benefits. These divergent results underline two things: first, the significance of maintaining a balanced mTOR activity for optimal brain health, and second, the existence of a 'golden window' for therapeutic intervention.
The multi-faceted role of mTOR signaling in neurodegenerative diseases has emerged as a pivotal avenue for scientific exploration. The intricate interplay between dysregulated mTOR signaling and its manifestations across various neurodegenerative disorders, including Alzheimer's and Parkinson's, offers fascinating insights. Yet, as with many aspects of groundbreaking science, the picture remains complex.
The core mechanisms surrounding protein degradation - whether via ubiquitin-proteasome pathways or lysosomal degradation like autophagy - are central to our understanding of how neurodegenerative diseases progress. Rapamycin's ability to modulate these pathways, facilitating autophagy and potentially counteracting the detrimental effects of misfolded proteins, has demonstrated promising therapeutic potential. Moreover, the insights gained from various animal models, including the J20 AD and 3×Tg mouse models, present both hopeful findings and the need for further nuanced exploration, especially concerning the timing and dosing of interventions.
Neurodegeneration remains one of modern medicine's most formidable adversaries. Despite concerted global efforts, we are still in search of interventions that can significantly alter the course of these devastating diseases. As we probe deeper, the idea of prevention, rather than just treatment, becomes an area of intrigue. Leveraging mTOR inhibitors to delay the onset of neurodegenerative diseases is a promising strategy that requires closer examination.
Latest Longevity Research Straight to your Inbox
Sign up for The Longevity Blueprint, a weekly newsletter from Healthspan analyzing the latest longevity research.
Sign up for The Longevity Blueprint, a weekly newsletter from Healthspan analyzing the latest longevity research.