Rapamycin
The most powerful tool to stop the acceleration of aging caused by mTOR dysfunction and cellular senescence.
As researchers continue to search for a cure for osteoarthritis, recent studies have uncovered a fascinating link between hypertrophic and senescent chondrocytes - the cells that make up our cartilage - and the progression of the disease. But what causes this degenerative condition, and how can we stop it in its tracks? In this article, we delve into the science behind these cranky chondrocytes and explore their pivotal role in this debilitating condition. We also explore the exciting potential of microparticle rapamycin therapy, which delivers the drug directly to the affected joints and encourages a shift towards greater joint health and improved mobility.
rapamycin
13 mins
By: Daniel Tawfik, Jacob Rose
Osteoarthritis is a widespread and painful joint disease that affects 1 in 7 people in the United States and is thought to be a major driver of disability as we age. This disorder is characterized by the loss of cartilage and subsequent bone damage surrounding an affected joint, leading to intense pain and loss of mobility.The challenging reality of osteoarthritis is that it seldom appears in isolation. Strikingly, neurodegeneration and osteoarthritis are often recognized as comorbidities, hinting at shared cellular dysfunction that may underlie many diseases of aging.However, the level of cohesive research that surrounds age-related diseases in varying organs is promising, and it may be that the reasons these diseases occur so concurrently will lead to a common treatment for them.Some of those aspects have already been uncovered, and even though this article will focus on hypertrophic chondrocytes, senescence in the cartilage, and how the secretions of these cells drive osteoarthritis, we are coming to understand that there are red threads connecting these diseases of aging.
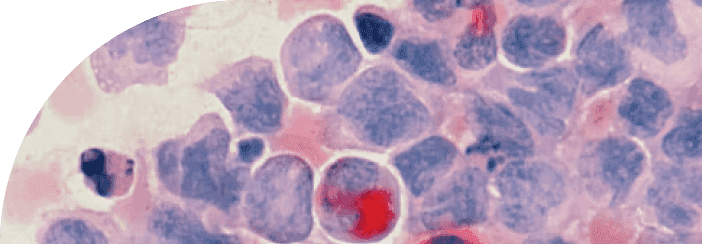
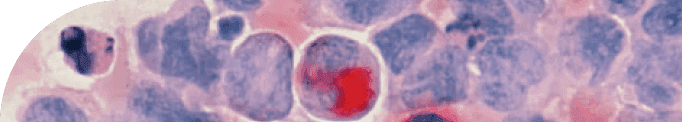
Osteoarthritis pathology is driven by two major biological processes: the gradual deterioration of cartilage and the excessive growth of the underlying bone. To understand the connection between these processes, let's first explore the maturation of articular cartilage and bone formation.Articular cartilage is the smooth, rubber-like tissue that covers the ends of bones in our joints, allowing for smooth movement and shock absorption. This cartilage is maintained by chondrocytes, specialized cells responsible for producing and regulating the extracellular matrix (ECM), which forms the structural framework for cartilage. As we age or experience joint injuries, the balance between ECM production and degradation can be disrupted, leading to the loss of cartilage and the onset of osteoarthritis.Simultaneously, the underlying bone can undergo excessive growth, a process known as bone remodeling. This occurs when the balance between bone formation, driven by cells called osteoblasts, and bone resorption, carried out by osteoclasts, becomes disrupted. In osteoarthritis, this imbalance results in the formation of bony outgrowths, or osteophytes, around the joint. These osteophytes contribute to joint stiffness, pain, and reduced mobility, which are hallmark symptoms of osteoarthritis.Chondrocytes within articular cartilage are considered quiescent, which means that they are typically non-dividing and metabolically inactive. However, they are still able to carry out their main function of secreting and maintaining the ECM, which is critical for the health and function of the cartilage. The ECM itself is a complex mixture of proteins and other molecules, including collagen and proteoglycans, which give articular cartilage its unique mechanical properties and resistance to wear and tear.Chondrocytes are largely responsible for the deposition of the proteins that make up the ECM, like collagen and aggrecan. These proteins, paired with water and a natural lubricant, form the smooth, low-friction material that keeps our bones from rubbing up against one another.The ECM is an incredibly important structural bed that cells lie in. Typically every cell type or tissue has preferences for what their bed is made of. For chondrocytes to get ample rest and stay quiescent, there must be an abundance of type II Collagen.This particular type of collagen is critical for the structural support and mechanical properties of articular cartilage, and it is necessary for chondrocytes to be able to maintain a healthy and quiescent state.Without sufficient amounts of type II collagen in the ECM, chondrocytes may become more active and begin to divide or produce different types of ECM molecules, which can lead to damage or degradation of the cartilage tissue. Therefore, ensuring that the ECM contains an abundance of type II collagen is critical for articular cartilage's proper functioning and health.Collagen II is largely specific to cartilage and provides just the right amount of cushion for chondrocytes to live in a material that bears weight without being too squishy. Additionally, Collagen II provides preferred anchor points for chondrocytes. Without these anchor points, chondrocytes will activate, move, and divide in attempts to find places to bed down.We see in models of osteoarthritis that the level of Collagen II is dramatically reduced and is replaced with other types of Collagens like I and X that provide a very different ECM bed leading to altered chondrocyte function.Chondrocytes hold a fascinating dual role as they are not only vital for cartilage health but also serve as the origin of mineralized bone. Contrary to the general notion that excessive cell growth, or hypertrophy, contributes to aging, chondrocyte hypertrophy is essential for forming healthy bone. Chondrocyte proliferation and hypertrophy kick off a complex sequence known as endochondral ossification, which refers to bone growth, particularly around joints.Endochondral ossification commences as chondrocytes exit dormancy, multiply, and undergo hypertrophy. These enlarged chondrocytes actively produce Collagen X, playing a pivotal role in bone development. They function as architects, displacing soft cartilage to create space for the sturdy bone structure.Once the hypertrophic chondrocytes have carved out a nice space, they mineralize their environment and undergo programmed cell death (apoptosis), leaving holes for further mineralization, blood vessel infiltration, and the appearance of classic bone cells like osteocytes.Hypertrophic chondrocytes are needed when actively forming our skeleton, but what happens when the landscapers come out of retirement in response to age or damage?It's fascinating to consider the unique mechanical sensitivity of cells within bone and cartilage. While all cells are somewhat aware of the physical forces in their microenvironment, those residing in cartilage and bone are particularly attuned to their surroundings and anchor points.The remarkable sensitivity of chondrocytes in detecting changes to their environment is essential for responding to cartilage damage, which can occur due to natural aging, injuries, or multiple surgeries on the same joint. When this damage takes place, dormant chondrocytes spring into action upon recognizing the absence of specific extracellular matrix (ECM) proteins and anchor points, setting off a cascade of events that ultimately alters their behavior and triggers cartilage degradation.Ironically, this process that aims to repair the damage ends up displacing what should be long-lasting cartilage. This results in the cartilage becoming mineralized, more porous, and prone to higher friction. Scientists have observed this phenomenon as cartilage recedes and new mineralized bone growths, called osteophytes, emerge to take its place. Consequently, our joints end up with mineralized and vascularized sections rubbing against each other, causing pain and limited mobility, which are the hallmarks of osteoarthritis.
For those familiar with the biology of aging, it may come as no surprise that the burden of cellular senescence is significantly higher in patients with osteoarthritis. Research has consistently shown a connection between cellular processes related to hypertrophy (cell enlargement) and senescence (when cells lose their ability to divide).Mikhail Blagosklonny, a renowned researcher in the field of aging, proposed the concept of cellular hyperfunction as a driver of aging and age-related diseases. According to Blagosklonny's theory, cellular hyperfunction occurs when cells become overactive and continue to perform their functions at an excessive rate, ultimately contributing to the development of age-related pathologies like osteoarthritis. This overactivity is a key characteristic of senescent cells.Senescent cells undergo a series of distinct changes, which include stopping cell division, developing resistance to programmed cell death, adopting an altered and enlarged shape, and increasing the secretion of various molecules. Among these secreted molecules are those that alter the extracellular matrix (ECM) and promote inflammation, further contributing to the development and progression of osteoarthritis.The Senescence-Associated Secretory Phenotype (SASP) is a complex mix of molecules released by senescent cells, which includes lipids, metabolites, proteins, and a witch's brew of inflammatory molecules and growth factors. These signaling compounds play a crucial role in affecting the extracellular matrix (ECM) and the behavior of neighboring cells. However, the SASP can also have detrimental effects on tissues, contributing to the development of pathological changes, such as chronic inflammation and tissue degradation – both of which are commonly seen in osteoarthritis.The SASP is a classic example of cellular hyperfunction. In the case of the SASP, the excessive secretion of various growth factors and inflammatory molecules by senescent cells leads to the pathological changes observed in osteoarthritis and other age-related conditions.In the context of chondrocytes, research has shown that certain "landscaping" proteins secreted by enlarged, hypertrophic chondrocytes are also released by senescent chondrocytes. These proteins contribute to changes in the cartilage, which can have significant implications for joint health.One group of proteins that plays a crucial role in this process is the Matrix Metalloproteinases (MMPs). MMPs are designed to break down the extracellular matrix (ECM) by cleaving specific bonds, including those that hold Collagen II proteins together. As these bonds are severed, the ECM, which can be thought of as the "bed" in which the cells reside, becomes less stable and supportive. In other words, if the cellular "bed" isn't comfortable, the cells won't be able to function optimally, leading to negative effects on the cartilage and joint health overall.The microenvironment surrounding a cell plays a critical role in determining its fate, and cells that are unable to function properly can take on various forms. Ideally, our immune system swiftly removes these dysfunctional cells from our bodies. However, the most persistent and problematic cells can become cancerous or senescent.A single senescent cell can disrupt its local environment by secreting MMP13, which cleaves Collagen 2, and makes the surrounding cells uncomfortable, initiating a cascade of negative effects. Chondrocytes, being highly sensitive to physical cues in their microenvironment, might mistakenly activate "landscaping" processes in response. This action can lead to the expansion of the damaged area and perpetuate a painful cycle, eventually progressing to full-blown osteoarthritis.It's important to note that our joints also contain other cell types that secrete extracellular matrix (ECM) components, such as fibroblasts found in connective tissues. Like chondrocytes, these cells can become senescent, accumulate with age, and modify their microenvironment through the SASP.In the realm of biology, understanding the intricate mechanisms behind cellular processes is crucial for developing targeted treatments for various health conditions. Researchers are currently making significant strides in understanding cellular senescence and the extracellular matrix (ECM), which play important roles in age-related diseases.Dysfunctional, or "cranky," cells can be found throughout our bodies, and addressing the issues within their microenvironments – or the "beds" they reside in – is a promising approach for tackling age-related diseases. By improving the conditions of these cellular "beds," scientists hope to reduce the likelihood of senescent cell accumulation, which contributes to the development and progression of various health conditions. Continued research in this area has the potential to unlock new therapeutic strategies for a wide range of age-related diseases, ultimately improving our overall quality of life as we age.
Rapamycin has been identified as a potential therapeutic agent for various diseases due to its ability to inhibit the mammalian target of rapamycin (mTOR) protein. Inhibition of mTOR has been shown to promote autophagy and reduce cellular senescence. In the context of osteoarthritis, these effects are particularly relevant, as autophagy activation can help maintain healthier joint tissues, while reduced cellular senescence can slow down the aging and deterioration of chondrocytes, the cells responsible for maintaining cartilage health in joints.However, administering rapamycin directly to the affected joint tissues can be challenging due to the drug's poor solubility and rapid clearance. In a recent study titled, "Rapamycin microparticles induce autophagy, prevent senescence and are effective in the treatment of Osteoarthritis," the researchers behind the study developed a microparticle formulation that can help overcome these challenges by providing a sustained-release system that allows for localized and controlled delivery of rapamycin to the joint tissues. This approach aims to maximize the drug's therapeutic effects in the targeted area.The research team pursued this route because they believed that a localized, sustained-release formulation of rapamycin could be more effective in preventing the onset of osteoarthritis following an articular joint injury. By combining the known effects of rapamycin on cellular processes with a novel drug delivery system, the researchers hoped to improve the potential therapeutic outcomes for patients suffering from osteoarthritis.The microparticle formulation of rapamycin was found to be effective in preventing cellular senescence and inducing autophagy in articular chondrocytes. By preventing senescence, the aging and deterioration of these cells can be slowed down, reducing the likelihood of osteoarthritis development. Additionally, the activation of autophagy helps break down and recycle damaged or unnecessary cellular components, further promoting joint tissue health.In the study, the researchers observed reduced inflammation markers and successful prevention and treatment of post-traumatic osteoarthritis in a mice model. To further validate the relevance of these findings for human disease, the researchers tested the microparticle formulation of rapamycin on chondrocytes isolated from osteoarthritic patients. The results supported the potential applicability of this novel therapy for human use.This study provides new insights into developing therapies that target senescence prevention and autophagy activation for the treatment of trauma-induced osteoarthritis. The sustained-release microparticle formulation of rapamycin has shown promise in animal models and human-derived cells.
Osteoarthritis, a common age-related disease, is often overshadowed by other health conditions or dismissed due to the availability of pain relief treatments. However, recent research has revealed connections between osteoarthritis, aging, and cellular senescence.Osteoarthritis is primarily driven by abnormal joint remodeling, usually in response to mechanical stress. While this process is a natural part of joint development, it shouldn't occur once our joints are fully formed. Mature cartilage is meant to be a permanent feature in our joints, with the resident cells mostly responsible for maintaining the extracellular matrix (ECM) over time. Mechanical damage and senescent cells can disrupt the microenvironment, triggering bone formation and leading to symptomatic osteoarthritis.The presence of senescent cells has been linked to various age-related diseases since their discovery by Leonard Hayflick. The impact of senescence extends beyond the Senescence-Associated Secretory Phenotype (SASP) and can affect different cell types in diverse ways, resulting in a wide variety of senescent cells. This heterogeneity is a fundamental aspect of senescence and should be considered when developing treatments targeting senescent cells.Much of the existing data on senescence and the SASP has been derived from fibroblast studies. By integrating this knowledge with emerging research on other cell types, scientists can gain valuable insights that will pave the way for more targeted and effective therapeutic options for age-related diseases, including osteoarthritis.Biologists and doctors see the red threads that link aging organs and are determining the best way to treat the system, not play symptomatic whack-a-mole by treating the worst symptoms first. This is already being tested by prohibiting hypertrophy and, more recently, treating the ECM. Maintaining where our cells sleep, especially in diseases like osteoarthritis, is an avenue that we will likely see more of in the future as medicine and recent biological discoveries coincide.More treatments for underlying causes of disease and aging are needed, and the supplements like rapamycin that aim to tackle the source of the problem instead of the symptom will be where the most success occurs.
Citations
\
Latest Longevity Research Straight to your Inbox
Sign up for The Longevity Blueprint, a weekly newsletter from Healthspan analyzing the latest longevity research.
Sign up for The Longevity Blueprint, a weekly newsletter from Healthspan analyzing the latest longevity research.