Rapamycin
The most powerful tool to stop the acceleration of aging caused by mTOR dysfunction and cellular senescence.
This review article investigates the potential of rapamycin, an mTOR pathway inhibitor, in alleviating symptoms associated with menopause, a natural biological process signifying the end of menstrual cycles and marked by a range of physical and emotional changes. By examining the biological underpinnings of menopause, including hormonal shifts and their systemic effects, the article sets the stage for understanding rapamycin's mechanism of action. Specifically, it explores how rapamycin's regulation of the mTOR pathway may mitigate menopausal symptoms by influencing ovarian function and extending the reproductive lifespan. Drawing on animal studies and initial human research, the review assesses the evidence for rapamycin's efficacy in improving menopausal outcomes. This article contributes to the ongoing discussion on menopause management and the search for effective treatments, emphasizing rapamycin's role in addressing the challenges of reproductive aging.
rapamycin
11 mins
By: Shreshtha Jolly, Shriya Bakhshi
Menopause, a natural biological milestone, signifies the end of the menstrual cycle for individuals assigned female at birth and typically begins in the 40s or 50s, extending over several years.
This transformative phase is characterized by hormone fluctuations, accompanied by various physical and emotional changes. Recently, rapamycin has emerged as a promising avenue for alleviating menopausal symptoms, sparking significant interest in the scientific community.
This in-depth article will delve into the research exploring the relationship between rapamycin and menopause. We will start by examining the biological processes of menopause, exploring its mechanisms and symptoms, to lay the foundation for understanding its complexities. Following this, we will transition to an exploration of rapamycin, covering its traditional applications and its emerging role in addressing menopausal issues.
By dissecting the underlying biological mechanisms through which rapamycin impacts menopausal symptoms, we aim to provide a comprehensive and nuanced insight into this intriguing topic, highlighting the potential of rapamycin as a transformative agent in managing the multifaceted challenges of menopause.
Menopause signifies the transition from a reproductive to a non-reproductive phase, marking an essential and natural part of their journey. It is conventionally defined as occurring when a woman has not had a menstrual period for twelve consecutive months. [1]
Women typically experience menopause between the ages of 40 and 55, with the average onset age being 51. Factors such as smoking and chronic illnesses can lead to an earlier onset of menopause. The transition, involving changes in the ovaries, can last from 2 to 8 years before menopause and up to 1 year after the final menstrual period. To better understand this process, let's first review the role of ovaries in reproduction.
The ovaries, two small organs located in the lower abdomen of individuals assigned female at birth, are crucial for reproductive potential. They produce eggs needed for reproduction. During each menstrual cycle, an egg is released from one of the ovaries in a process called ovulation. If the egg is fertilized by sperm, pregnancy can occur.
Throughout their lives, women have a finite number of eggs in their ovaries. At fetal development, they have the maximum number of eggs, which reduces to around 1 to 2 million at birth. With age, a process called atresia decreases the number of eggs, leaving a few hundred to a few thousand by the time of menopause. [2]
Besides producing eggs, the ovaries also generate hormones like estrogen and progesterone, which regulate the menstrual cycle, maintain bone health, and influence the growth and development of breasts and other reproductive organs.
As a woman ages, the function of the ovaries naturally changes. Menopause marks the stage in their life when the ovaries gradually cease releasing eggs and producing hormones, leading to the end of menstrual periods and a decrease in fertility. [2]
Estrogen and progesterone impact several physiological systems in our bodies. When the levels of these hormones drop during menopause, these systems are affected, leading to a range of physical and emotional symptoms. Individuals may experience mood and sleep disturbances, hot flashes, and urogenital symptoms. [2]
Mood Disturbances
The period around menopause can be a vulnerable time for individuals to experience mood swings. Studies indicate that the risk of depression increases during menopause, with a threefold higher chance of developing a major depressive episode compared to before menopause. A major depressive episode is characterized by intense and persistent sadness or low mood lasting at least two weeks, along with a loss of interest in activities, changes in appetite or weight, sleep disturbances, fatigue, feelings of worthlessness, difficulty concentrating, and thoughts of death or suicide, significantly impacting daily life and functioning. Anxiety symptoms, which can precede depression, may also increase the risk of depression during midlife. [3][4][5]
Sleep Disturbances
As individuals age, sleep quality tends to decline, a process further exacerbated by menopause. Many report increased sleep difficulties, with problems being more pronounced around the menstrual cycle. Studies, such as the longitudinal one conducted by Kravitz et al. (2008), have shown that the likelihood of experiencing sleep problems increases with menopause. However, early morning awakenings tend to decrease from late perimenopause to postmenopause. While hormonal changes contribute to sleep difficulties, factors like sleep habits and mood disorders also play a significant role, emphasizing the importance of identifying the specific nature of sleep problems during menopause. [6]
Hot Flashes
Hot flashes, sudden feelings of warmth often most intense over the face, neck, and chest, are common during menopause. They can cause sweating and a rapid heartbeat, usually followed by a cold chill. About 85% of individuals experience hot flashes during menopause, with frequency and intensity peaking during the late transition phase and gradually decreasing thereafter. [5] [6]
Urogenital Symptoms
Estrogen-sensitive tissues in the urinary and genital areas become fragile during menopause, leading to symptoms like vaginal dryness or pain during sex, which affect 27% to 60% of women. Menopause can also cause narrowing and shortening of the vagina and uterus, as well as bladder issues like urinary incontinence. Unlike hot flashes, these symptoms do not improve over time without treatment. Menopausal hormone therapy, involving synthetic estrogen, has proven effective in managing vaginal atrophy and dryness, although its efficacy for other menopausal symptoms is limited. [7] [8]
As menopause presents a range of symptoms, treatments targeting them holistically are being explored, with rapamycin gaining attention as a potential option.
As outlined in our previous discussions, rapamycin is a medication that targets a complex known as mTOR within our bodies. mTOR acts as a cell's 'mission control,' orchestrating various physiological processes such as cell growth, proliferation, survival, protein synthesis, and the rate of cellular aging. It operates through two types of complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), which mediate these effects.
The functions of mTOR vary with age; it operates optimally to promote cell growth and protein synthesis during youth but can become overactive as we age. This overactivity, or dysregulation, is associated with various age-related diseases, including cancer, diabetes, and neurodegenerative disorders. Rapamycin acts as a molecular 'brake' on mTORC1, preventing mTOR dysregulation and thereby offering protection against numerous age-related conditions.
But, beyond its established role in managing age-related diseases, rapamycin has also been identified as a promising candidate for managing menopausal symptoms.
Research indicates that rapamycin may significantly impact female reproductive health, including its potential effects on ovarian function and fertility. In various animal models, studies have shown that rapamycin can extend the reproductive lifespan and delay the onset of menopause-like changes, offering promising insights for human application.
For instance, Dou et al. (2013) administered rapamycin to mice and observed the effects on both young (8 weeks old) and middle-aged (8 months old) groups. The treatment caused temporary disturbances in ovarian function, but remarkably, all mice regained normal fertility within two months post-treatment. The most intriguing findings emerged when these mice were allowed to mate naturally post-treatment: the ovarian function in both young and middle-aged mice improved and lasted longer, particularly in those over 12 months old, suggesting that even a short-term rapamycin regimen could preserve and enhance egg quality in the ovaries, extending their functional period. [9]
Given that reproductive aging in women typically starts around the age of 31, the study's findings propose a potential strategy of administering rapamycin during the perimenopausal stage. This could improve ovarian function in women, mirroring the prolonged reproductive lifespan seen in the treated mice, and potentially delaying the onset of menopause.
To comprehend how rapamycin achieves its effects, it is essential to delve into its action mechanism, specifically in regulating mTOR pathways crucial for reproductive biology. In women, the ovaries contain numerous eggs, each within an ovarian follicle—a small, fluid-filled sac. During the menstrual cycle, these follicles mature and release eggs, which, if not fertilized, are expelled during menstruation. As time progresses, the ovarian reserve, or the total number of remaining follicles, decreases, leading to irregular menstrual cycles and eventually to menopause, marking the cessation of egg release. [9]
Recent research has illuminated the mTOR pathway's crucial role in activating and growing early-stage ovarian follicles, emphasizing its importance in the timing and development of these follicles. Investigations, particularly using genetic mouse models, have explicitly shown that disruptions in the mTOR pathway can lead to reproductive aging and menopausal symptoms.
At the heart of this regulation are the proteins TSC1 (tuberous sclerosis complex 1) and TSC2 (tuberous sclerosis complex 2), which form a complex that acts as a regulatory brake on mTORC1 activity, preventing its overactivation. However, when mutations lead to the removal or dysfunction of the TSC1/TSC2 complex, this regulatory control is lost. The resultant overactive mTORC1 pathway accelerates the activation of ovarian follicles, leading to their premature depletion. [10]
This research demonstrates that the hyperactivity of mTORC1 is associated with menopausal symptoms, and rapamycin, by inhibiting mTORC1, could provide a therapeutic avenue to delay or alleviate these symptoms. This linkage highlights rapamycin's potential as both a means to understand reproductive aging and a feasible treatment option for managing menopause, paving the way for new research and therapeutic approaches in women's health.
Experiments with mice have shown that rapamycin can reverse the overactivation of early ovarian follicles, reduce follicle growth in the ovaries, and decrease the number of eggs released. This suggests that rapamycin may slow the depletion of eggs from the resting pool each cycle, potentially extending the overall egg reserve and delaying menopause.
Rapamycin is now under investigation for its ability to delay menopause in humans, with pilot studies such as the 'Validating Benefits of Rapamycin for Reproductive Aging Treatment' (VIBRANT) trial in progress. This trial aims to evaluate the efficacy of low-dose rapamycin in delaying ovarian reserve depletion. It will involve 50 healthy women aged 38 to 45 who have regular menstrual cycles but are not seeking pregnancy. Participants will be randomly assigned to receive either weekly oral rapamycin or a placebo, with both participants and researchers blinded to the allocation. The treatment will last three months, followed by nine months of observation, during which ovarian reserve will be assessed through transvaginal ultrasound. [11]
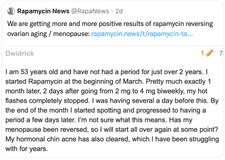
A patient's positive response to rapamycin in treating ovarian aging, as featured on Rapamycin News.
These efforts contribute to a better understanding of rapamycin’s impact on female reproductive health, notably in improving ovarian function and fertility. Animal model studies have already indicated that short-term rapamycin treatment can prolong reproductive lifespan, enhance egg quality, and delay menopause-like changes. This effect is believed to be due to rapamycin’s influence on the mTOR pathways that regulate early ovarian follicle activation and development. By mitigating the excessive activation of these follicles and decelerating egg release, rapamycin holds promise for postponing menopause. Ongoing human trials, like the VIBRANT trial, aim to further explore rapamycin’s capacity to prolong the ovarian reserve in women, thereby offering new avenues for improving ovarian function and delaying menopause.
Exploring rapamycin's role in managing menopausal symptoms marks a significant development in women's health, indicating a shift in our approach to reproductive aging. Although the understanding of rapamycin's full potential is in the early stages, initial findings highlight its ability to influence the mTOR pathway, providing a promising method to enhance ovarian function and delay menopause.
The implications of this research for patients are profound. Should rapamycin prove effective in human trials, it could revolutionize the management of menopausal symptoms, offering women greater control over their reproductive health and aging process. However, the journey does not end with clinical trials. The next steps involve comprehensive studies to ascertain the long-term effects of rapamycin on the menopausal transition, including its safety, efficacy, and impact on quality of life. Additionally, understanding the individual variability in response to rapamycin will be crucial for personalized treatment plans.
Moving forward, it's crucial to adopt a patient-focused strategy, ensuring that any interventions not only sustain ovarian function but also improve the overall well-being and satisfaction during the menopausal phase. The exploration into rapamycin's potential in menopause management offers a promising path for enhancing women's healthcare, aiming for a future where menopause management is optimized through innovative and efficacious treatments.
TAKE HOME POINTS
Menopause is a natural biological milestone indicating the end of the menstrual cycle, typically beginning in the 40s or 50s. It is characterized by hormone fluctuations leading to various physical and emotional changes.
Menopause marks the transition to a non-reproductive phase, defined by the absence of menstrual periods for twelve consecutive months. It occurs between ages 40 and 55, influenced by factors such as smoking and chronic illnesses.
Ovaries play a crucial role in reproduction, producing eggs and hormones. Their function and egg reserve decline with age, leading to menopause.
Signs and Symptoms of Menopause: A drop in estrogen and progesterone levels leads to mood and sleep disturbances, hot flashes, and urogenital symptoms.
Rapamycin targets the mTOR complex, a crucial regulator of cell growth and aging, by acting as a molecular brake on mTORC1.
Rapamycin shows the potential to extend the reproductive lifespan and delay menopausal changes observed in animal studies. It could improve ovarian function and delay the onset of menopause by preserving egg quality and extending the functional period of ovaries.
Mechanism of Rapamycin Preserving Follicular Health: Rapamycin regulates the mTOR pathway, crucial for the activation and growth of ovarian follicles. Inhibition of mTORC1 by rapamycin could delay or alleviate menopausal symptoms by preventing the premature depletion of ovarian follicles.
Research and Trials on Rapamycin Delaying Ovarian Reserve Depletion: Animal studies show rapamycin's potential to slow egg depletion and extend the reproductive lifespan. Human trials, like the VIBRANT trial, aim to evaluate rapamycin's efficacy in delaying ovarian reserve depletion in women.
Latest Longevity Research Straight to your Inbox
Sign up for The Longevity Blueprint, a weekly newsletter from Healthspan analyzing the latest longevity research.
Sign up for The Longevity Blueprint, a weekly newsletter from Healthspan analyzing the latest longevity research.